Abstract
This study studied the cultivation of granules from an expanded granular sludge bed reactor that simultaneously transforms sulfates, nitrates, and oxygen to elementary sulfur, nitrogen gas, and carbon dioxides, respectively. The living cells accumulate at the granule outer layers, as revealed by the multicolor staining and confocal laser scanning microscope technique. The microbial community comprises sulfate-reducing bacteria (SRB, Desulfomicrobium sp.), heterotrophic (Pseudomonas aeruginosa and Sulfurospirillum sp.), and autotrophic denitrifiers (Sulfurovum sp. and Paracoccus denitrificans) whose population dynamics at different sulfate and nitrate loading rates are monitored with the single-strand conformation polymorphism and denaturing gradient gel electrophoresis technique. The Desulfomicrobium sp. presents one of the dominating strains following reactor startup. At high sulfate and nitrate loading rates, the heterotrophic denitrifiers overcompete autotrophic denitrifiers to reduce SRB activities. Conversely, suddenly reducing nitrate loading rates completely removes the heterotrophic denitrifier Sulfurospirillum sp. from the granules and activates the autotrophic denitrifiers. The physical fixation of different groups of functional strains in granules fine-tunes the strains’ activities, and hence the reactor performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial wastewaters can contain high levels of sulfate and nitrate. Biological elimination of sulfate and/or nitrate from these wastewaters presents as a cost-effective and environmentally friendly alternative to the chemical removal processes. Biological sulfate removal can be accomplished by anaerobically reducing the sulfate to sulfide with sulfate-reducing bacteria (SRB). Biological nitrate removal can be accomplished by reducing nitrate to nitrogen gas with autotrophic or heterotrophic denitrifiers.
Weijma et al. (2002) observed that sulfate reduction is favored over methanogenic reaction when the chemical oxygen demand (COD)/\(\operatorname{SO} _4^{2 - } \) ratio is lowered from 6 to 0.34 in an expanded granular sludge bed (EGSB) reactor. Dries et al. (1998) respectively controlled the feed stoichiometry at high C/S ratio and the solution pH at alkaline conditions to increase sulfate removal and to restrict inhibition by undissociated H2S to SRB in an acetate-fed EGSB reactor. The acclimated sulfidogenic granular sludge can degrade 80–90% of sulfate loaded at a rate of 10.4 kg S m−3 day−1 after 60 days of start-up. De Smul et al. (1999) considered sulfate removal in an EGSB reactor at 33°C with sufficient ethanol dose and concluded that the removal ratio of sulfur was in the range 80–90% and 60–70% at loading rates of 4 or 7 kg S m−3 day−1, respectively. De Smul and Verstraete (1999) observed a maximum sulfate removal rate of 9.5 kg S m−3 day−1 in their formate-fed EGSB reactor. By stripping H2S gas from the solution with N2 and feeding it with methanol as sole carbon and energy source at 65°C and pH 7.5, Weijma et al. (2000) obtained maximum of sulfite and sulfate removal rates in EGSB reactors of 18 kg \(\operatorname{SO} _3^{2 - } \) m−3 day−1 and 5.5 kg \(\operatorname{SO} _4^{2 - } \) m−3 day−1, respectively. Vallero et al. (2003) applied a three-compartment baffled reactor to examine the degradation of volatile fatty acid mixture under sulfidogenic (COD/\(\operatorname{SO} _4^{2 - } \) = 1:2) condition. The COD and sulfate removal rates were 85% and 30%, respectively, at 0.50 kg COD kg−1 VSS day−1. They thereby studied a two-stage process to minimize competition between autotrophic and heterotrophic denitrifiers. Chuang et al. (2005) proposed the application of a two-stage system, including a UASB sulfate-reducing reactor to transform sulfate to sulfide, and a floated bed micro-aerobic reactor (DO < 0.5 mg l−1) to partially oxidize sulfide to elemental sulfur. Kim et al. (2004) performed heterotrophic and autotrophic denitrification processes in two separate reactors and revealed that the removal rates of nitrogen were greater than those utilizing single-stage reactors with either obligated heterotrophic or autotrophic processes alone. However, the adoption of two-stage process acquires excess equipment and controlling units, thus raising the operation and installation costs.
