Abstract
This study aimed at examining and comparing the relevance of various methods in order to discriminate different cellular states of Lactobacillus bulgaricus CFL1 and to improve knowledge on the dynamics of the cellular physiological state during growth and acidification. By using four fluorescent probes combined with multiparametric flow cytometry, membrane integrity, intracellular esterase activity, cellular vitality, membrane depolarization, and intracellular pH were quantified throughout fermentations. Results were compared and correlated with measurements of cultivability, acidification activity (Cinac system), and cellular ability to recover growth in fresh medium (Bioscreen system). The Cinac system and flow cytometry were relevant to distinguish different physiological states throughout growth. Lb. bulgaricus cells maintained their high viability, energetic state, membrane potential, and pH gradient in the late stationary phase, despite the gradual decrease of both cultivability and acidification activity. Viability and membrane integrity were maintained during acidification, at the expense of their cultivability and acidification activity. Finally, this study demonstrated that the physiological state during fermentation was strongly affected by intracellular pH and the pH gradient. The critical pHi of Lb. bulgaricus CFL1 was found to be equal to pH 5.8. Through linear relationships between dpH and cultivability and pHi and acidification activity, pHi and dpH well described the time course of metabolic activity, cultivability, and viability in a single analysis.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
During fermentation, lactic acid bacteria are subjected to inhibitory compounds such as lactic acid and must cope with acidification. Such environmental conditions affect the cellular physiological state, thus leading to suboptimal growth, which often corresponds to a decrease of maximal cell densities (Amrane and Prigent 1999; Cachon et al. 1998). They also act on the performance and the survival potential of lactic acid starters (Baati et al. 2000; Wang et al. 2005).
In order to understand the different cellular events that occur during growth and acidification, various studies have been carried out at population, cellular, and molecular levels. At a population level, cultivability is often determined, but it is a time-consuming task and does not display the heterogeneity within bacterial populations or the diversity of bacterial physiological states (Ben Amor et al. 2002; Kaprelyants et al. 1996; McDougald et al. 1998). Some methods based on metabolic activity are available to describe the behavior of bacterial populations. Among them, the Cinac system (Corrieu et al. 1988) is widely employed for characterizing lactic acid bacteria by determining their acidification activity (Béal and Corrieu 1994). It also gives an indirect estimation of cell concentration (Wang et al. 2005) and has been successfully applied to the quantification of the resistance to freezing, freeze drying, and storage of lactic acid and probiotic bacteria (Fonseca et al. 2000). However, as some cells may be viable but have a less active metabolism, this method can lead to an underestimation of viable cells. Measuring cellular ability to recover growth by using automated spectrophotometric methods such as the Bioscreen system is another reliable tool to quantify a starter’s activity. It has been used by Champagne et al. (1999) to quantify the growth of lactococci and lactobacilli, and by Gabier et al. (2005) to characterize yeast proliferation during storage at 4 °C. In the literature, no information was found about the use of these methods to measure the consequences of acidification during growth of lactic acid bacteria.
At the molecular level, the responses of lactic acid bacteria to acid stress have been studied using genetic, transcriptional, proteomic, and membrane fatty acid analyses. These methods make it possible to demonstrate the ability of cells to develop an acid tolerance response and to reorganize their metabolism (Budin-Verneuil et al. 2005; Guillouard et al. 2004) and membrane fatty acid composition (Fozo et al. 2004; Wang et al. 2005) in response to acid stress. They are, however, laborious and thus inappropriate to gaining an understanding of the dynamic modifications of the cellular state during acidification.
Another approach consists in analyzing the dynamic response of lactic acid bacteria to acidification by integrating molecular data within physiological investigations of metabolic and energetic parameters at a cellular level. This approach makes it possible to identify the energetic balance between catabolism and anabolism in Lactobacillus bulgaricus (Mercade et al. 2004), the dynamic response of catabolic pathways to autoacidification in Lactococcus lactis (Even et al. 2002), and the relationship between acid tolerance, cytoplasmic pH, and ATP levels in cultures of L. lactis (O’Sullivan and Condon 1999). Methods based on fluorescence measurements combined with flow cytometry aim at increasing knowledge on bacterial physiology and on the complex nature of lactic acid bacteria viability (Breeuwer and Abee 2000). They also permit a rapid and quantitative analysis of microbial heterogeneity by determining the size, shape, nucleic acid and protein contents (Davey and Kell 1996), membrane integrity (Baatout et al. 2007; Graça da Silveira et al. 2002), intracellular enzyme activity (Ananta et al. 2005; Bunthof et al. 2001), cellular vitality (Bouix and Leveau 2001; Bunthof et al. 2000), intracellular pH (pHi) (Breeuwer et al. 1996; Siegumfeldt et al. 1999), and membrane potential (Jepras et al. 1995; Novo et al. 2000). Among these different parameters, pH-dependent probes such as carboxyfluorescein diacetate succinimydil ester (cFDA-SE) are used in combination with fluorescence ratio imaging microscopy (FRIM) (Siegumfeldt et al. 1999), spectrofluorimetry (Gabier et al. 2005), and flow cytometry (Lahtinen et al. 2006) to analyze changes in intracellular pH after a sudden acid stress (Shabala et al. 2006; Siegumfeldt et al. 2000) or to assess viability of lactic acid bacteria (Rechinger and Siegumfeldt 2002). Simultaneous staining with different fluorochromes associated with multiparametric flow cytometry makes it possible to differentiate individual cells according to various criteria. The association of the esterase substrate carboxyfluorescein diacetate (cFDA), which quantifies the intracellular esterase activity (Riis et al. 1995) and the cellular vitality (Bunthof et al. 2000), with propidium iodide (PI), which discriminates cells with compromised membranes (Bunthof et al. 1999), or with the anionic, lipophilic distributional probe DIBAC4(3) (Jepras et al. 1995), facilitates measurement of the viability and mortality of stressed bacteria. It has been used for viability assessment of bile salt-stressed Bifidobacterium (Ben Amor et al. 2002), high-pressure stressed Lactobacillus rhamnosus (Ananta et al. 2004), acid-stressed Streptococcus macedonicus (Papadimitriou et al. 2006), frozen lactobacilli (Rault et al. 2007), and sucrose- and trehalose-stressed L. rhamnosus (Sunny-Roberts and Knorr 2008).
