Abstract
A mutant Aspergillus carbonarius selected for temperature tolerance after UV treatment, when grown in shake flasks, produced mycelia bearing yellow pigment. Since the mutant was affected in sterol biosynthetic pathway, the pigment was apparently produced to maintain membrane fluidity and rigidity for growth sustenance in low-pH culture broth. Nuclear magnetic resonance analyses characterizing the pigment as a partially saturated canthaxanthin, containing β-ionone end rings, suggested its application as a retinoid. When tested for this property in retinoic acid receptor expressing prostate cancer cell line, LNCaP, the fungal partially saturated canthaxanthin induced apoptosis. Low apoptosis percentage in DU145 prostrate cancer cells that does not express functional retinoic acid receptor-β (RAR-β) suggested binding specificity of the partially saturated canthaxanthin for RAR-β.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Some microorganisms, especially Aspergilli, reduce the pH of culture media to extreme acidity during growth. The internal pH homeostasis, for growth sustenance under such an extreme condition, is maintained either through the cytoplasmic membrane P-adenosine triphosphatases which expel free protons (Serrano 1988; Hesse et al. 2002) or by incorporating sterols, squalene, polyisoprenes (dolichol, ubiquitin), saturated fatty acids, and unusual lipids into the lipid bilayers (Lazrak et al. 1988; Albers et al. 2001) for strongly reducing permeability to water and small molecules. Carotenoids like zeaxanthin, astaxanthin, and canthaxanthin also have the ability to incorporate into membranes and change fluidity and integrity (Wisniewska and Subczynski 1998; Socaciu et al. 2002). While the polar end groups and hydrophobic middle chain of carotenoids have physiological roles in influencing structural and mechanical properties of lipid bilayers to decrease diffusion of free ions (Gabrielska and Gruszecki 1996), the β-ionone rings find application as retinoids for cancer treatment (Bertram 1999; Altucci and Gronemeyer 2001; Garattini et al. 2007).
Retinoids, a group of natural and synthetic derivatives of vitamin A (Niles 2004), mediate cell signaling through retinoic acid and retinoid X nuclear receptors to restore normalcy in abnormal animal cells (de Luca 1991; Li et al. 1998; Altucci and Gronemeyer 2001). It involves binding of the retinoid β-ionone end rings to the ligand binding pockets of the receptors (Bertram 1999; Egea et al. 2002). Though efficacy of several synthetic retinoids for cancer therapy have been reported based on studies using cell line models (Huang et al. 1997; Sun et al. 1999; Altucci and Gronemeyer 2001; Bertram and Vine 2005), the occurrence of stereoisomers and toxicity restricted their clinical use (Garattini et al. 2007). Hence, efforts are being made to identify natural retinoids (McCormick et al. 1999) and Gundersen and Blomhoff (2001) have elaborately discussed qualitative and quantitative analysis of different natural retinoids.
When we isolated a mutant Aspergillus carbonarius for temperature tolerance, it was found to accumulate a yellow pigment during growth in acidic pH. The pigment was characterized as partially saturated canthaxanthin containing β-ionone rings. Like a retinoid, it also induced apoptosis in cell lines containing retinoid receptors. The details are described in the paper.
Materials and methods
Isolation of A. carbonarius mutant
Spores of A. carbonarius (Accession No. CFTRI 1047) were spread on potato dextrose agar plates and inverted on transilluminator (302 nm) to obtain ~50% kill. After placing the plates at 4°C overnight in the dark to avoid photoactivated DNA repair, they were incubated at 42°C for 48 h (Gordon et al. 2000). The colonies that emerged 24 h after incubation at 30°C were isolated and grown in corn flour medium (4% corn flour, 0.5% (NH4)2HPO4, and 0.5% NH4H2PO4, pH 5.5) under shake flask conditions (200 rpm for 48 h). Under this culture condition, the biomass of some of the mutants attained a yellow color. One such mutant (accession No. CFTRI UV-10046) was used for the study.
