Abstract
The effect of rapid and slow chilling on survival and nisin sensitivity was investigated in Escherichia coli. Membrane permeabilization induced by cold shock was assessed by uptake of the fluorescent dye 1-N-phenylnapthylamine. Slow chilling (2°C min−1) did not induce transient susceptibility to nisin. Combining rapid chilling (2,000°C min−1) and nisin causes a dose-dependent reduction in the population of cells in both exponential and stationary growth phases. A reduction of 6 log of exponentially growing cells was achieved with rapid chilling in the presence of 100 IU ml−1 nisin. Cells were more sensitive if nisin was present during stress. Nevertheless, addition of nisin to cell suspension after the rapid chilling produced up to 5 log of cell inactivation for exponentially growing cells and 1 log for stationary growing cells. This suggests that the rapid chilling strongly damaged the cell membrane by disrupting the outer membrane barrier, allowing the sensitization of E. coli to nisin post-rapid chilling. Measurements of membrane permeabilization showed a good correlation between the membrane alteration and nisin sensitivity. Application involving the simultaneous treatment with nisin and rapid cold shock could thus be of value in controlling Gram negatives, enhancing microbiological safety and stability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nisin is the best known and studied bacteriocin produced by lactic acid bacteria, and is the only bacteriocin recognized as safe by the World Health Organization in food industry. It exhibits antimicrobial activity toward a wide range of Gram-positive bacteria, in particular against foodborne pathogens in food, such as Listeria monocytogenes, Staphylococcus aureus, and Bacillus cereus (Brewer et al. 2002; Lopez-Pedemonte et al. 2003; Sobrino-Lopez and Martin-Belloso 2006). It shows, however, little or no activity against Gram-negative bacteria, yeasts and molds. Gram-negative bacteria are resistant to nisin because their cell walls are far less permeable than those of Gram-positive bacteria. The outer membrane of Gram-negative bacteria is unique among biological membranes as it is a complex barrier system for many antibiotics, dyes, and detergents, which normally have a deleterious effect on the inner membrane. This resistance can be overcome by treatments that alter the outer membrane, thus render the outer membrane of Gram-negative bacteria permeable (Alakomi et al. 2000), consequently sensitizing cells to nisin. Such treatments include exposure to chelating agents (EDTA; Belfiore et al. 2007), sublethal heat (Modi et al. 2000), or post treatment with high pressure (Helander and Mattila-Sandholm 2000b). Unlike the other physical treatments, chilling-induced nisin sensitivity has not been well studied yet, the effect of chilling shock remained insufficient to produce a useful transient susceptibility to nisin (Boziaris and Adams 2000, 2001). Therefore, by producing a lethal cold shock, we investigated the combined effect of a lethal chilling and a slow chilling with nisin on the inactivation of a Gram-negative bacterium, Escherichia coli TG 1. This bacterium was chosen as representative of foodborne pathogens. The relationship between nisin sensitivity and increase in membrane permeability induced by cold shock was also studied, by measuring the uptake of the fluorescent probe 1-N-phenylnaphthylamine (NPN), one of typical fluorescent dyes widely used to monitor the integrity and/or the permeabilization of the outer membrane (Hancock et al. 1991; Helander and Mattila-Sandholm 2000a; Tsuchido et al. 1989). Indeed, NPN is a hydrophobic probe of which the quantum yield is greatly increased in phospholipid as opposed to aqueous environments, thus enhanced uptake of NPN occurs only when the outer membrane of cells is altered.
Materials and methods
Organism, culture condition, and chemicals
E. coli TG 1 was obtained from the Microbiology Laboratory Culture Collection, ENSBANA, Dijon, France. Cultures were grown in 10 ml of 10% reconstituted skim milk and incubated at 37°C to log (2 × 107 colony-forming units (CFU) ml−1) or stationary phase (2 × 109 CFU ml−1).