On the basis of this information, a large range of methods is made available for the characterization of the cellular physiological state, but only a few of them can be applied to characterize the dynamic of cellular events during growth and acidification. The present work aimed at applying some of them to analyze the growth dynamic of a lactic acid bacteria, Lb. bulgaricus CFL1, in order to improve knowledge on changes in the physiological state during growth. The relevance of the Cinac system, the Bioscreen system, and of multiparametric flow cytometry at rendering an efficient discrimination between different physiological states of Lb. bulgaricus CFL1 will be examined. Finally, a dynamic scheme of the time course of the cellular state during acidification will be proposed and discussed.
Materials and methods
Bacterial strain and preculture conditions
Lactobacillus delbrueckii subsp. bulgaricus CFL1 (INRA, Thiverval-Grignon, France) was used throughout this study. Frozen cells were stored at −80 °C in MRS broth (AES-Chemunex, Combourg, France) supplemented with 15% glycerol. Before inoculation in the bioreactor, cells were subcultured twice for 24 h and 8.5 h, at 42 °C in 5 ml MRS broth.
Fermentation conditions
The culture medium was composed of 60 g/L sweet whey powder (Eurosérum, Port-sur-Saône, France) to be close to industrial dairy media that are used for starter’s production. It was adjusted to pH 5 with 50% H2SO4, heat treated at 110 °C for 20 min, and centrifuged at 7,000 ×g and 4 °C for 30 min. After filtration (0.45 µm), it was supplemented with 20 g/L yeast extract (Organotechnie, La Courneuve, France) and introduced into a 2-L bioreactor (Setric Génie Industriel, Toulouse, France). This medium composition was chosen to avoid protein coagulation and to allow sampling, while remaining close to dairy media. After sterilization at 110 °C for 20 min, the initial pH was adjusted at pH 6 and inoculation was done at an initial level of 20 ± 5.5 CFU/mL to allow overnight fermentation. Culturing was done at 42 °C without pH control and with stirring at 100 rpm. During fermentation, pH was measured and acidification rate (dpH / dt, in min−1) was calculated with the WCIDUS software (INRA).
The fermentation kinetics were established and the reproducibility was assessed according to three descriptors: the maximal acidification rate (Vm, in min−1), the time needed to reach this maximal rate (tm, in min), and the pH reached at tm. In order to analyze different physiological parameters, samples were taken from the bioreactor at different times from the beginning of the acidification at tm −150 min, up to tm +600 min.
HPLC measurements
Lactose, glucose, galactose, and lactic acid concentrations were quantified using high performance liquid chromatography (HPLC, Waters Associates, Millipore, Molsheim, France). Before the HPLC analyses, each sample was combined with 120 g/L trichloroacetic acid (Prolabo, Paris, France), centrifuged at 4,000 ×g for 30 min at 4 °C and filtered (0.22 µm). The HPLC analysis was made on a cation exchange column (Aminex Ion Exclusion HPX-87H 300 × 7.8 mm, Biorad, Richmond, USA) at 35 °C with propionic acid (10 g/L) as an internal standard. The mobile phase was 0.005 M H2SO4 and the flow rate was set at 0.6 mL/min (LC-6A pump, Shimadzu, Courtaboeuf).
Measurement of cultivability
Cultivability was evaluated by plate counts. After serial dilutions in peptoned water (bactopeptone, 1 mg/L, Difco Laboratories, Le Pont-de-Claix, France), cells were plated onto solid MRS agar (AES-Chemunex) and incubated at 42 °C for 48 h under anaerobic conditions (Genbox 96124, bioMérieux, Marcy l’Etoile, France). Each result (N, in CFU/mL) was the geometrical mean of at least three counts.
Fluorescent probes and staining protocols
Viability and mortality assessment
Carboxyfluorescein diacetate (cFDA) was used to assess L. delbrueckii viability according to Rault et al. (2007). The nucleic acid dye propidium iodide (PI) made it possible to quantify damaged and dead cells. Live/dead assays were done by dual staining of each sample to differentiate viable, dead, and stressed cells. Before staining, cell suspensions were diluted in Chemsol B13 buffer (AES-Chemunex) to reach 106 cells/mL. One milliliter of the diluted suspension was first supplemented with 10 µL PI (1.496 mM in distilled water, Sigma Aldrich, Lyon, France) and incubated for 20 min at 40 °C. Also, 10 µL cFDA (0.217 µM in acetone, Invitrogen-Molecular Probes, Eragny-sur-Oise, France) was then added and incubation took place for 10 min before direct analysis by flow cytometry. Such an incubation period was necessary for the optimal staining of the cells with these two probes (Rault et al. 2007). It did not lead to any change to the size, shape, or counts of the cells except for a higher fluorescence.
Depolarization assessment
Bis (1.3 dibutylbarbituric acid) trimethine oxonol (DIBAC4(3)) was used to assess the depolarization state of the cells (Ben Amor et al. 2002). Dual staining was performed in order to differentiate dead cells with propidium iodide and depolarized cells with DIBAC4(3) in a single analysis. Moreover, 1 mL of a bacterial suspension (106 cells) was diluted in Chemsol B13 buffer (AES-Chemunex) and incubated with 10 µL PI (1.496 mM in distilled water, Sigma Aldrich) for 10 min at 40 °C. Five microliters of DIBAC4(3) (96.78 µM in dimethylsulfoxyde, Invitrogen-Molecular Probes) was then added and incubation took place for 20 min before direct analysis by flow cytometry.