Proton permeability determination and sterol extraction
Mycelia of A. carbonarius mutant obtained after 36-h growth in the medium buffered to pH 3.0 and 5.5 were used for permeability studies. Washed mycelia (1 g) were placed in 50-ml distilled water acidified to pH 3.0 with 0.1 M HCl. Changes in conductivity and pH of the distilled waters were measured after 60-min incubation. Using the equation, \({\text{pH}} = - \log \left[ {{\text{H}}^ + } \right]\), concentration of H+ ions in the distilled waters was obtained. The mycelia were dried at 50°C to represent the values in terms of milligram dry biomass. Based on standard deviation (SD) of three independent determinations, mean ± SD was obtained.
Free sterols from 1-g dried fungal biomass were extracted in methanol–chloroform (1:2 v/v) by pulverization with neutralized sand using mortar and pestle (Stoudt and Foster 1954; Folch et al. 1957). After filtration, the solvent was removed by flash evaporation and the extract was reconstituted in 1.0 ml hexane. Gas chromatography analysis was carried out (Nemec et al. 1997) and sterol concentration was obtained using ergosterol (Sigma, USA) as standard.
Extraction and purification of the pigment
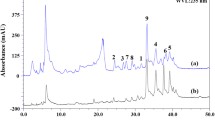
The biomass grown in corn flour medium for 36 h was separated from the medium by filtration through nylon mesh (250 μm). Following washing with distilled water, they were dried at 50°C. The pigment from 1.0-g biomass was extracted with 50 ml absolute ethanol taken in conical flasks. For this, the flasks were placed on a platform shaker (200 rpm) for 30 min at room temperature. Two sequential extractions were performed and the pooled extract was subjected to preparative thin-layer chromatography (TLC; Merck) with the solvent system made of isooctane, acetone, and diethyl ether (6:2:2). The yellow spot from the TLC plate (pigment) was eluted into ethanol and the procedure was repeated until the compound eluted as a single peak after high performance liquid chromatography (HPLC; Shimadzu LC-10A; isocratic elution at 1-ml min−1 flow rate in 30-cm × 4.0-mm C18 Supelco column) using 95:5 HPLC-grade methanol and acetone (Merck).
Spectroscopic methods
Mass spectrum was recorded with liquid chromatography–mass spectrometry (LC–MS; Waters, TOF) at a flow rate of 200 μl min−1 with 90% methanol as mobile phase. 1H and 13C nuclear magnetic resonance (NMR) spectra of the purified compound dissolved in CDCl3 were collected with 500-MHz (Inova-500) and 75-MHz (Gemini-300) NMR spectrometers, respectively. Distortionless enhancement of polarization transfer (DEPT) was performed with Gemini-300 spectrometer and heteronuclear single quantum coherence (HSQC) and correlation spectroscopy (COSY) with Inova-500 spectrometer.
2, 2-Diphenyl-1-picrylhydrazyl free radical scavenging assay
The pigment dissolved in 0.2 ml ethanol (concentration 20–100 μg ml−l) was made to 1 ml with 0.8-ml 100-mM Tris HCl buffer (pH 7.4). One milliliter 500 μM 2, 2-diphenyl-1-picrylhydrazyl prepared in ethanol was added, shaken vigorously, and incubated for 30 min at room temperature. Absorbance of the resulting solution was measured at 517 nm (UV–visible spectrophotometer, Shimadzu 160A, Japan) using pigment and substrate blanks. All the assays were carried out in triplicate.
Lipid peroxidation inhibitory studies
Lipid peroxidation inhibitory activity of ethanol extract was measured by the method described by Duh and Yen (1997). Egg lecithin (5 mg ml−l in phosphate buffer, pH 7.4) was sonicated (Hielscher GmbH, UP 50H ultraschallprozessor) for 30 min to obtain small membrane liposome vesicles. One-milliliter liposome mixture was treated with varying pigment concentration (30–100 μg ml−l) and lipid peroxidation was induced by the addition of 10 μl l-ascorbic acid (200 mM) and FeCl3 (400 mM). After incubation for 1 h at 37°C (Buchi Heating-bath B-490, Switzerland), 2 ml 0.25 N HCl containing 15% trichloroacetic acid and 0.375% thiobarbituric acid was added and boiled for 15 min. Absorbance of the filtered supernatant was measured at 532 nm. Blank and controls were maintained without liposome mixture and sample, respectively.