The EGSB reactor enables wastewater to pass through at high upflow velocity. The granules in the EGSB reactor, if they retain SRB, and both heterotrophic and autotrophic denitrifiers can accomplish simultaneous removal of sulfate, nitrate, and COD. To the authors’ best knowledge, no previous study has addressed simultaneous sulfate/nitrate/COD removal in a single EGSB reactor. The correlation of microbial community and the noted reactor performance is also lacking. This study aims to cultivate granules from a single EGSB reactor acclimatized with SRB and heterotrophic and autotrophic denitrifiers. The performance of simultaneous sulfate, nitrate, and COD removal using the cultivated granules is determined. The interior structure of the granules was revealed with multicolor staining and confocal laser scanning microscope (CLSM) technique, and the microbial community structure for granule was examined with single-strand conformation polymorphism (SSCP) and denaturing gradient gel electrophoresis (DGGE).
Materials and methods
Experimental setup
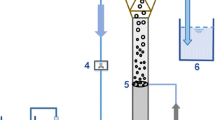
The EGSB reactor comprised Plexiglass with a diameter of 80 mm and a height of 200 cm, giving a working volume of 4.0 l (Chen et al. 2008). The reactor was maintained at 28 ± 1°C. A peristaltic pump introduced influent from the bottom of the column. A gas-washing device containing NaOH solution accumulated the H2S gas that was produced. The reflux ratio was fixed at 6:1, producing an upflow velocity of 1.8 m h−1.
The seed sludge was accumulated from the anaerobic sludge thickener of the Wenchang Wastewater Treatment Plant, Harbin, China, which was screened with 0.2 mm Tyler mesh to eliminate most grits. Synthetic wastewater comprising 1,000 mg l−1 of \(\operatorname{SO} _4^{2 - } \) and of lactate to make COD/\(\operatorname{SO} _4^{2 - } \) = 2:1 was prepared. Phosphate was added at N/P = 5:1, and bicarbonate was utilized to maintain the influent pH at 8.0 ± 0.3. The micronutrients in 1 l of feed wastewater included: 500 mg (each) of H3BO3, ZnCl2, (NH4)6Mo7O24·4H2O, NiCl·6H2O, AlCl3·6H2O, CoCl2·6H2O, and CuSO4·5H2O; 1,000 mg of NaSeO3·5H2O; 1,500 mg of FeCl3·6H2O; 5,000 mg of MnCl2·4H2O, and 5 ml of 37% HCl solution.
Parallel batch tests were performed in 100-ml culture bottles. The granules cultivated at the end of continuous tests were centrifuged (3,000 rpm and 10 min) to eliminate supernatant. The obtained granules were washed five times with milli-Q water. Three sets of batch tests were conducted. Batch tests A–C were (performed OR conducted OR undertaken) with 25 ml granules with 25 ml substrates with compositions the same as the aforementioned feed wastewater. In test A, the sulfate and nitrate were fed without addition of COD. Sulfate, nitrate, and lactate were applied in test B. In test C, the nitrate and COD were fed without addition of sulfate. All bottles were sealed and incubated on a shaken table at 35°C and sampled regularly.
Community structure analysis
The community structures of cultivated granules were studied with SSCP and DGGE techniques by following procedures utilized in Chen et al. (2008). Briefly, the total DNA was extracted, concentrated, and purified. For SSCP assessment, the amplified target DNA was the variable region V1-V3 of the 16S ribosomal RNA gene. The primers were Eub-8F and Eub-533R-ph. Bacterial 16S ribosomal DNA (rDNA) library was contracted for microbial composition analysis. The PCR primers were Eub-8F and Eub-926R, corresponding to 8–926 bp of 16S rDNA in Escherichia coli. The primer pair nosZ-F and nosZ-R was adopted for quantification of denitrifying microorganisms in granule samples. DGGE tests were undertaken with the Bio-Rad universal mutation detection system and 8% (w/v) polyacrylamide gels.
Sequence classification was conducted online using sequence matching software in RDP (Ribosomal Database Project; Maidak et al. 1999) and the BLAST program on the National Center for Biotechnology Information (NCBI) website (Altschul et al. 1997).