Vitality measurement
Measuring the ability of the cells to release intracellular carboxyfluorescein (cF) upon energization by lactose addition can be employed as a vitality measurement (Bunthof et al. 2000). Cells were diluted in a pH 7.3 Mac Ilvaine buffer (citric acid 0.1 M, Fisher Chemical, Elancourt, France; disodium dihydrogenophosphate 0.2 M, J.T. Baker, Deventer, The Netherlands) to reach 106 cells/mL. Two milliliters of diluted cells was supplemented with 10 µL of cFDA (2.17 µM in acetone, Invitrogen-Molecular Probes) and incubated for 10 min at 40 °C. They were centrifuged and the pellets were resuspended in 2 mL fresh pH 7.3 Mac Ilvaine buffer with 10 mM of lactose added. One milliliter of this suspension was directly analyzed with flow cytometry to determine the initial fluorescence intensity (IF0). Another 1 mL was reincubated for 10 min at 40 °C to allow cF release before a second flow cytometric analysis (IF10). The fluorescence difference between these two measurements was associated to cF efflux and then to cellular vitality. The loss of fluorescence (ΔIF10, in %) was considered as a measurement of cellular vitality and was calculated by using the following formula:
pHi measurement
The fluorochrome 5(6-)-carboxyfluorescein diacetate succinimidyl ester (cFDA-SE) was used for pHi measurements (Rechinger and Siegumfeldt 2002). The method was first calibrated by using pH-equilibrated cells with known pHi, as previously described (Siegumfeldt et al. 2000). Cell suspensions were diluted in a pH 7.3 Mac Ilvaine buffer to reach 107 cells/mL, which was the optimal cell concentration for this probe. One milliliter of the diluted suspension was incubated with 5 µL cFDA-SE (8.96 µM in acetone, Invitrogen-Molecular Probes) for 10 min at 40 °C. After staining, 100 µL of cell suspension was diluted in 1 mL Mac Ilvaine buffers ranging from pH 4 to pH 7.5. Intracellular pH was then equilibrated with the buffer pH by addition of valinomycin and nigericin (Sigma Aldrich) to a final concentration of 1 µM each. After 1 min incubation at 25 °C, fluorescence intensity was measured by flow cytometry.
Calibration curves were established by plotting the logarithm of the mean fluorescence intensity (log IF) as a function of pH of equilibrated cells, in the range of pH 4 to pH 7.5 (Fig. 1). A linear relationship, involving 95% confidence intervals in brackets, was established between these two parameters:
Calibration curve of Lb. bulgaricus CFL1 for pHi measurements. Results were obtained by plotting the logarithm of the mean fluorescence intensity (log IF) after staining with cFDA-SE, as a function of intracellular pH (pHi), which was fixed using pH-equilibrated cells by addition of valinomycin and nigericin. Each point represents the mean of six measurements, with error bars indicating the standard deviation
As confidence intervals were low and the coefficient of determination high, this linear relationship was satisfactory.
Analyses of intracellular pH of samples were carried out according to the same protocol. After staining with cFDA-SE, 100 µL of cell suspension was diluted in 1 mL of a buffer adjusted to the external pH of the cells. The fluorescence intensity was measured with flow cytometry and from the linear calibration curve (Fig. 1) which made it possible to quantify the intracellular pH.
Flow cytometry and data analyses
Flow cytometry analyses were performed with a Cyflow cytometer (AES-Chemunex) equipped with a specific volumetric counting system, an air-cooled argon ion laser emitting at 488 nm, and four band-pass filters: a forward-angle light scatter (FSC) combined with a diode collector, a side-angle light scatter (SSC), and two fluorescence signals collected with photomultiplier tubes. A 530 nm band-pass filter (515 to 545 nm) was used to collect the green fluorescence of carboxyfluorescein or DIBAC4(3) (FL1 channel), and a 630 nm long-pass filter to collect the red fluorescence of propidium iodide (FL2 channel). The FACSFlow solution (Becton Dickinson, Le Pont-de-Claix, France) was used as sheath fluid. Flow cytometry analyses were performed by using logarithmic gains and specific detector settings adjusted on a sample with unstained cells in order to eliminate cellular autofluorescence. A combination of FSC and SSC was used to discriminate the bacteria from the background.
Data were collected and analyzed with the Flowmax software (Partec, Sainte-Geneviève-des-Bois, France). The subpopulations were identified by using dot plots of FSC, SSC, green fluorescence, and red fluorescence, which separate the different events (Rault et al. 2007). Data were analyzed with the aid of statistical tables given by the Flowmax software, which indicated numbers (in cells/mL) and percentages of stained cells determined by each detector along with the mean of fluorescence intensity of each fluorescent signal, and the associated coefficients of variation. Each result corresponded to the mean of at least two cytometric measurements.
Measurement of growth recovery in fresh medium
The Bioscreen C system (Labsystems, Cergy-Pontoise, France) was used to determine the ability of the cells to recover growth in fresh medium on the basis of continuous optical density measurements at 600 nm (Gabier et al. 2005). From a previous determination of viable cell concentrations by cFDA staining and flow cytometry, the inoculation rate was normalized at 105 viable cells/mL, corresponding to OD600nm = 0.173 ± 0.02. In addition, 200 µL of cell suspension was introduced in triplicate into Bioscreen C wells and incubated at 42 °C for 48 h. The optical density at 600 nm was measured every 15 min and registered by the Biolink software (Labsystems). The time necessary to reach an optical density of 0.2 (tOD0.2, in min) was chosen to characterize the ability of Lb. bulgaricus CFL1 cells to recover growth in fresh medium. The higher the tOD0.2, the longer the latency phase and the lower the ability of the cells to recover growth in fresh medium.
Specific acidification activity measurements
The Cinac system (Ysebaert, Frépillon, France) was used to assess the acidification activity of the cells (Corrieu et al. 1988). Acidification was measured in triplicate at 42 °C in 100 g/L dry skim milk (EPI-Ingredient, Ancenis, France), heat treated at 110 °C for 20 min in 150-mL flasks, and stored at 4 °C before use (Streit et al. 2007). From a previous determination of viable cell concentrations by cFDA staining and flow cytometry, flasks were inoculated at an initial concentration of 105 viable cells/mL. The pH was continuously measured by the Cinac system and led to the determination of the time necessary to reach pH 5.5 (tpH5.5, in min), as a descriptor to characterize the acidification activity of bacterial suspensions. The higher the tpH5.5, the lower the acidification activity.