Cell lines
Human cervical cancer cells (HeLa) negative for retinoic acid receptor (RAR) expression (Bartsch et al. 1992; Geisen et al. 1997), prostate cancer cells (LNCaP) positive for RARs (Campbell et al. 1998), and prostate cancer cells (DU145) negative for RAR-β (Campbell et al. 1998; Singh et al. 2003) were obtained from American Type Culture Collection for use in this study. The cells were cultured in Roswell Park Memorial Institute 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen) and 2 mM glutamine. Cells were maintained at 37°C with 5% CO2.
3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide cell proliferation and fluorescence-activated cell sorting assays
Cells seeded in two 96-well microtiter plates for 24 h were treated with growth medium containing 1.27 and 2.14 μM partially saturated canthaxanthin, respectively. Control cells were treated with 0.5% ethanol. Metabolic activity of the cells was measured, after 48 h treatment, using 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium (MTT) cell proliferation kit (Roche) following the manufacturer’s protocol.
Apoptosis of the cells treated to the pigment was estimated by fluorescence-activated cell sorting assays (FACS). For this, 24-well plates containing seeded cells were treated with the medium containing partially saturated canthaxanthin (1.27 and 2.14 μM) dissolved in ethanol such that the concentration of ethanol in the medium did not exceed 0.5%. As vehicle control, cells were treated with medium containing 0.5% ethanol. Cells grown in the medium were also used as control. After 48 h, floating and adherent cells harvested were washed twice in phosphate-buffered saline and suspended in 1× binding buffer for staining with annexin-V and propidium iodide (APOAF apoptosis detection kit; Sigma Aldrich). The fluorescence of 10,000 cells was sorted and analyzed using EPICS XL-MCL flow cytometer (Coulter). The data were plotted on two axis design, which had four quadrants. Quadrant 1 quantitated population of dead but nonapoptotic cells (annexin-V negative and propidium iodide positive), quadrant 2, population of apoptotic cells (positive for both annexin-V and propidium iodide), quadrant 3, live cells (negative for both annexin-V and propidium iodide), and quadrant 4, cells at the onset of apoptosis (stains positive for annexin alone).
Results
Role of the pigment in the organism
A mutant A. carbonarius selected for temperature tolerance, when grown in corn flour medium buffered to pH 3.0, produced yellow-pigmented mycelia. Such a pigmentation was absent in mycelia grown at pH 5.5. Since the wild type grown at pH 3.0 did not exhibit pigmentation, the sterol concentrations were analyzed to predict the role of the pigment in the mutant fungus. The mutant mycelia grown at pH 5.5 had ~22% less sterol (1.148 ± 0.012 mg g−1 dcm) compared to that estimated in the wild type (1.466 ± 0.026 mg g−1 dcm). While the sterol concentration remained unaffected in the wild-type mycelium grown at pH 3.0, only negligible quantities of sterol were estimated in mutant mycelia grown in pH 3.0 medium.
Pigmented mycelia of the mutant fungus placed in distilled water, acidified to pH 3.0, increased the pH to 3.27. The pH-5.5-grown unpigmented mycelia, under these experimental conditions, increased the pH of the distilled water to 4.01. Since the acidified distilled water contained only H+ ions, the increase in pH was solely due to their loss as shown by the decreased conductivity of distilled water. The loss of H+ was more significant in unpigmented mycelia (20.552 ± 0.100-μM mg−1 biomass) compared to those pigmented (7.771 ± 0.116-μM mg−1 biomass). Thus, it appeared that the pigment functioned to prevent ion influx into mutant cells for higher tolerance to extreme environmental acidity.
