Abstract
Pleurotus ostreatus showed atypical laccase production in submerged vs. solid-state fermentation. Cultures grown in submerged fermentation produced laccase at 13,000 U l−1, with a biomass production of 5.6 g l−1 and four laccase isoforms. However, cultures grown in solid-state fermentation had a much lower laccase activity of 2,430 U l−1, biomass production of 4.5 g l−1, and three laccase isoforms. These results show that P. ostreatus performs much better in submerged fermentation than in solid-state fermentation. This is the first report that shows such atypical behavior in the production of extracellular laccases by fungi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laccases (ρ-diphenol: oxygen oxidoreductase; EC 1.10.3.2) are enzymes that oxidize phenolic compounds and are produced by plants, bacteria, insects, and fungal species such as the white-rot fungus Pleurotus ostreatus. These enzymes can be used for various biotechnological and environmental applications. Solid-state fermentation (SSF) represents the growth of microorganisms in the absence or near-absence of free water (Viniegra-González et al. 2003; Hölker et al. 2004). It has been reported that SSF is a better system than submerged fermentation (SMF) for producing enzymes including laccases. Ramírez et al. (2003) found that laccase production by P. ostreatus grown on wheat bran and vinasse in SSF was twice as high (20 U l−1) and with a greater number (three) of isoforms than those cultures grown in SMF (two). The production of laccase by a wild strain of Aspergillus niger and by two transformants (C28eco3 and C28eco3–13) with the laccase gene IV from Trametes versicolors was studied in SMF and SSF using different concentrations of glucose and maltodextrin (Téllez-Jurado et al. 2005). Laccase titers were higher when glucose was used as a carbon source. However, repression of laccase production was evident for SMF upon increasing glucose from 10 to 50 g l−1. In contrast, the production of the highest laccase activity for strain C28eco3 (362 U l−1) and C28eco3–13 (592 U l−1) was shown when 50 g l−1 of glucose was used as a carbon source in SSF. Cultures of A. niger have been used to study the production of several enzymes in SSF and SMF. It has been reported that pectinase (Antier et al. 1993), esterase (Asther et al. 2002), invertase (Romero-Gómez et al. 2000), pectin lyase (Acuña-Argüelles et al. 1995), and tannase (Aguilar et al. 2001) activities were higher in cultures grown in SSF than those cultures grown in SMF. Similarly, the production by A. niger of β-fructofuranosidase (Ashokkumar and Gunasekaran 2002), exopectinase (Díaz-Godínez et al. 2001), endopectinase (Acuña-Argüelles et al. 1995), and polygalacturonase (Maldonado and Strasser de Saad 1998) was higher in SSF than in SMF. Enzyme production by A. niger and Penicillium citrinum in SSF using culture media embedded in an inert support (i.e., polyurethane foam) was increased, as compared to that in SSF on a biodegradable support (i.e., coffee pulp, sugar cane bagasse or maize straw) and to that in SMF (Zhu et al. 1996; Díaz-Godínez et al. 2001). It has been reported that the higher pectinase titer in SSF than in SMF might be due to (a) a lower level of pectinase degradation as the result of low protease production and (b) a higher amount of biomass produced under SSF conditions (Díaz-Godínez et al. 2001; Viniegra-González et al. 2003).
Téllez-Téllez et al. (2005) and Palmieri et al. (2003) reported that the number and type of laccase isoforms produced depend on the fungal species, culture medium, and growth conditions. In the present research, production of laccase and the number of laccase isoforms made by P. ostreatus grown in SSF and in SMF were evaluated. A number of unexpected results were observed, including the relative production of laccase in SSF vs. SMF and different numbers of isoforms made under the two conditions.