Nisin was purchased from Sigma-Aldrich (Sigma Chemical, St. Louis, MO, USA). A stock suspension of 104 IU ml−1 of nisin, corresponding to 400 μg of pure nisin per ml (Papagianni et al. 2006), was prepared by dissolving 0.1 g of a commercial 2.5% nisin powder in 10 ml of 0.02 N HCl solutions (pH = 2). This stock solution was then filter-sterilized with 0.2 μm-pore-size Millipore filters (Nalgene, Rochester, NY, USA) and stored at 4°C.
In experiments where cold shock treatment was combined with nisin, the bacteriocin was added to samples either immediately before or immediately after chilling.
Chilling treatments
Chilling was conducted directly in phosphate-buffered saline (PBS), using the protocol as described previously by Cao-Hoang et al. (2008). Cultures were harvested in log or stationary growth phase and centrifuged (2,800×g for 10 min at 37°C). The pellets were resuspended in PBS to a cell concentration of about 107 CFU ml−1. Two rates of temperature variation were performed: a slow temperature downshift of 2°C min−1, and an instantaneous cold shock of 2,000°C min−1. For the slow chilling, 100 μl of cell suspension was added to bottles containing 9.9 ml of PBS at 37°C, with or without nisin, then they were placed in a cryostat at 37°C (Julabo-F81, SeelBach, Germany). Temperature changes were planned and monitored using a program to obtain a linear and progressive temperature decrease from 37°C to 0°C.
For the instantaneous cold shock (2,000°C min−1), bottles containing 9.9 ml of PBS were precooled to −0.5°C in a cryostat (Julabo-F81, SeelBach, Germany). A 0.1 ml of cell suspensions was then rapidly introduced into the bottles to obtain a final temperature of 0°C. In this case, the cell suspensions reached 0°C nearly instantaneously, from which we estimated a chilling rate of 2,000°C min−1.
The temperature variation was followed using a T-thermocouple (TCSA, Dardilly, France) inserted into an uninoculated tube (blank) that was placed into the cryostat along with the inoculated samples. This thermocouple was connected to an InstruNet 100 acquisition card (GWI, Somerville, USA). Measurements were recorded with this system, which could then be evaluated using a spreadsheet to calculate the chilling rates. When the target temperature of 0°C was obtained, samples were transferred directly to a 37°C water bath for warming at 2°C min−1; this rate did not influence the cell viability.
Treatments with nisin
For cold treatments in the presence of nisin, the stock solution was diluted appropriately in sterile bottles containing PBS at 37°C (for the slow chilling) or at −0.5°C (for the instantaneous chilling) before 0.1 ml of cell suspension was added, yielding final nisin concentrations ranging from 25 to 1,000 IU ml−1 and final volume of 10 ml. This range of concentration was chosen because it is commonly used in the literature (Boziaris and Adams 2001; Liang et al. 2002). When nisin was added after treatments, the nisin solution was added after the temperature stress had been carried out and the suspension was back at 37°C.
Permeability assay based on the uptake of 1-N-phenylnaphthylamine
NPN (Sigma-Aldrich), as a 1 mmol l−1 solution in acetone, was added to 9.9 ml of PBS to give a final concentration of 10 μmol l−1. The fluorescent intensity of the samples was measured at 420 nm, following excitation at 350 nm, using a spectrofluorometer (Fluorolog-3, Jobin Yvon, Inc., USA). Sample temperatures were monitored by a T-thermocouple placed in the sample and controlled using a Peltier system (Wavelength Electronics Inc., Bozeman, USA). The results were expressed as NPN uptake factors, which were calculated as the ratio of background-corrected (subtracted by the value in the absence of NPN) fluorescent values of the bacterial suspension and of the buffer.
Viability determinations
Cell viabilities were determined by the colony-forming units method. Cell suspensions taken after treatment were diluted serially then plated simultaneously onto Luria-broth agar medium (Sigma-Aldrich) and incubated at 37°C for 24 h before counting. Log reductions were calculated as the difference between the logarithmic counts of colonies in untreated and treated samples. Each experiment was repeated at least three times.