Staining and CLSM imaging
Proteins in the samples were stained using fluoresceinisothiocyanate (FITC). Concanavalin tetramethylrhodamine conjugate (ConA) was adopted to bind to α-mannopyranosyl and α-glucopyranosyl sugar residues. The cells were probed in their entirety by the wall-permeable nucleic acid stain SYTO 63. The dead cells were stained using cell-wall-impermeable stain SYTOX blue. Lipids were stained by Nile red, while β-polysaccharides were stained by calcofluor white. The CLSM (Leica TCS SP2 Confocal Spectral Microscope Imaging System, Germany) technique was employed to visualize cell or EPS distributions in bio-samples. Staining details are available in Chen et al. (2007).
Chemical analysis
An ion chromatographer (Dionex ICS-3000) was applied to measure the concentrations of nitrate, nitrite, sulfate, and thiosulfate in the collected liquor samples after 0.45-μm filtration. Sample separation and elution were performed by an IonPac AG4A AS4A-SC 4 mm analytical column using carbonate/bicarbonate eluent (1.8 mmol dm−3 Na2CO3/1.7 mmol dm3 NaHCO3 at 1 cm3 min−1) and a sulfuric regeneration (H2SO4, 25 mmol dm−3 at 5 cm3 min−1). Sulfide concentration was measured by potential titration by Sure-Flow™ combination silver/sulfide electrodes. Alkalinity was measured by titration by diluted hydrochloric acid (HCl). A pH/ORP meter (pHS-25) was adopted to measure the pH and oxidation–reduction potential (ORP) of each liquid sample. The suspended solids and volatile suspended solids were measured based on standard methods.
Results
Reactor performance
Figure 1 depicts the sulfate and nitrate loading rates and the removal ratios over the EGSB testing period (112 days). The influent with 1,000 mg l−1 of \(\operatorname{SO} _4^{2 - }\) and with lactate to make COD/\(\operatorname{SO} _4^{2 - } \) = 2:1 was initially fed at a flow rate of 8.8 l day−1, leading to sulfate loading rate (SLR) of 0.7 kg m−3 day−1 (stage I). The pH of influent was maintained at 8.0, while the initial alkalinity was at 1,500 mg l−1. The sulfate removal reached 80% in the 10 days of operation. Nitrate was then added at a low level (240 mg l−1 of \(\operatorname{N} \operatorname{O} _3^ - \)) to the reactor, yielding a nitrate loading rate (NLR) of 0.22 kg m−3 day−1. The nitrate removal exceeded 99% within 1 week. The influent SLR level was increased to 1.6 and 2.1 kg m−3 day−1 on days 18 and 27, respectively, while the influent NLR level was increased to 0.8 and 1.7 kg m−3 day−1 on days 22 and 27, respectively. The sulfate and nitrate removal levels were 78% and >99%, respectively, after day 30 (stage II).
On day 62, the NLR had increased to 2.1 kg m−3 day−1 (stage III). This action decreased the sulfate removal rate to less than 35% in 5 days. The influent alkalinity was raised to 2,000 mg l−1 after day 65. The sulfate removal recovered to >78% on day 71 and onward.
Straw yellow, granular and filamentous insoluble solids were observed in the suspended liquor and in the effluent from EGSB reactor after granule formation. The insoluble solids were sampled and air-dried, soaked in acid and then alkali solutions, and then dissolved in carbon bisulfide. Piperidine reacted with the dissolved to form products in red color, confirming that the collected insoluble solid was elemental sulfur (S0). The quantity of S0 produced in the reactor was estimated by mass balance calculation by following the procedures in Chen et al. (2008). The conversion rate of S0 in EGSB reactor was in the range 10–40%.
The SLR was reduced to 1.0 kg m−3 day−1 on day 83, with the NLR adjusted to 0.6, 1.0, and 1.7 kg m−3 day-1 on days 83, 94, and 102, respectively (stage IV). A drop in sulfate removal rate was noted initially. The sulfate removal rate recovered to >90% since day 94.