Materials and methods
Organism and culture conditions
P. ostreatus (ATCC 32783) was used. A liquid medium previously optimized for producing laccases by this fungus in SMF was prepared containing (in gram per liter): glucose, 10; yeast extract, 5; KH2PO4, 0.6; MgSO4–7H2O, 0.5; K2HPO4, 0.4; CuSO4–5H2O, 0.25; FeSO4–7H2O, 0.05; MnSO4–H2O, 0.05; ZnSO4–7H2O, 0.001. The pH was adjusted to 6.0 using 0.1 M NaOH. All cultures were inoculated with three mycelial plugs (4 mm diam) taken from the periphery of a colony grown on PDA at 25 °C for 7 days. The cultures were incubated at 25 °C for 25 days on a rotary shaker at 120 rpm. SSF was carried out in a flask of 250 ml containing 1 g of polyurethane foam of low density (PUF; 17 kg m−3) cubes (0.5 × 0.5 × 0.5; Díaz-Godínez et al. 2001) as an inert support impregnated with 30 ml of sterile culture medium. Previously, the cubes were washed twice with distilled water, oven-dried at 60 °C for 24 h, and then autoclaved at 120 °C for 15 min. SMF was undertaken in flasks of 250 ml containing 30 ml of culture medium.
Enzymatic extract and biomass evaluation
The enzymatic extract (EE) of cultures grown under SMF conditions was obtained by filtration using filter paper (Whatman No. 4) and that of cultures grown under SSF conditions was obtained by using a Millipore filter (0.45 μm) and a syringe of 50 ml. The cubes were washed three times with distilled water to remove any remains of EE and then oven-dried at 60 °C for 24 h. In both fermentation systems, the biomass (X) was determined as difference of weight (gram per liter; Díaz-Godínez et al. 2001). Alternatively, 30 ml of the EE that showed the highest activity of laccase under SMF conditions was added to 1 g of unused PUF, incubated for 1 h at room temperature, and then washed and oven-dried as indicated above. Nitrogen content was determined in such an oven-dried PUF and in an unused PUF using the Kjeldhal method, which showed that there was no immobilization of enzymes in the support.
Evolution of biomass X = X(t) was done using the Velhurst–Pearl or logistic equation:
where μ is the maximal specific growth rate, and X max is the maximal (or equilibrium) biomass level achieved when dX/dt = 0 for X > 0. The solution of Eq. 1 is as follows;
where, C = (X max − X 0)/X 0, and X = X 0, the initial biomass value.
Estimation of kinetic parameters in the above equations was performed using a non-linear least square-fitting program “Solver” (Excel, Microsoft; Díaz-Godínez et al. 2001; Viniegra-González et al. 2003). Y E /X is the yield of enzyme per unit of biomass produced, estimated as the relation between maximal laccase activity (E max) and X max. Enzymatic productivity (P) was evaluated by using the time of E max. The specific rate of enzymatic production was calculated by the equation: \(qP = \left. {\left[ {\left( \mu \right)\left( {Y_{{E \mathord{\left/ {\vphantom {E X}} \right. \kern-\nulldelimiterspace} X}} } \right)} \right]} \right)\).
Enzyme assays
Laccase activity was determined by changes in the absorbance at 468 nm, using 2,6-dimethoxyphenol (DMP) as substrate. The assay mixture contained 950 μl substrate (2 mM DMP in 0.1 M phosphate buffer at pH 6.0) and 50 μl EE, which were incubated at 40 °C for 1 min (Téllez-Téllez et al. 2005). Protease activity was measured by changes in the absorbance at 280 nm, produced by the release of aromatic amino acids, using casein as substrate (Kunitz 1947). One enzymatic unit (U) of laccase or protease activity is defined as the amount of enzyme, which gives an increase of 1 U of absorbance per minute in the reaction mixture. The activity was expressed in unit per liter of EE. The laccase specific activity (E spec) is reported as unit per milligram of extracellular protein (unit per milligram protein) estimated as the relation between maximal laccase activity (E max) and the extracellular protein content observed at the same time. The total extracellular protein was measured in the EE by the Bradford (1976) method using bovine serum albumin as standard. Laccase activity was detected through zymograms using DMP as substrate, by the modified sodium dodecyl sulfate polyacrylamide gel electrophoresis technique (Laemmli 1970). Gel composition, running conditions, and handling of the gel after running were performed as previously reported (Téllez-Téllez et al. 2005).