Results
The effects of chilling and chilling in combination with nisin on cell inactivation
Nisin added before treatments
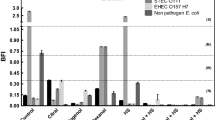
Figure 1a and b shows the effect of nisin and chilling with or without nisin on the survival of E. coli in exponential and stationary growth phases, respectively. Treatment alone with nisin was not effective against E. coli, as the maximal log reduction at highest nisin concentration tested (100 and 1,000 IU ml−1 for exponentially and stationary growing cells, respectively) was 0.12. The slow chilling did not cause any significant reduction in bacterial populations for both exponentially and stationary growing cells. The instantaneous chilling induced a reduction of 1.05 log for E. coli in exponential growth phase (Fig. 1a) and of 0.13 log for cells in stationary growth phase (Fig. 1b). The combination of nisin and the rapid chilling had a more pronounced bactericidal effect than did either nisin or the rapid chilling alone. When nisin was present in the medium during the rapid cold shock, a dose-dependent increase in lethality was observed in most cases. Cells in exponential growth phase were very sensitive to the rapid chilling in the presence of nisin, where there was a 6.05-log reduction in the number of survivors at nisin concentration of 100 IU ml−1 (Fig. 1a). At the same level of concentration of nisin, a reduction of 1.32 log was obtained with stationary growing cells, while it was 2.28 log at 1,000 IU ml−1 nisin (Fig. 1b). However, this synergistic effect of chilling and nisin on cell survival was not observed while combining slow chilling with nisin, regardless of the concentrations of nisin added and the cell growth phase. The maximal reduction obtained was 0.15 log with cells in exponential growth phase and at 100 IU ml−1 nisin (Fig. 1a).
Effect of chilling and chilling in combination with nisin on inactivation of E. coli in a exponential and b stationary growth phases. Gray bars, population reduction after treatment with nisin alone; black bars, population reduction after slow chilling combined with nisin; white bars, population reduction after rapid chilling combined with nisin (error bars represent the standard deviation)
Nisin added after treatments
From the previous results (“Nisin added before treatments”), it appeared that the rapid chilling sensitized E. coli to the action of nisin, but not the slow chilling. In this experiment, nisin was added to cell suspensions immediately after the rapid chilling followed by rewarming to 37°C. At 25 IU ml−1 nisin, a reduction of 3.01 log was observed with cells in exponential growth phase when nisin was added after treatments (Fig. 2a), compared with 3.39 log reduction when present during treatments (Fig. 1a). Increasing nisin concentration resulted also in higher rates of cell inactivation, up to 5.03 log reduction at 100 IU ml−1 with cells in exponential growth phase (Fig. 2a) and 1.24 log reduction at 1,000 IU ml−1 with stationary growing cells (Fig. 2b).
Effect of stress on NPN uptake
NPN was added to unstressed cells and to physically stressed cells before or after the thermal treatments and its fluorescence measured (Table 1). The slow chilling did not cause significant NPN uptake for cells in both exponential and stationary growth phases. Cells that were cold-shocked rapidly showed higher fluorescence intensities than cells that were cold-shocked slowly. The increase in NPN uptake was 57.3 and 49.7 when NPN was present during or after the rapid chilling, respectively, for exponentially growing cells, and 16.8 and 11.4, respectively, for stationary growing cells (Table 1). The NPN uptake factor was far greater for exponentially growing cells than for stationary growing cells, confirming important OM damage of exponentially growing cells. It remained approximately tenfold higher than that of untreated control, even when NPN was added after the instantaneous cold shock (49.7 compared with 5.4).