Characterization of the pigment
The pigment purified from mutant A. carbonarius mycelia exhibited double-shouldered absorption spectrum with λ max at 414 nm in ethanol. The LC–MS resolved a major peak at m/z of 572. Assignment of individual protons and carbons after 1H and 13C-1D NMR, DEPT, COSY, and 13C-HSQC analyses (Table 1) showed aliphatic chains in the pigment, indicated by the number of 13C peaks in the range 34.6–14.2 ppm and corresponding proton peaks in 0.95–2.04 ppm. The most downfield 13C peak at 180.4 ppm, without any proton cross peaks in HSQC, identified as a quaternary carbon in DEPT spectrum, showed the presence of ketone group. The olefinic group, probably in conjugation, was deciphered from the number of 13C peaks between 128.4 and 131.0 ppm and corresponding proton cross peaks in the range 5.98–6.5 ppm. Similarly, a bunch of 13C peaks between 34.6 and 14.2 ppm and the corresponding proton peaks between 0.95 and 2.04 ppm indicated the presence of saturated hydrocarbons. The carbonyl group at C1 and C1′ (13C peak at 180.2 ppm) suggested a symmetrical structure for the compound. The 13C at 32.5 and the corresponding proton peak at 2.39 ppm (triplet, 2H) showed the occurrence of C2 and C2′ (–CH2–). Likewise, C3 and C3′ were confirmed by 13C peak at 35.0 ppm and the quaternary carbons C4 and C4′ by the 13C peaks at 33.6 ppm. 13C peaks at 128.1 and 128.6 ppm confirmed double bonds connecting two sets of quaternary carbon C5, C5′ and C6, C6′. The olefinic groups C7, C8, and C10 and corresponding C7′, C8′, and C10′ were identified with the 13C peaks at 131.0, 128.4, and 130.4 ppm and the corresponding protons at 6.5 (doublet, H), 6.3 (doublet, H), and 5.98 (multiplet, H), respectively. Existence of saturated carbon chains were confirmed by 13C peaks at 26.0 ppm (1H at 2.04 ppm) for C11 and C11′, 34.6 ppm (1H at 1.32 ppm) for C12 and C12′, 30.2 ppm (1H at 1.36 ppm) for C13 and C13′, and 32.8 ppm (1H at 1.10 ppm) for C15 and C15′. The multiplet nature of the proton peaks further supported the presence of saturated carbon chain in the compound. The 27.8-, 18.0-, and 14.2-ppm peaks corresponded to methyl groups at C16, C17, C18, and C19 (C16′ or C17′, C18′, and C19′) positions. The respective singlet proton cross peaks at 1.32, 2.00, and 1.64 ppm suggested that they were attached to quaternary carbons. Similarly, the –CH3 at C20, C20′ were confirmed by 13C peaks at 23.0 (1H at 0.95 ppm).
Based on the NMR data, the pigment was identified as partially saturated canthaxanthin (11, 12, 13, 14, 15, 11′, 12′, 13′, 14′, 15′ decahydro β, β carotene-4, 4′ dione), a polar carotenoid with keto groups at C1 and C1′ (Fig. 1). A molar extinction coefficient of 51,158.7 M−1 (log ɛ of 4.7089) was also calculated for the pigment in absolute ethanol using its molecular mass and λ max at 414 nm.
Functional significance of the pigment as a retinoid
Though a canthaxanthin, it exhibited only a weak free radical quenching and lipid peroxidation prevention activities (Table 2). This was predicted due to its partial saturation. Its structural homology with known retinoids, identified through the occurrence of β-ionone rings, suggested its application as a drug. Preliminary screening of the fungal partially saturated canthaxanthin for apoptosis in RAR-expressing LNCaP cells showed its ability for such an application since changes in morphology could be observed in cells treated to 1.27- and 2.14-μM concentrations (Fig. 2). HeLa cells, lacking the receptor, did not exhibit any morphological variations (Fig. 2). FACS and MTT cell proliferation assays were carried out to further evidence the above observation that the partially saturated canthaxanthin required RARs, like the known retinoids, for reactivity.