Results
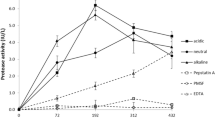
P. ostreatus (ATCC 32783) was grown in both SMF and SSF modes. As shown in Fig. 1a, growth in SSF began earlier but reached a lower level (4.5 g l−1) than growth in SMF (5.5 g l−1). Also, μ was higher in SSF (0.033 h−1) than in SMF (0.022 h−1). Figure 1b shows that glucose consumption was more rapid in SSF than in SMF and became exhausted at 290 h in SSF and at 430 h in SMF. Production of laccase began earlier in SSF (Fig. 2a) but was rather poor in SMF. The P value observed in SMF was approximately 3.6-fold higher than in SSF. The activity began in the stationary growth phase and reached a much higher level. Details are shown in Table 1. The E spec was approximately fivefold higher in cultures grown in SMF than in those cultures grown in SSF. The pH showed an increase of only 0.5 U during growth, and protease was only formed in SSF (Fig. 2b).
Zymograms shown in Fig. 3 revealed that cultures grown in SMF showed up to four isoforms during the course of the experiment. On the other hand, SSF cultures grown showed one isoform in the lag phase, two or possibly three isoforms in the exponential phase, and two in the stationary phase.
Discussion
Growth curves were fitted by Eq. 1 with high correlation coefficients (R 2 > 0.98) showing that the model was adequate for this process. Cultures grown in SMF had higher biomass production, which might be due to oxygen and space limitation inside of the polyurethane foam of SSF. On the other hand, the μ in SSF was somewhat higher than that observed in SMF, which might be related to the fact that solid substrates are the natural habitat of the fungus.
Since the ultimate consumption of carbon source was the same in both fermentation systems, the difference in biomass production is not related to the amount of assimilated glucose. The culture medium acted as a buffer in both fermentation systems, suggesting that the pH is not responsible for differences in laccase and biomass production observed in SMF and in SSF.
It has been reported that the number and type of laccase isoforms produced by Pleurotus is species dependent (Téllez-Téllez et al. 2005). Díaz-Godínez et al. (2001) and Aguilar et al. (2001) reported that the undesirable production of proteases by A. niger was low in SSF and that the breakdown of other enzymes by these proteases was high in SMF. However, in our study with P. ostreatus, protease activity was only observed in cultures grown in SSF. Contrary to findings by others (Ramírez et al. 2003; Téllez-Jurado et al. 2005), our culture showed higher growth and greater production of laccase in SMF than in SSF. Cultures of A. niger showed a higher productivity and production of several enzymes in SSF than in SMF (Antier et al. 1993; Acuña-Argüelles et al. 1995; Maldonado and Strasser de Saad 1998; Romero-Gómez et al. 2000; Aguilar et al. 2001; Díaz-Godínez et al. 2001; Ashokkumar and Gunasekaran 2002; Asther et al. 2002; Viniegra-González et al. 2003; Hölker et al. 2004). The zymograms showed that P. ostreatus had different laccases production patterns, which depended on the culture conditions. The differences observed in the production of laccases might be mainly due to the presence of oxygen in the fermentation system. The fact that SMF showed higher laccase production than SSF might be a result of the stress caused by the little amount of oxygen available in SMF. Similar laccases production patterns have been reported in other studies. Téllez-Téllez et al. (2005) observed that P. ostreatus produced two laccase isoforms when it was grown on Petri dishes. However, Tlecuitl-Beristain et al. (2008) reported that P. ostreatus produced four laccase isoforms in SMF. Those isoforms observed during the lag and exponential phases might be related to the substrate degradation and those shown in the stationary phase related to mushroom morphogenesis and pigmentation processes (Temp and Eggert 1999). The zymogram patterns of laccases and laccases activity observed by us indicate enzymatic stability during the cultivation period and a high qP during the stationary phase of the cultures grown in SMF. This absence of protease activity in cultures grown in SMF probably is responsible for the high laccase activity. However, further studies such as the use of PUF of a bigger pore size in SSF and evaluation of the effect of the oxygen concentration in SMF on the growth and laccase production of the fungus will give us in the future a better understanding of the atypical behavior of P. ostreatus in SSF.