Discussion
In this work, we have explored the potential of a combined treatment with chilling and nisin against the Gram-negative bacterium E. coli. Numerous work has studied the synergistic action of different treatments and nisin (Liang et al. 2002; Modi et al. 2000; Rodriguez et al. 2005; Ter steeg et al. 1999). However, only several of them contributed to studying the effect of chilling and nisin in combination on cell inactivation. Combining 2,500 IU ml−1 nisin with chilling induced a 0.5-log reduction in E. coli population (Boziaris and Adams 2000). Treatment with 5 μg ml−1 of nisin at 0°C without high pressure resulted in only 0.1 log reduction of E. coli colonies (Ter steeg et al. 1999). These results are comparable to the results obtained in our study when cells were chilled slowly, indicating that slow chilling did not render the cells sensitive to the action of nisin. Indeed, access of nisin to its site of action, the cytoplasmic membrane, is prevented in Gram-negative bacteria by the presence of the outer membrane (Garcera et al. 1993; Jack et al. 1995). It has also been shown that nisin is far less effective against rigid cell membrane due to the reduced temperature (Abee et al. 1994), and the pore formation by nisin should be hindered by the increase in rigidity due to low temperature. In our work, combining the rapid chilling and nisin showed an obvious synergistic effect, achieving significant inactivation for both cells in exponential and stationary growth phases (more than 6 log reductions at 100 IU ml−1 nisin with exponentially growing cells vs. 2 log reductions at 1,000 IU ml−1 with stationary growing cells). This suggests that the rapid chilling causes lethal injury for cells in exponential growth phase, where a 1-log reduction was observed immediately after the treatment, and sublethal injury for stationary growing cells renders cells sensitive to the effect of nisin. The rapid chilling, which is instantaneous, may not permit cells to reorganize the membrane by forming the lateral phase separation of phospholipid and protein domains, which occurs during the slow chilling, consequently resulting in loss of membrane integrity and outer membrane alteration (Cao-Hoang et al. 2008). Once the outer membrane has been permeabilized, nisin may bind the phospholipid head groups, form transient transmembrane pores, and locally immobilize the cytoplasmic membrane (Ter steeg et al. 1999), thus increasing the cell injury during the rapid chilling. The required levels of nisin that synergistically inactivate E. coli during the rapid chilling are also higher for cells in stationary growth phase than those in exponential growth phase. Thus, a more lethal treatment is needed to obtain high inactivation rate of stationary phase cells.
When nisin was added after the rapid chilling, cells were still sensitive with nisin but were less affected than when nisin was present during treatments. These findings are not really similar with those observed by Boziaris and Adams (2001), who reported a transient sensitivity to nisin in cells subjected to rapid chilling, which was canceled as soon as cells were returned to initial temperature. In our study, high inactivation rates (about 4 log for exponentially growing cells at 100 IU ml−1 and 1 log for stationary growing cells at 1,000 IU ml−1) were still obtained when nisin was added to cells submitted to the instantaneous chilling followed by a 3-h incubation at 37°C (results not shown). It points out the lethal effect of the instantaneous chilling on the outer membrane of E. coli, which produced an important loss of outer membrane permeability barrier that does not permit cells to repair, allowing the nisin molecule to gain access even after the stress. This result correlated well with the uptake of NPN during and after the stresses.
This study confirms that the rapid chilling has a critical effect on membrane damage, which has recently been proven in the work of Cao-Hoang et al. (2008), who observed an irreversible rigidification of the rapid cold-shocked cell membrane by using the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene. It illustrates also the efficacy of the rapid cold shock and nisin in combination on inactivation of the Gram-negative bacterium E. coli. Utilization of nisin with such a lethal cold shock in food processing treatments could increase the efficiency of the process, thus it needs to be examined with other Gram-negative bacteria and pathogenic contaminants in food for exploring its application in food processing treatments.