Results
Dynamic analysis of Lb. bulgaricus CFL1 physiological characteristics
Fermentation kinetics
In order to analyze various physiological parameters at different fermentation times, six cultures were run during this work. Their reproducibility was assessed by considering the changes in pH and acidification rate (Fig. 2a). The maximal rate of acidification Vm was equal to −0.0104 ± 0.0004 min−1. The corresponding pHm was 5.17 ± 0.04 and the time tm to reach Vm was equal to 663 ± 25 min. After Vm was achieved, the pH slowly decreased to a minimum of pH 3.7 ± 0.05, which was reached 619 ± 25 min after tm. During the fermentations, the cells consumed 14.5 ± 1.2 g/L of lactose and produced 8.2 ± 0.7 g/L of galactose and 8.1 ± 0.6 g/L of lactic acid. As 95% confidence intervals were low as compared to the mean values of the descriptors, it was considered that the reproducibility of the cultures was satisfactory which made it possible to analyze and compare physiological characteristics obtained from the six different batch cultures.
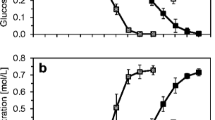
Time course of Lb. bulgaricus CFL1 physiological characteristics throughout fermentation performed without pH control. a pH (—) and acidification rate (dpH/dt, in min−1
, - - -); b cultivability (□, in CFU/mL), total counts of viable cells (●, in viable cells/mL), and relative percentages of viable (■, in %), dead (○, in %), injured (△, in %), and depolarized ( , in %) cells; c specific acidification activity (■, tpH5.5 in min), cells’ ability to recover growth in a fresh medium (△, tOD0.2 in min) and cellular vitality (
, in %) cells; c specific acidification activity (■, tpH5.5 in min), cells’ ability to recover growth in a fresh medium (△, tOD0.2 in min) and cellular vitality ( , ΔIF10 in %); d intracellular pH (■), extracellular pH (□), and pH gradient (dpH = pHi − pHext) (
, ΔIF10 in %); d intracellular pH (■), extracellular pH (□), and pH gradient (dpH = pHi − pHext) ( ). Results are the means of at least three independent measurements and were expressed as a function of the time tm to reach the maximal acidification rate
). Results are the means of at least three independent measurements and were expressed as a function of the time tm to reach the maximal acidification rate
In order to normalize the data obtained from these six cultures, results were expressed as a function of the time tm (in min) to reach the maximal acidification rate which was considered as a reference time.
Effect of pH decrease on cultivable, viable, stressed, dead, and depolarized cells
Viability, mortality, injury, and depolarization were established by using multiparametric flow cytometry. The results were associated with plate count determinations and are shown in Fig. 2b. Before the pH started to decrease, from tm −660 min to tm −200 min, the cultivability rose exponentially from 20 ± 5.5 CFU/mL to 2 · 105 ± 4 · 104 CFU/mL. Then, when pH decreased to pH 4.1, cultivability continued to increase to 1.3 · 108 ± 9.8 · 106 CFU/mL at tm +180 min. Once pH was below 4.1, cultivability dropped steadily to 2 · 107 ± 8 · 105 CFU/mL at tm +600 min, whereas viable cell counts remained stable at 1 · 108 ± 8 · 106 viable cells/mL. As shown in Fig. 2b, percentages of viable cells remained high and constant (89.9 ± 1.6%) despite the drop of cultivability at the end of growth. Percentages of dead and injured cells were therefore quite stable, at mean levels of 6.8 ± 1.5% and 4.6 ± 1.4%, respectively. Low cell depolarization was observed during the culture (4.3 ± 1.9% depolarized cells). In light of these results, it appears obvious that neither viability nor membrane potential was affected by the pH decrease, whereas cultivability was negatively influenced during the stationary phase.
Effect of pH decrease on specific cellular activities and cellular vitality
Cellular activity was determined at different growth times by using three kinds of parameters: the specific acidification activity of the cells as measured by the Cinac system, the cells’ ability to recover growth in a fresh medium as measured with the Bioscreen system, and cellular vitality as assessed by cF extrusion with multiparametric flow cytometry. As shown in Fig. 2c, a slight increase of cellular activities was observed at the beginning of acidification between tm −300 min and tm −60 min, i.e., when pH decreased from pH 6 to pH 5.6. The maximal values of cellular activities and vitality were reached around 60 min before tm, at pH 5.6. This cellular activity remained high up to the maximal acidification point tm, with a minimal tpH5.5 value of 226 ± 13 min, a minimal tOD0.2 of 125 ± 30 min, and high cellular vitality ranging between 40% and 50% of cF extrusion. These results showed a positive effect of acidification during the exponential phase between pH 6 and pH 5.1. After tm was reached, pH decreased to pH 3.7 and both cellular activities and vitality continuously decreased. The time to reach pH 5.5 in milk increased from 217 ± 5 min at tm to 402 ± 6 min at tm +550 min. In the same period, the tOD0.2 increased from 130 ± 10 min up to 285 ± 10 min and cellular vitality decreased from 37 ± 5% to 15 ± 10%. On the basis of these results, these three cellular activity descriptors made it possible to discriminate distinct physiological states corresponding to different harvesting times. They also suggested relationships between these three descriptors.
Incidence of pH decrease on intracellular pH and pH gradient
The intracellular pH (pHi) was measured during the fermentations and compared to extracellular pH (pHext). Results are shown in Fig. 2d. It was noticeable that pHi always remained higher than pHext. Significant differences were observed throughout fermentation, in terms of pHi and pH gradient (dpH) as a consequence of pHext decrease. Intracellular pH decreased concomitantly with extracellular pH, but at different rates. This decrease led to a significant increase in dpH value, from 0.5 at tm −200 min to a maximum of 1.7 at tm +180 min, i.e., at the end of the exponential phase. Once tm +180 min was reached, pHi and dpH both decreased to minimum values of pHi = 4.8 and dpH = 1.1, when pHext reached pH 3.7 at tm +500 min.
Relationships between physiological parameters
From previous analyses, some descriptors may be related throughout the growth curve of Lb. bulgaricus CFL1.