Flipping of plasma membrane phosphatidylserine (PS) from the inside surface to the outside surface is an early event in apoptosis (Fadok et al. 1992) and specificity of annexin-V–fluorescein isothiocyanate conjugate binding to PS is used to probe apoptotic cells. Since cell membrane integrity excludes propidium iodide in viable and apoptotic cells, whereas necrotic cells are permeable to propidium iodide, dual-parameter FACS analysis distinguishes between viable, apoptotic, and necrotic cells. Simultaneous MTT cell proliferation assay that measures cellular metabolic activity supplement results of the FACS assay by further quantitating the percentage of live cells.
FACS and MTT assays with prostate cancer cell lines, LNCaP (positive for RARs) and DU145 (does not express functional RAR-β), treated to 1.27 and 2.14 μM partially saturated canthaxanthin, showed that LNCaP cells exhibited 9% and 68% apoptosis, respectively. Only 4% and 23% apoptosis could be estimated in DU145 treated to such pigment concentrations (Fig. 3). Absence of apoptosis assayed in cells grown in the medium containing the highest concentration (0.5%) alcohol (control) suggested that the effect was solely due to partially saturated canthaxanthin. Since MTT assays determined 85 ± 0.7% and 35 ± 1.9% metabolically active LNCaP cells and 96 ± 0.4% and 76 ± 0.9% viable DU145 cells, the results corroborated each other for the conclusion of retinoid-like activity for the fungal partially saturated canthaxanthin.
Discussion
Low pH and elevated temperature affect growth of fungi by altering membrane fluidity and integrity causing increased proton influx (Vigh et al. 1998; Burgstaller 1997; van de Vossenberg et al. 1995). For survival, adaptation to these extreme environmental conditions involve incorporation of primarily sterols, fatty acids of varied saturation, and polyisoprenes into the membrane (Petrovic et al. 1999; Albers et al. 2001; Haines 2001). It appeared that the A. carbonarius mutant isolated for temperature tolerance was also affected in sterol biosynthesis and partially saturated canthaxanthin was synthesized from isoprenoid precursors to regulate membrane fluidity, similar to carotenoids of chloroplast during xanthophyll cycle (Gruszecki and Strzalka 1991). Apparently, the partially saturated canthaxanthin incorporated in the mutant A. carbonarius cell membrane in a manner similar to zeaxanthin in lipid bilayers (Wisniewska and Subczynski 1998; Socaciu et al. 2000; Socaciu et al. 2002).
Significance of the β-ionone containing partially saturated canthaxanthin with retinoid properties synthesized by the mutant A. carbonarius, identified in this study, is new to literature. Ability of the fungal partially saturated canthaxanthin to react with retinoic acid receptor containing LNCaP cells and cause apoptosis suggested its retinoid-like activity for cellular signaling (Klaholz and Moras 1998; Teicher et al. 1999; Mailfait et al. 2000; Egea et al. 2002; Nagao 2004). It has been shown that cell signaling by retinoids leading to apoptosis occurs largely through RAR-β (Campbell et al. 1998). Low apoptosis in DU145 cells expressing nonfunctional RAR-β further evidenced the functionality of partially saturated canthaxanthin as a retinoid for possible use as a drug. From 100-g dry fungal biomass (36 h old culture), 130 to 150 mg partially saturated canthaxanthin could be obtained. Since the value corresponded to that present in unrefined red palm oil, so far reported for highest carotenoid content (Dawson 2000), the A. carbonarius mutant appears a good source for the xanthin.