References
Abee T, Rombouts FM, Hugenholtz J, Guihard G, Letellier L (1994) Mode of action of nisin Z against Listeria monocytogenes Scott A grown at high and low temperatures. Appl Environ Microbiol 60:1962–1968
Alakomi HL, Skytta E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM (2000) Lactic acid permeabilizes Gram-negative bacteria by disrupting the outer membrane. Appl Environ Microbiol 66:2001–2005
Belfiore C, Castellano P, Vignolo G (2007) Reduction of Escherichia coli population following treatment with bacteriocins from lactic acid bacteria and chelators. Food Microbiol 24:223–229
Boziaris IS, Adams MR (2000) Transient sensitivity to nisin in cold-shocked Gram negatives. Lett Appl Microbiol 31:233–237
Boziaris IS, Adams MR (2001) Temperature shock, injury and transient sensitivity to nisin in Gram negatives. J Appl Microbiol 91:715–724
Brewer R, Adams MR, Park SF (2002) Enhanced inactivation of Listeria monocytogenes by nisin in the presence of ethanol. Lett Appl Microbiol 34:18–21
Cao-Hoang L, Dumont F, Marechal PA, Le-Thanh M, Gervais P (2008) Rates of chilling to 0 degrees C: implications for the survival of microorganisms and relationship with membrane fluidity modifications. Appl Microbiol Biotechnol 77:1379–1387
Garcera MJ, Elferink MG, Driessen AJ, Konings WN (1993) In vitro pore-forming activity of the lantibiotic nisin. Role of protonmotive force and lipid composition. Eur J Biochem 212:417–422
Hancock RE, Farmer SW, Li ZS, Poole K (1991) Interaction of aminoglycosides with the outer membranes and purified lipopolysaccharide and OmpF porin of Escherichia coli. Antimicrob Agents Chemother 35:1309–1314
Helander IM, Mattila-Sandholm T (2000a) Fluorometric assessment of gram-negative bacterial permeabilization. J Appl Microbiol 88:213–219
Helander IM, Mattila-Sandholm T (2000b) Permeability barrier of the Gram-negative bacterial outer membrane with special reference to nisin. Int J Food Microbiol 60:153–161
Jack RW, Tagg JR, Ray B (1995) Bacteriocins of gram-positive bacteria. Microbiol Rev 59:171–200
Liang Z, Mittal GS, Griffiths MW (2002) Inactivation of Salmonella typhimurium in orange juice containing antimicrobial agents by pulsed electric field. J Food Prot 65:1081–1087
Lopez-Pedemonte TJ, Roig-Sagues AX, Trujillo AJ, Capellas M, Guamis B (2003) Inactivation of spores of Bacillus cereus in cheese by high hydrostatic pressure with the addition of nisin or lysozyme. J Dairy Sci 86:3075–3081
Modi KD, Chikindas ML, Montville TJ (2000) Sensitivity of nisin-resistant Listeria monocytogenes to heat and the synergistic action of heat and nisin. Lett Appl Microbiol 30:249–253
Papagianni M, Avramidis N, Filioussis G, Dasiou D, Ambrosiadis I (2006) Determination of bacteriocin activity with bioassays carried out on solid and liquid substrates: assessing the factor “indicator microorganism”. Microb Cell Fact 5:30
Rodriguez E, Arques JL, Nunez M, Gaya P, Medina M (2005) Combined effect of high-pressure treatments and bacteriocin-producing lactic acid bacteria on inactivation of Escherichia coli O157:H7 in raw-milk cheese. Appl Environ Microbiol 71:3399–3404
Sobrino-Lopez A, Martin-Belloso O (2006) Enhancing inactivation of Staphylococcus aureus in skim milk by combining high-intensity pulsed electric fields and nisin. J Food Prot 69:345–353
Ter steeg PF, Hellemons JC, Kok AE (1999) Synergistic actions of nisin, sublethal ultrahigh pressure, and reduced temperature on bacteria and yeast. Appl Environ Microbiol 65:4148–4154
Tsuchido T, Aoki I, Takano M (1989) Interaction of the fluorescent dye 1-N-phenylnaphthylamine with Escherichia coli cells during heat stress and recovery from heat stress. J Gen Microbiol 135:1941–1947
Acknowledgments
This work was supported by the Agence Universitaire de la Francophonie (AUF), the Vietnamese and French Ministries of Education and Training, and the Bourgogne region.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao-Hoang, L., Marechal, P.A., Le-Thanh, M. et al. Synergistic action of rapid chilling and nisin on the inactivation of Escherichia coli . Appl Microbiol Biotechnol 79, 105–109 (2008). https://doi.org/10.1007/s00253-008-1402-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1402-9