Relationship between viable and cultivable cell concentrations
Viable cell counts (V, in cells/mL) assessed by PI/cFDA double staining were compared with plate counts (N, in CFU/mL) during fermentations of Lb. bulgaricus CFL1. Figure 3a represents the relationship between the mean values of the logarithm of viable and stressed cell counts (log V) and the logarithm of CFU concentrations (log N). A strong linear correlation was observed throughout the exponential growth phase, i.e., until 1.3 · 108 ± 9.8 · 106 CFU/mL were reached. The corresponding linear equation was established as follows:
Relationships between physiological parameters characterizing Lb. bulgaricus CFL1 cultures performed without pH control. a relationship between the logarithm of plate counts (N, in CFU/mL) and the logarithm of viable cell counts (V, in cells/mL) during the exponential phase (■) and the stationary phase (□). The bold line shows linear regression during the exponential phase; b relationships between acidification activity (tpH5.5, in min) and cells’ ability to recover growth (tOD0.2, in min) (▲) or cellular vitality (ΔIF10, in %) (△); c relationship between acidification activity (tpH5.5, in min) and intracellular pH of the cells (pHi) (●); d relationship between the logarithm of plate counts (N, in CFU/mL) and the pH gradient (dpH = pHi – pHext) (♦). Results corresponded to at least three independent measurements, with error bars indicating the standard deviation for each variable
As the slope was close to 1 and the intercept was not significantly different from 0, viable cell counts gave similar results to those of plate counts during the exponential growth phase. However, during the stationary phase, viable cell counts remained stable at 1 · 108 ± 8 · 106 CFU/mL whereas plate counts decreased from 1.3 · 108 ± 9.8 · 106 to 2 · 107 ± 8 · 105 CFU/mL. This result confirmed that the physiological state of cells deteriorated at pH lower than pH 4.1.
Relationship between cellular activity and vitality descriptors
As shown in Fig. 3b, the time necessary to reach pH 5.5 (tpH5.5, in min) as measured by the Cinac system, the time necessary to reach an optical density of 0.2 (tOD0.2, in min) as measured by the Bioscreen system, and cellular vitality (ΔIF10, in %) as measured by flow cytometry were correlated. The corresponding linear relationships were determined as follows:
From these relationships we see that when tpH5.5 increased from 200 to 400 (±4.6) min, tOD0.2 was enhanced from 120 to 350 (±15) min, whereas the cellular vitality decreased from 40% to 15% (±5.3). As the coefficients of determination were lower than 0.92, the coefficients of variation were calculated. They were higher for cellular vitality (29.1%) than for Bioscreen data (7.4%) or Cinac measurements (1.7%). It was thus considered that specific acidification activity measurement represented the most relevant method for measuring activity during fermentation of Lb. bulgaricus CFL1.
Relationship between acidification activity and intracellular pH
The specific acidification activity (tpH5.5, in min) observed throughout fermentation was related to intracellular pH (pHi). By correlating these two descriptors (Fig. 3c), the following equation was established:
This linear relationship indicates that a good acidification activity was achieved when intracellular pH was high. Conversely, the decrease of pHi as a result of acidification of the medium led to a decrease in metabolic activity followed by a degradation of the acidification activity of the cells.
Relationship between pH gradient and cultivability of Lb. bulgaricus CFL1
As shown in Fig. 3d, a positive relationship was observed between the pH gradient (dpH) and the logarithm of CFU concentration (log N). It was expressed by the following equation:
From this equation, it becomes obvious that cultivability increased with the intracellular pH gradient. This last parameter may be a key parameter that explains the stop in growth and the following loss of cultivability observed during the stationary phase.
Discussion
Relevance of different methods to characterize the physiological state of Lb. bulgaricus CFL1
The relevance of different methods, including cultivability, multiparametric flow cytometry, and cellular activity measurements, was investigated in order to characterize dynamic changes in physiological state during growth of Lb. bulgaricus CFL1. From flow cytometry combined with cFDA, PI, and DIBAC4(3) staining, we first demonstrated that the percentage of viable cells, the membrane integrity, and the membrane potential of the cells remained high and stable throughout growth and the stationary phase, despite continuous pH decrease. By using the same fluorescent probes, such limited effects of low pH on membrane parameters have been demonstrated by Baatout et al. (2007) on E. coli that was found to become smaller and rounder as a consequence of acidification but was able to maintain intracellular characteristics. Differences in the resistance to acid stress were observed with other microorganisms. Papadimitriou et al. (2006) and Baatout et al. (2007) have demonstrated that injured or dead subpopulations with damaged membranes appear when a sudden acid stress is applied respectively on Streptococcus macedonicus and Cupriavidus metallidurans. Nevertheless, despite their interest for these studies, these three descriptors did not differentiate different physiological states of Lb. bulgaricus during growth and acidification.
Flow cytometry analyses of viable cells were correlated with plate counts during the exponential phase but not during the stationary phase. This result indicates that, in the stationary phase, some cells enter a non-cultivable state but are still viable and active. Differences between viable cell counts and plate counts have previously been shown with stressed cells (Bunthof et al. 1999). According to McDougald et al. (1998), this non-cultivable state is characterized by the ability of the cells to keep their membranes while retaining their RNA and DNA contents, proton motive force, and energetic and metabolic activities. This ability was confirmed in our work through PI staining which showed that the cells maintained their membrane integrity. The ability of multiparametric flow cytometry to detect viable but not cultivable subpopulations has been demonstrated in bile salt-stressed Bifidobacterium (Ben Amor et al. 2002), in high-pressure stressed L. rhamnosus GG (Ananta et al. 2004), in probiotic bacteria during storage at 4 °C (Lahtinen et al. 2006), in acid-stressed S. macedonicus (Papadimitriou et al. 2006), and in sucrose-stressed L. rhamnosus (Sunny-Roberts and Knorr 2008).