Cultivated granule
Granules were produced in EGSB and developed to a size of approximately 0.5 mm on day 25 near the end of stage I. The granules further developed to reach a size of about 3 mm on day 112. Figure 2 illustrates the appearance of the outer layer (Fig. 2a) and the fluorescent staining results (Fig. 2b–h) probed at 360 μm from the outer surface of the granule cultivated on day 110.
The CLSM images of EGSB granules. a Phase contrast photograph. b Combined image of individual images in c–h. c CLSM image of proteins (FITC). d CLSM image of lipids (Nile red), e β-polysaccharides (calcofluor white). f CLSM image of α-polysaccharides proteins (Con A). g CLSM image of total cells (SYTO 63). h CLSM image of dead cells (SYTOX Blue)
The granules were in near-round shape and had a compact surface structure comprising of many micro colonies and channels (photograph not shown). The integrity coefficients of all granules were measured as approximately 1.1% (data not shown), revealing that the structures of the incubated granules were strong. The SS and VSS were raised to 9–12 g l−1 and 5.5–6.5 g l−1, respectively, after 32 days of start-up. The corresponding VSS/SS ratio was in the range 0.6–0.7, revealing that the granules contained good levels of organic compounds.
Conversely, the formed granule had very loose interior structures, in contrast to the noted compact and relatively smooth outer layers. Cells (total cells in Fig. 2g and dead cells in Fig. 2h), together with proteins (Fig. 2c), lipids (Fig. 2d), β-polysaccharides (Fig. 2e), and α-polysaccharides (Fig. 2f) accumulated uniformly in the central regime of the granule. Figure 2b is the combined image of Fig. 2c–h. Most cells were located at the outer layer of the granule to minimize the mass transfer limitation of nutrients.
Therefore, stable granules with compact outer layer and loose interior structure were successfully developed in the EGSB reactor.
Microbial community in EGSB reactor
Figure 3 displays the SSCP bands displayed by sludge and granular samples accumulated from the EGSB reactor. Dominant populations were enriched, as shown by the bright SSCP bands. Partial dominant bands in Fig. 3 were cut out for DNA sequencing (Table 1). The sequences with similarity exceeding 97% were considered as the same operational taxonomic unit (OUT). Thus, a total of 19 OTUs were obtained. The proportions of the sequences were: Chloroflexi, 13%; Fimuicutes, 8.7%; Bacteroidetes, 26.1%; Proteobacteria, 52.2%.
The bands 12, 13, and 16 in Fig. 3 correspond to anaerobes Xenohaliotis, Succinispira, Anaerolinea, and Allisonella in the reactor. Band 4 in SCCP corresponds to Desulfomicrobium sp., which is an SRB that can reduce sulfate using ethanol or H2 as electron donors (Rozanova et al. 1988). Sulfurovum sp. (band 10) is a chemolithotrophic bacterium that uses sulfur, sulfide, or thiosulfate as electron donors and nitrate as electron acceptor (Inagaki et al. 2004). Sulfurospirillum sp. (band 9) is a heterotrophic denitrifier that utilizes sulfate and nitrate as electron acceptors (Hubert and Voordouw 2007; Kodama et al. 2007).
The DGGE identified two denitrifiers in the EGSB reactor, which are the Pseudomonas aeruginosa and Paracoccus denitrificans that have the NosZ gene (Fig. 4). The P. aeruginosa presents a heterotrophic denitrifier that can utilize various organic substrates. The Paracoccus sp. can reduce nitrate by both autotrophic and heterotrophic pathways.
Discussion
Complex interactions among acidogenic bacteria, SRB, autotrophic denitrifiers, and heterotrophic denitrifiers present in a simultaneous desulfurization and denitrification EGSB reactor. Briefly, the autotrophic and heterotrophic denitrifiers in the same reactor compete on the same nitrogen source. With limited nitrate or COD, the autotrophic denitrifier could outweigh the activities of heterotrophic denitrifier, forming excess nitrite to inhibit the SRB. Excess nitrate and COD caused heterotrophic denitrifiers to dominate over autotrophic denitrifiers, thus possibly impeding SRB by overcompeting the COD or yielding excess nitrite. The autotrophic denitrifiers are inhibited if the SRB produces insufficient quantities of reduced forms of sulfur. However, maintaining all functional strains in prescribed quantities and activities in a continuous flow reactor is extremely difficult for long-term operation because different strains can have very different physiological attributes or become mutually inhibitory at various environmental constraints. The bio-granules provide physical fixation and separation of competitive strains (Adav and Lee 2008).