References
Acuña-Argüelles ME, Gutiérrez-Rojas M, Viniegra-González G, Favela-Torres E (1995) Production and properties of three pectinolytic activities produced by Aspergillus niger in submerged and solid-state fermentation. Appl Microbiol Biotechnol 43:808–814
Aguilar CN, Augur C, Favela-Torres E, Viniegra-González G (2001) Production of tannase by Aspergillus niger Aa-20 in submerged and solid-state fermentation: influence of glucose and tannic acid. J Ind Microbiol Biotechnol 26:296–302
Antier P, Minjares A, Roussos S, Raimbault M, Viniegra G (1993) Pectinase-hyperproducing mutants of Aspergillus niger C28B25 for solid-state fermentation of coffee pulp. Enzyme Microb Technol 15:254–260
Ashokkumar B, Gunasekaran P (2002) Beta-fructofuranosidase production by 2-deoxyglucose resistant mutants of Aspergillus niger in submerged and solid-state fermentation. Indian J Exp Biol 40:1032–1037
Asther M, Haon M, Roussos S, Record E, Delattre M, Lesage-Meessen L, Labat M, Asther M (2002) Feruloyl esterase from Aspergillus niger: a comparison of the production in solid state and submerged fermentation. Process Biochem 38:685–691
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Díaz-Godínez G, Soriano J, Augur C, Viniegra-González G (2001) Exopectinases produced by Aspergillus niger in solid-state and submerged fermentation: a comparative study. J Ind Microbiol Biotechnol 26:271–275
Hölker U, Höfer M, Lenz J (2004) Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl Microbiol Biotechnol 64:175–186
Kunitz M (1947) Crystalline soy bean trypsin inhibitor. General properties. J Gen Physiol 30:291
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Maldonado MC, Strasser de Saad AM (1998) Production of pectinesterase and polygalacturonase by Aspergillus niger in submerged and solid state systems. J Ind Microbiol Biotechnol 20:34–38
Palmieri G, Cennamo G, Faraco V, Amoresano A, Sannia G, Giardina P (2003) Atypical laccase isoenzymes from copper supplemented Pleurotus ostreatus cultures. Enzyme Microb Technol 33:220–230
Ramírez NE, Vargas MC, Ariza JC, Martínez C (2003) Caracterización de la lacasa obtenida por dos métodos de producción con Pleurotus ostreatus. Rev Colombiana Biotecnol 2:64–72
Romero-Gómez S, Augur C, Viniegra-González G (2000) Invertase production by Aspergillus niger in submerged and solid-state fermentation. Biotechnol Lett 22:1255–1258
Téllez-Jurado A, Arana-Cuenca A, González-Becerra AE, Viniegra-González G, Loera O (2005) Expression of a heterologous laccase by Aspergillus niger cultured by solid-state and submerged fermentations. Enzyme Microb Technol 38:665–669
Téllez-Téllez M, Sánchez C, Loera O, Díaz-Godínez G (2005) Differential patterns of constitutive intracellular laccases of the vegetative phase for Pleurotus species. Biotechnol Lett 27:1391–1394
Temp U, Eggert C (1999) Novel interaction between laccase and cellobiose dehydrogenase during pigment synthesis in the white rot fungus. Appl Environ Microbiol 65(5):389–395
Tlecuitl-Beristain S, Sánchez C, Loera O, Robson GD, Díaz-Godínez G (2008) Laccases of Pleurotus ostreatus observed at different phases of its growth in submerged fermentation: Production of a novel laccase isoform. Myc Res. doi:https://doi.org/10.1016/j.mycres.2008.03.001
Viniegra-González G, Favela-Torres E, Aguilar CN, Rómero-Gómez SJ, Díaz-Godínez G, Augur C (2003) Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem Eng J 13:157–167
Zhu Y, Knol W, Smits JP, Bol J (1996) Medium optimization for nuclease P1 production by Penicillium citrinum in solid-state fermentation using polyurethane foam as inert carrier. Enzyme Microb Technol 18:108–112
Acknowledgements
We thank Dr. A. L. Demain for critical reading of the manuscript and the Mexican Council of Science and Technology (CONACYT) for supporting this research (Project No. 47396). M. Téllez-Téllez was supported by a CONACYT scholarship (No. 177867).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Téllez-Téllez, M., Fernández, F.J., Montiel-González, A.M. et al. Growth and laccase production by Pleurotus ostreatus in submerged and solid-state fermentation. Appl Microbiol Biotechnol 81, 675–679 (2008). https://doi.org/10.1007/s00253-008-1628-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1628-6