The three methods of measuring cellular activity discriminated different physiological states during Lb. bulgaricus growth. They all displayed that cellular activity was high at the end of the log phase, up to the maximal acidification point, and then decreased gradually throughout the stationary phase. A high correlation was observed between the acidification activity as measured by the Cinac system, the ability of the cells to recover growth as measured by the Bioscreen system, and cellular vitality as analyzed by flow cytometry. Even if these relationships have never previously been described, it can be proposed that these three methods led to similar results in terms of cellular activity. The interest of the Cinac and Bioscreen systems is well known as they are often used to quantify respectively the acidification activity of lactic acid bacteria (Fonseca et al. 2000; Streit et al. 2007) or the growth of different microorganisms (Champagne et al. 1999; Gabier et al. 2005). They were used here for the first time to measure cellular activity during growth. Cellular vitality, associated with cF efflux activity, has previously been shown to be a good and rapid indicator of the cellular activity of stressed L. lactis (Bunthof et al. 1999). However, we found high variability with this method in this study. With the lowest variability between the different cultures performed, the Cinac system was found to be the most relevant method for specific activity measurements throughout growth.
As viability remained high and constant, the decrease of cultivability and cellular activity in the stationary phase indicated that cellular activity and viability were dissociated. This observation is in agreement with the results of Bunthof et al. (1999) with stressed L. lactis. Consequently, to achieve a complete description of the cellular physiological state, analyses of both viability and cellular activity of lactic acid bacteria are needed.
As proposed by Hutkins and Nannen (1993), intracellular pH plays a central role in both cellular activity and viability. In order to elucidate some mechanisms involved in this dissociation, the dynamics of intracellular pH and the pH gradient throughout growth were investigated. pHi had previously been determined by using ratios of cFDA-SE fluorescence combined with spectrofluorimetry (Gabier et al. 2005), FRIM (Shabala et al. 2006; Siegumfeldt et al. 2000), or flow cytometry (Lahtinen et al. 2006), but the pHext range had been limited to between pH 5 and pH 8. In our study, we demonstrated that this probe was efficient to pH 3.8. This method thus represents a reliable approach for the assessment of intracellular pH in acidic conditions.
High correlations between dpH and cultivability and between pHi and cellular activity were demonstrated. The first one was consistent with previous reports based on other methods that displayed a good relationship between the pH gradient and cultivability after a sudden acid stress (Rechinger and Siegumfeldt 2002). The second correlation, which relates pHi and acidification activity measured by the Cinac system, is established here for the first time. Consequently, pHi measurements by flow cytometry allowed indirect and reliable analyses of viability, cultivability, and cellular activity during Lb. bulgaricus growth.
Progress of physiological events during Lb. bulgaricus growth
These different observations made it possible to identify distinct physiological states corresponding to different harvesting times. A dynamic scheme of the time course of the cellular state during fermentation is proposed in Fig. 4. It identifies four major periods during fermentation.
The first period, P1 [from tm −200 min to tm −100 min], corresponds to the exponential growth phase which was observed in both CFU and viability measurements. It was characterized by the decrease of external pH from pH 6.1 to pH 5.8, but by a slight increase of pHi from pH 6.6 to pH 6.7 and of dpH from 0.5 to 0.9. P1 was associated with a small increase in cellular acidification activity, which may be related to the pHi increase. During this period, the cellular strategy was mainly devoted to cellular activity and growth. The reduced acidification induced an increase of pHi which made it possible to maintain both a high energetic level and a high acidification activity.
The second phase, P2 [from tm −100 min to tm], was characterized by a rapid decrease of pHext down to pH 5.1 associated with a concomitant decrease in intracellular pH down to pH 6.3. The pH gradient continued to increase. This acidification remained associated with growth up to the maximal acidification point (tm). Acidification activity was stabilized at a high value, i.e., a minimum of tpH5.5, as shown in Fig. 4, indicating that the internal pH was maintained at values compatible with growth and cellular activity, without consuming too much energy for the proton gradient. This result is consistent with the conclusions of previous studies on lactic acid bacteria, based on other methods of pHi measurements (Even et al. 2002; Mercade et al. 2004; O’Sullivan and Condon 1999). The maximum acidification point occurred when pHext reached pH 5.1 and pHi pH 6.3. These values, combined with the high cellular activity, may explain the slowing down of growth during the next phase.
During the third period, P3 [from tm to tm +180 min], external and intracellular pH decreased down to pH 4.1 and pH 5.8, respectively while dpH increased to a maximum of 1.7. Membrane integrity and viability remained high and stable whereas the growth rate and acidification activity were reduced and the cells entered their stationary phase at the end of this period. In light of these observations, one may propose that intracellular pH and the maximal pH gradient may explain the stopping of growth. This hypothesis is in agreement with previous studies (Even et al. 2002; Mercade et al. 2004). The authors indicated that a pHi decrease leads to an inhibition of glycolytic enzymes and thus to a decrease of intracellular energy production. At the same time, the increase of pH gradient, to counter cytoplasmic acidification, requires energy for the ATPase function (Hutkins and Nannen 1993). These events combine to reduce available energy for biomass synthesis and lead to an uncoupling of growth and acidification (Even et al. 2002; Mercade et al. 2004). Consequently, growth stopped when the pHi reached a critical value, when maximal dpH was attained, and when catabolism and energy production slowed down (Even et al. 2002). The critical pHi of Lb. bulgaricus CFL1 (5.8) was higher than that reported by Hutkins and Nannen (1993) or Mercade et al. (2004), who reported critical pHi of Lb. bulgaricus ranging between 4.4 and 4.7. It was also higher than those observed for different strains of Lactococcus lactis and Streptococcus thermophilus, which ranged between 5 and 5.5 (Nannen and Hutkins 1991). The critical pH gradient of Lb. bulgaricus CFL1 (1.7) was higher than that observed by Mercade et al. (2004) for another Lb. bulgaricus strain (1.1) or by Nannen and Hutkins (1991) for L. lactis (0.97 to 1.4) and S. thermophilus (0.89 to 1.21). Even if they appeared to be strain and species dependent, these high values of critical pHi and dpH for Lb. bulgaricus CFL1 may explain the reduced growth observed for this strain (1.3 · 108 CFU/mL at the end of the log phase). This result confirmed that the cellular strategy of Lb. bulgaricus CFL1 during growth was devoted mainly to maintaining a high energetic state characterized by high pHi and dpH at the expense of a high bacterial concentration. Finally, the critical pHi, together with the elevated pH gradient, induced a decrease of cultivability and acidification activity of Lb. bulgaricus CFL1 during the stationary phase.