As demonstrated in Fig. 3, regardless of the sulfate and nitrate loading rates, the Xenohaliotis, Succinispira, Anaerolinea, and Allisonella presented as the acidogenic strains in the reactor. The Desulfomicrobium sp. (band 4 in Fig. 3) presented SRB for sulfate reduction. The Sulfurospirillum sp. (band 9 in Fig. 3) and P. aeruginosa (band 1 in Fig. 4) are the heterotrophic denitrifiers for nitrate reduction. The Sulfurovum sp. (band 10 in Fig. 3) and P. denitrificans (band 2 in Fig. 4) were likely the autotrophic denitrifiers for reducing sulfide to elementary sulfur (S0).
The SRB Desulfomicrobium sp. presented one of the dominating strains in the reactor since reactor startup. The SLR level was maintained steadily high (2.0 kg m−3 day−1) in stage II, during days 30–61. The removal rate was sufficient (>78%), showing a minimal COD limit or production (S2− or \(\operatorname{NO} _2^ - \)) inhibition effects on SRB. Because the production of S0 was minimal, the two heterotrophic denitrifiers Sulfurospirillum sp. and P. aeruginosa corresponded to the high nitrate removal rate (>99%) previously noted.
The sulfate removal rate was reduced in stage III with further increase in NLR at 2.1 kg m−3 day−1. This occurrence was very likely attributable to the overcompetition of heterotrophic denitrifier with SRB at high NLRs. The activity of heterotrophic denitrification remained high, corresponding to the raised population of the heterotrophic denitrifiers Sulfurospirillum sp. and P. aeruginosa at stage III. The removal rate of sulfate recovered after day 68 when the influent alkalinity was increased from 1,500 to 2,000 mg l−1. Additionally, the S0 conversion rate increased to 40% or more during days 70–74 (Fig. 1). The populations of genus Sulfurovum sp. and P. denitrificans rose, as indicated in Figs. 3 and 4. Their enrichment lowered the demand for COD by heterotrophic denitrifiers, thus restoring the activity of SRB. However, the S0 conversion rate became low again on the following days, revealing that the activities of autotrophic denitrifiers could not eventually compete with those of heterotrophic denitrifiers at the high NLRs. The SRB had 5–6 days to recover its reduction capability to reduce sulfate to sulfide, as revealed by the accumulation of thiosulfate in the transition stage (Fig. 1).
In stage IV, the SLR level was lowered to 1.0 kg m−3 day−1, and NLR was reduced to 0.6 kg m−3 day−1. At this stage, the fed COD must be in excess in reaction. However, the sulfate removal rate still dropped correspondingly on days 93–98, while the heterotrophic denitrifier Sulfurospirillum sp. disappeared in stage IV. That is, the heterotrophic denitrifier failed to compete with autotrophic denitrifiers in the granules at low NLR. The nitrite yielded by autotrophic denitrifiers (Reyes-Avila et al. 2004) then inhibited the SRB because the sulfide concentration was low (Fig. 1) and the COD was in excess. However, the SRB then restored its activity at increased NLRs, probably owing to the reactivation of heterotrophic denitrifiers at higher NLRs. The dominating strain at this stage was P. aeruginosa, while Sulfurospirillum sp. was not detected in the granules.
Experimental results show that because all strains were physically retained within the granules, the activity of each strain could be selectively tuned with adjusted feed compositions. Chen et al. (2008) demonstrated very high activities of anaerobic granules that could simultaneously remove sulfide, nitrate, and COD. This investigation also reveals the possibility of simultaneously converting sulfate/nitrate/COD to S0/N2/CO2 using EGSB granules. Such characteristics allow the so-acclimated granules greater tolerance of feed fluctuations than the suspended growth counterparts.