References
Albers S, van de Vossenberg JLCM, Driessen AJM, Konings WN (2001) Bioenergetics and solute uptake under extreme conditions. Extremophiles 5:285–294
Altucci L, Gronemeyer H (2001) The promise of retinoids to fight against cancer. Nat Rev Cancer 1:181–193
Bartsch D, Boye B, Baust C, Hausen H, Schwarz E (1992) Retinoic acid-mediated repression of human papillomavirus 18 transcription and different ligand regulation of the retinoic acid receptor gene in nontumorigenic and tumorigenic HeLa hybrid cells. EMBO J 11:2283–2291
Bertram JS (1999) Carotenoids and gene regulation. Nutr Rev 57:182–191
Bertram JS, Vine AL (2005) Cancer prevention by retinoids and carotenoids: independent action on a common target. Biochim Biophys Acta 1740:170–178
Burgstaller W (1997) Transport of small ions and molecules through plasma membrane of filamentous fungi. Crit Rev Microbiol 23:1–46
Campbell MJ, Park S, Uskokovic MR, Dawson MI, Koeffler HP (1998) Expression of retinoic acid receptor-b sensitizes prostate cancer cells to growth inhibition mediated by combinations of retinoids and a 19-nor hexafluoride vitamin D3 analog. Endocrinology 139:1972–1980
Dawson MI (2000) The importance of vitamin A in nutrition. Curr Pharm Des 6:311–325
de Luca LM (1991) Retinoids and their receptors in differentiation, embryogenesis and neoplasia. FASEB J 5:2924–2933
Duh PD, Yen GC (1997) Antioxidative activity of three herbal water extracts. Food Chem 60:639–645
Egea PF, Mitschelar A, Moras D (2002) Molecular recognition of agonist ligands by RXRs. Mol Endocrinol 16:987–997
Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 148:2207–2216
Folch J, Lees M, Stanley GHS (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Gabrielska J, Gruszecki LW (1996) Zeaxanthin (dihydroxy-b-carotene) but not b-carotene rigidifies lipid membranes: a 1H-NMR study of carotenoid-egg phosphatidylcholine liposomes. Biochim Biophys Acta 1285:167–174
Garattini E, Gianni M, Terao M (2007) Retinoids as differentiating agents in oncology: a network of interactions with intracellular pathways as the basis for rational therapeutic combinations. Curr Pharm Des 13:1375–1400
Geisen C, Denk C, Gremm B, Baust C, Karger A, Bollag W, Schwarz E (1997) High-level expression of the retinoic acid receptor b gene in normal cells of the uterine cervix is regulated by the retinoic acid receptor a and is abnormally down-regulated in cervical carcinoma cells. Cancer Res 57:1460–1467
Gordon CL, Khalaj V, Ram AFJ, Archer DB, Brookman JL, Trinci APJ, Jeenes DJ, Doonman JH, Wells B, Punt PJ, van den Hondel CAMJJ, Robson GD (2000) Glucoamylase: green fluorescent protein fusion to monitor protein secretion in Aspergillus niger. Microbiology 146:415–426
Gruszecki WI, Strzalka K (1991) Does the xanthophyll cycle take part in the regulation of fluidity of the thylakoid membrane? Biochim Biophys Acta 1060:310–314
Gundersen TE, Blomhoff R (2001) Qualitative and quantitative liquid chromatographic determination of natural retinoids in biological samples. J Chromatogr A 935:13–43
Haines TH (2001) Do sterol reduce proton and sodium leaks through lipid bilayers? Prog Lipid Res 40:299–324
Hesse SJA, Ruijter GJG, Dijkema C, Visser J (2002) Intracellular pH homeostasis in filamentous fungi. Eur J Biochem 269:3485–3494
Huang C, Ma W, Dawson MI, Rincon M, Flavell RA, Dong Z (1997) Blocking activator protein-1 activity, but not activating retinoic acid response element, is required for the antitumor promotion effect of retinoic acid. Proc Natl Acad Sci U S A 94:5826–5830
Klaholz BP, Moras D (1998) A structural view of ligand binding to the retinoid receptors. Pure Appl Chem 70:41–47
Lazrak T, Wolf G, Albrecht AM, Nakatani Y, Ourisson G, Kates M (1988) Bacterioruberins reinforce reconstituted Halobacterium lipid membranes. Biochim Biophys Acta 939:160–162