The last phase, P4 [from tm +180 min to tm +550 min], was characterized by a significant loss of cultivability, whereas viable cell counts remained stable. This loss of cultivability was well correlated and explained by the pH gradient decrease. Despite this subsequent loss of cultivability, a slight acidification was still observed, with pHext and pHi decreasing to pH 3.7 and pH 4.8, respectively. The pHi decrease was related to the gradual loss of acidification activity, as shown in the previous phase. During this period, cellular viability was stable and was not affected by acidification, which acted only on cultivability and acidification activity. The strategy of Lb. bulgaricus cells was here mainly devoted to maintaining their membrane integrity, membrane polarization, and a positive pH gradient, at the expense of their cultivability and acidification activity.
Finally, these results show that the time course of the cellular state during fermentation was significantly affected by pHi and the pH gradient. These two parameters explain the time course of metabolic activity, cultivability, and viability during growth of Lb. bulgaricus CFL1 well.
As a final conclusion, this work displayed that certain physiological and viability analyses produce good discrimination of different physiological states during Lb. bulgaricus CFL1 growth. Among them, the Cinac system was found to be a relevant method for specific activity measurements throughout growth. It displayed the lowest coefficient of variation, as compared to Bioscreen and vitality measurements. Measurements of intracellular pH and pH gradient by flow cytometry also gave major pieces of information to improve the understanding of the dynamic of Lb. bulgaricus growth and acidification. Close relationships have been displayed between dpH and cultivability and between pHi and acidification activity. Consequently, these measurements made it possible to analyze, in a short lapse of time, viability, cultivability, and acidification activity.
By considering the physiological events that occurred during growth, this study pointed out that Lb. bulgaricus was capable of maintaining its viability, energetic state, membrane potential, and pH gradient even after the end of growth. Cell viability and membrane integrity were therefore not affected by acidification in contrast to cultivability and acidification activity which appeared to be strongly affected by pHi and the pH gradient during acidification.
In the future, this approach may be useful for improving knowledge on the cellular state during fermentations performed under pH control. As a complex medium was used in this study, it may be interesting to enlarge this approach by using a chemically defined medium containing all the growth requirements. Moreover, in order to better understand the mechanisms that induced these different behaviors, this study will be strengthened by analyzing the membrane fatty acid composition and the proteome of each specific physiological state.
References
Amrane A, Prigent Y (1999) Differentiation of pH and free lactic acid effects on the various growth and production phases of Lactobacillus helveticus. J Chem Technol Biot 74:33–40
Ananta E, Heinz V, Knorr D (2004) Assessment of high pressure induced damage on Lactobacillus rhamnosus GG by flow cytometry. Food Microbiol 21:567–577
Ananta E, Voigt D, Zenker M, Heinz V, Knorr D (2005) Cellular injuries upon exposure of Escherichia coli and Lactobacillus rhamnosus to high-intensity ultrasound. J Appl Microbiol 99:271–278
Baati L, Fabre-Gea C, Auriol D, Blanc PJ (2000) Study of the cryotolerance of Lactobacillus acidophilus: effect of culture and freezing conditions on the viability and cellular protein levels. Int J Food Microbiol 59:241–247
Baatout S, Leys N, Hendrickx L, Dams A, Mergeay M (2007) Physiological changes induced in bacteria following pH stress as a model for space research. Acta Astronaut 60:451–459
Béal C, Corrieu G (1994) Viability and acidification activity of pure and mixed starters of Streptococcus salivarius subsp. thermophilus 404 and Lactobacillus delbrueckii subsp. bulgaricus 398 at the different steps of their production. Lebensm Wiss Technol 27:86–92
Ben Amor K, Breeuwer P, Verbaarschot P, Rombouts FM, Akkermans ADL, De Vos WM, Abee T (2002) Multiparametric flow cytometry and cell sorting for the assessment of viable, injured, and dead Bifidobacterium cells during bile salt stress. Appl Environ Microbiol 68:5209–5216
Bouix M, Leveau JY (2001) Rapid assessment of yeast viability and yeast vitality during alcoholic fermentation. J Inst Brew 107:217–225
Breeuwer P, Abee T (2000) Assessment of viability of microorganisms employing fluorescence techniques. Int J Food Microbiol 55:193–200
Breeuwer P, Drocourt JL, Rombouts FM, Abee T (1996) A novel method for continuous determination of intracellular pH in bacteria with the internally conjugated fluorescent probe 5 (and 6-)-carboxyfluorescein succinimidyl ester. Appl Environ Microbiol 62:178–183
Budin-Verneuil A, Pichereau V, Auffray Y, Ehrlich Dusko S, Maguin E (2005) Proteomic characterization of the acid tolerance response in Lactococcus lactis MG1363. Proteomics 5:4794–4807
Bunthof CJ, Van den Braak S, Breeuwer P, Rombouts FM, Abee T (1999) Rapid fluorescence assessment of the viability of stressed Lactococcus lactis. Appl Environ Microbiol 65:3681–3689
Bunthof CJ, Van den Braak S, Breeuwer P, Rombouts FM, Abee T (2000) Fluorescence assessment of Lactococcus lactis viability. Int J Food Microbiol 55:291–294
Bunthof CJ, Bloemen K, Breeuwer P, Rombouts FM, Abee T (2001) Flow cytometric assessment of viability of lactic acid bacteria. Appl Environ Microbiol 67:2326–2335
Cachon R, Antérieux P, Diviès C (1998) The comparative behavior of Lactococcus lactis in free and immobilized culture processes. J Biotechnol 63:211–218