References
Adav SS, Lee DJ (2008) Physiological characterization and interactions of isolates in phenol-degrading aerobic granules. Appl Microbiol Biotechnol 78:899–905
Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Chen C, Ren NQ, Wang AJ, Yu ZG, Lee DJ (2008) Simultaneous biological removal of sulfur, nitrogen and carbon using EGSB reactor. Appl Microbiol Biotechnol 78:1057–1063
Chen MY, Lee DJ, Tay JH, Show KY (2007) Staining of extracellular polymeric substances and cells in bioaggregates. Appl Microbiol Biotechnol 75:467–474
Chuang SH, Pai TY, Horng RY (2005) Biotreatment of sulfate-rich wastewater in an anaerobic/micro-aerobic bioreactor system. Environ Technol 26:993–1001
De Smul A, Geothals L, Verstraete W (1999) Effect of COD to sulphate ratio and temperature in expanded-granular-sludge-blanket reactors for sulphate reduction. Process Biochem 34:407–416
De Smul A, Verstraete W (1999) Retention of sulfate-reducing bacteria in expanded granular-sludge-blanket reactors. Water Environ Res 71:427–431
Dries J, De Smul A, Goethals L, Grootaerd H, Verstraete W (1998) High rate biological treatment of sulfate-rich wastewater in an acetate-fed EGSB reactor. Biodegradation 9:103–111
Hubert C, Voordouw G (2007) Oil field souring control by nitrate-reducing Sulfurospirillum spp. that outcompete sulfate-reducing bacteria for organic electron donors. Appl Environ Microbiol 73:2644–2652
Inagaki F, Takai K, Nealson KH, Horikoshi K (2004) Sulfurovum lithotrophicm gen. nov., sp. nov., a novel sulfur-oxidizing chemolithoauthtroph within the epsilon-Protebacteria isolated from Okinawa Torugh hydrothermal sediments. Int J Syst Evol Microbiol 54:1477–1482
Kim S, Jung H, Kim KS, Kim IS (2004) Treatment of high nitrate-containing wastewaters by sequential heterotrophic and autotrophic denitrification. J Environ Eng ASCE 130:1475–1480
Kodama Y, Ha LT, Watanabe K (2007) Sulfurospirillum cavolei sp nov, a facultatively anaerobic sulfur-reducing bacterium isolated from an underground crude oil storage cavity. Int J Syst Evol Microbiol 57:827–831
Maidak BL, Cole JR, Parker CT, Garrity GM, Larsen N, Li B, Lilburn TG, McCaughey MJ, Olsen GJ, Overbeek R, Pramanik S, Schmidt TM, Tiedje JM, Woese CR (1999) A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res 27:171–173
Reyes-Avila J, Razo-Flores, Gomez J (2004) Simultaneous biological removal of nitrogen, carbon and sulfur by denitrification. Water Res 38:3313–3321
Rozanova EP, Nazina TN, Galushko AS (1988) Isolation of a new genus of sulfate-reducing bacteria and description of a new species of this genus, Desulfomicrobium apsheronum gen. nov., sp. nov. Mikrobiologiya 57:634–641
Vallero MVG, Lens PNL, Bakker C, Lettinga G (2003) Sulfidogenic volatile fatty acid degradation in a baffled reactor. Water Sci Technol 48:81–88
Weijma J, Bots EAA, Tandlinger G, Stams AJM, Pol LWH, Lettinga G (2002) Optilisation of sulphate reduction in a methanol-fed thermophilic bioreactor. Water Res 36:1825–1833
Weijma J, Pol LWH, Stams AJM, Lettinga G (2000) Performance of a thermophilic sulfate and sulfite reducing high rate anaerobic reactor fed with methanol. Biodegradation 11:429–439
Acknowledgement
This work was supported by National Nature Science Foundation of China (No. 50638020) and Ministry of Education of China (Development of simultaneous desulfurization and denitrification process and the control strategy for high strength wastewater, New Century Distinguished Young Scientist Supporting Plan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, C., Ren, N., Wang, A. et al. Microbial community of granules in expanded granular sludge bed reactor for simultaneous biological removal of sulfate, nitrate and lactate. Appl Microbiol Biotechnol 79, 1071–1077 (2008). https://doi.org/10.1007/s00253-008-1503-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1503-5