Li Y, Lin B, Agadir A, Liu R, Dawson MI, Reed JC, Fontana JA, Bost F, Hobbs PD, Zheng Y, Chen G, Shroot B, Mercola D, Zhang X (1998) Molecular determinants of AHPN (CD437)-induced growth arrest and apoptosis in human lung cancer cell lines. Mol Cell Biol 18:4719–4731
Mailfait S, Thoreau E, Belaiche D, Formstecher BS (2000) Critical role of the H6–H7 loop in the conformational adaptation of all-trans retinoic acid and synthetic retinoids within the ligand-binding site of RAR alpha. J Mol Endocrinol 24:353–364
McCormick DL, Rao KVN, Steele VE, Lubet RA, Kellof GJ, Bosland MG (1999) Chemoprevention of rat prostate carcinogenesis by 9-cis-retinoic acid. Cancer Res 59:521–524
Nagao A (2004) Oxidative conversion of carotenoids to retinoids and ther products. J Nutr 134:237S–240S
Nemec T, Jernejc K, Cimerman A (1997) Sterols and fatty acids of different Aspergillus species. FEMS Microbiol Lett 149:201–205
Niles RM (2004) Signaling pathways in retinoid chemoprevention and treatment of cancer. Mutat Res 555:81–96
Petrovic U, Gunde-Cimerman N, Plemenitas A (1999) Salt stress affects sterol biosynthesis in the halophilic black yeast Hortaea werneckii. FEMS Microbiol Lett 180:325–330
Serrano R (1988) H+-ATPase from plasma membranes of Saccharomyces cerevisiae and Avena sativa roots: purification and reconstitution. Methods Enzymol 157:533–544
Singh RP, Agarwal C, Agarwal R (2003) Inositol hexaphosphate inhibits growth, and induces G1 arrest and apoptotic death of prostate carcinoma DU145 cells: modulation of CDKI-CDK-cyclin and pRb-related protein-E2F complexes. Carcinogenesis 24:555–563
Socaciu C, Jessel R, Diehl HA (2000) Carotenoid incorporation into microsomes: yields, stability and membrane dynamics. Spectrochim Acta Part A 56:2799–2809
Socaciu C, Bojarski P, Aberle L, Diehl HA (2002) Different ways to insert carotenoids into liposomes affect structure and dynamics of the bilayer differently. Biophys Chem 99:1–15
Stoudt TH, Foster JW (1954) The microbiological synthesis of ergosterol. Appl Environ Microbiol 2:385–387
Sun S, Yue P, Lotan R (1999) Induction of apoptosis by N-(4-Hydroxyphenyl)retinamide and its association with reactive oxygen species, nuclear retinoic acid receptors and apoptosis-related genes in human prostate carcinoma cells. Mol Pharmacol 55:403–410
Teicher VB, Kucharski N, Martin H, Saag P, Sies H, Stahl W (1999) Biological activities of apo-canthaxanthinoic acids related to gap junctional communication. Arch Biochem Biophys 365:150–155
van de Vossenberg JLCM, Ubbink-Kok T, Elferink MGL, Driessen AJM, Konings WN (1995) Ion permeability of the cytoplasmic membrane limits the maximum growth temperature of bacteria and archaea. Mol Microbiol 18:925–932
Vigh L, Maresca B, Harwood JL (1998) Does the membrane’s physical state control the expression of heat shock and other genes? Trends Biochem Sci 23:369–374
Wisniewska A, Subczynski WK (1998) Effects of polar carotenoids on the shape of the hydrophobic barrier of phospholipid bilayers. Biochim Biophys Acta 1368:235–246
Acknowledgement
The authors thank Dr. Dinesh K Sukumaran, Director, Magnetic Resonance Center, Department of Chemistry, State University of New York, Buffalo, NY, USA for his assistance in NMR data and its analysis. NK, KRS, KSV, and RK were supported by fellowship grants from Indian Government research agencies, the Council of Scientific Industrial Research, Indian Council of Medical Research and University Grant Commission.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at https://doi.org/10.1007/s00253-008-1586-z
Rights and permissions
About this article
Cite this article
Kumaresan, N., Sanjay, K.R., Venkatesh, K.S. et al. Partially saturated canthaxanthin purified from Aspergillus carbonarius induces apoptosis in prostrate cancer cell line. Appl Microbiol Biotechnol 80, 467–473 (2008). https://doi.org/10.1007/s00253-008-1538-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1538-7