Champagne CP, Gaudreau H, Conway J, Chartier N, Fonchy E (1999) Evaluation of yeast extracts as growth media supplements for lactococci and lactobacilli by using automated spectrophotometry. J Gen Appl Microbiol 45:17–21
Corrieu G, Spinnler HE, Jomier Y, Picque D (1988) Automated system to follow up and control the acidification activity of lactic acid starters. French Patent FR 2:629–612
Davey HM, Kell DB (1996) Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analyses. Microbiol Rev 60:641–696
Even S, Lindley ND, Loubiere P, Cocaign-Bousquet M (2002) Dynamic response of catabolic pathways to autoacidification in Lactococcus lactis: transcript profiling and stability in relation to metabolic and energetic constraints. Mol Microbiol 45:1143–1152
Fonseca F, Béal C, Corrieu G (2000) Method of quantifying the loss of acidification activity of lactic acid staters during freezing and frozen storage. J Dairy Res 67:83–90
Fozo EM, Kajfasz JK, Quivey JRG (2004) Low pH-induced membrane fatty acid alterations in oral bacteria. FEMS Microbiol Lett 238:291–295
Gabier AC, Gourdon P, Reitz J, Leveau JY, Bouix M (2005) Intracellular physiological events of yeast Rhodotorula glutinis during storage at 4°C. Int J Food Microbiol 105:97–109
Graça da Silveira M, Vitória San Romão M, Loureiro-Dias MC, Rombouts FM, Abee T (2002) Flow cytometric assessment of membrane integrity of ethanol-stressed Oenococcus oeni cells. Appl Environ Microbiol 68:6087–6093
Guillouard I, Lim EM, Van de Guchte M, Grimaldi C, Penaud S, Maguin E (2004) Tolerance and adaptative acid stress of Lactobacillus delbrueckii ssp. bulgaricus. Lait 84:1–6
Hutkins RW, Nannen NL (1993) pH homeostasis in lactic acid bacteria. J Dairy Sci 76:2354–2365
Jepras RI, Carter J, Pearson SC, Paul FE, Wilkinson MJ (1995) Development of a robust flow cytometric assay for determining numbers of viable bacteria. Appl Environ Microbiol 61:2696–2701
Kaprelyants AS, Mukamolova GV, Davey HM, Kell DB (1996) Quantitative analysis of the physiological heterogeneity within starved cultures of Micrococcus luteus by flow cytometry and cell sorting. Appl Environ Microbiol 62:1311–1316
Lahtinen SJ, Ouwehand AC, Reinikainen JP, Korpela JM, Sandholm J, Salminen SJ (2006) Intrinsic properties of so-called dormant probiotic bacteria, determined by flow cytometric viability assays. Appl Environ Microbiol 72:5132–5134
McDougald D, Rice SA, Weichart D, Kjelleberg S (1998) Nonculturability: adaptation or debilitation. FEMS Microbiol Ecol 25:1–9
Mercade M, Duperray F, Loubière P (2004) Energetic analysis of cultures of Lactobacillus delbrueckii subsp. bulgaricus: identification of the type of control between catabolism and anabolism. Lait 84:39–47
Nannen NL, Hutkins RW (1991) Intracellular pH effects in lactic acid bacteria. J Dairy Sci 74:741–746
Novo DJ, Perlmutter NG, Hunt RH, Shapiro HM (2000) Multiparameter flow cytometric analysis of antibiotic effects on membrane potential, membrane permeability, and bacterial counts of Staphylococcus aureus and Micrococcus luteus. Antimicrob Agents Chemother 44:827–834
O’Sullivan E, Condon S (1999) Relationship between acid tolerance, cytoplasmic pH, and ATP and H+-ATPase levels in chemostat cultures of Lactococcus lactis. Appl Environ Microbiol 65:2287–2293
Papadimitriou K, Pratsinis H, Nebe-von-Caron G, Kletsas D, Tsakalidou E (2006) Rapid assessment of the physiological status of Streptococcus macedonicus by flow cytometry and fluorescence probes. Int J Food Microbiol 111:197–205
Rault A, Béal C, Ghorbal S, Ogier J-C, Bouix M (2007) Multiparametric flow cytometry allows rapid assessment and comparison of lactic acid bacteria viability after freezing and during frozen storage. Cryobiology 55:35–43
Rechinger KB, Siegumfeldt H (2002) Rapid assessment of cell viability of Lactobacillus delbrueckii subsp. bulgaricus by measurement of intracellular pH in individual cells using fluorescence ratio imaging microscopy. Int J Food Microbiol 75:53–60
Riis SB, Pedersen HM, Sorensen NK, Jakobsen M (1995) Flow cytometry and acidification power test as rapid techniques for determination of the activity of starter cultures of Lactobacillus delbrueckii subsp. bulgaricus. Food Microbiol 12:245–250
Shabala L, McMeekin T, Budde BB, Siegumfeldt H (2006) Listeria innocua and Lactobacillus delbrueckii subsp. bulgaricus employ different strategies to cope with acid stress. Int J Food Microbiol 110:1–7
Siegumfeldt H, Rechinger KB, Jakobsen M (1999) Use of fluorescence ratio imaging for intracellular pH determination of individual bacterial cells in mixed cultures. Microbiology 145:1703–1709
Siegumfeldt H, Rechinger KB, Jakobsen M (2000) Dynamic changes of intracellular pH in individual lactic acid bacterium cells in response to a rapid drop in extracellular pH. Appl Environ Microbiol 66:2330–2325
Streit F, Corrieu G, Béal C (2007) Acidification of fermented broth improves cryotolerance of Lactobacillus delbrueckii subsp. bulgaricus CFL1. J Biotechnol 128:659–667
Sunny-Roberts EO, Knorr D (2008) Evaluation of the response of Lactobacillus rhamnosus VTT E-97800 to sucrose-induced osmotic stress. Food Microbiol 25:183–189
Wang Y, Corrieu G, Béal C (2005) Fermentation pH and temperature influence the cryotolerance of Lactobacillus acidophilus RD758. J Dairy Sci 88:21–29
Acknowledgements
This work was financially supported by ARILAIT Recherches (Paris, France) and the Genetic Resources Board (BRG, Paris). The authors wish to thank Donald White for revision of the English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rault, A., Bouix, M. & Béal, C. Dynamic analysis of Lactobacillus delbrueckii subsp. bulgaricus CFL1 physiological characteristics during fermentation. Appl Microbiol Biotechnol 81, 559–570 (2008). https://doi.org/10.1007/s00253-008-1699-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1699-4