Abstract
The phosphoenolpyruvate: carbohydrate phosphotransferase system (PTS) catalyzes transport of carbohydrates by coupling carbohydrate translocation and phosphorylation. Enzyme I and HPr, encoded in ptsI and ptsH, respectively, are cytoplasmic proteins commonly used for transport of variety of PTS sugars. In this study, we investigated the role of SugR on the expression of the ptsI and ptsH which increases in the presence of PTS sugars in Corynebacterium glutamicum. Disruption of sugR resulted in the increased expression of ptsI and ptsH in the absence of PTS sugar. Introduction of a plasmid containing sugR gene complemented the effect of sugR disruption. SugR was purified and binding to the promoter regions of ptsI and ptsH was indicated by EMSA. DNase I footprinting analysis indicated the binding sites of SugR on the promoter region of divergently transcribed ptsI gene and fructose-pts operon. The binding sites contain a possible SugR binding motif which is conserved in the promoter regions of general and sugar-specific pts genes. Mutations in this motif resulted in the decrease of SugR binding to the ptsI promoter. These results suggest that SugR represses ptsI and ptsH in the absence of PTS sugar and derepression is the mechanism for the induction of the general components of PTS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many bacteria, the phosphoenolpyruvate (PEP): carbohydrate phosphotransferase system (PTS) catalyzes transport of carbohydrates by coupling carbohydrate translocation and phosphorylation (Postma et al. 1993; Kotrba et al. 2001a). The PTS consists of two cytoplasmic proteins, enzyme I (EI) and HPr, and membrane-bound sugar specific enzyme II complexes (EIIs). The phosphoryl group from PEP is sequentially transferred to EI, HPr, EIIs, and finally to the substrate as it is translocated across the membrane. In most cases, EI and HPr participate in the phosphorylation of all PTS carbohydrates and have been called the general PTS proteins (Postma et al. 1993). In addition to carbohydrate uptake, the PTS regulates the expression of many catabolic genes depending on its phosphorylation state (Postma et al. 1993; Kotrba et al. 2001a).
It is known that expression of general PTS proteins is increased in the presence of PTS sugar especially by glucose (Postma et al. 1993). The mechanism for the induction has been extensively studied in Escherichia coli and in Bacillus subtilis. In E. coli, ptsH, and ptsI are cotranscribed with crr, which encodes for the EIIA component of the glucose-EII. Global regulator Mlc is responsible for the glucose induction of the pts operon (Kim et al. 1999; Plumbridge, 1999; Tanaka et al. 1999). In the absence of glucose, Mlc represses the transcription of the pts operon. In the presence of glucose, transport of glucose results in the dephosphorylation of EIICBGlc and this dephosphorylated EIICBGlc interacts and inhibits the activity of Mlc, resulting in the induction of the pts operon (Lee et al. 2000; Nam et al. 2001; Tanaka et al. 2000). In B. subtilis, the ptsGHI operon expression is controlled by the GlcT antiterminator (Stülke et al. 1997). GlcT is active in its dephosphorylated form which is generated by the transport of glucose. Activated GlcT inhibits the rho-independent terminator preceding the ptsGHI operon and transcription proceeds to the 3′ end of ptsGHI.
Corynebacterium glutamicum is a high-GC Gram-positive bacterium which is widely used for the industrial production of amino acids (Kinoshita et al. 1957; Ikeda 2003; Kelle et al. 2005). We are investigating C. glutamicum R strain, which can provide high yields of lactate and succinate from sugar (Inui et al. 2004; Okino et al. 2005). To improve the production of organic acids, understanding the regulatory systems of the sugar transport and metabolism is important. Hence, we are investigating the regulation of PTS expression for efficient production of useful compounds.
In C. glutamicum, there are general components of PTS encoded in ptsI and ptsH, and fructose-, glucose-, and sucrose-specific EIIs which are encoded in ptsF, ptsG, and ptsS, respectively (Mori and Shiio, 1987; Dominguez and Lindley, 1996; Parche et al. 2001; Moon et al. 2005; Yukawa et al. 2007). Recently, we found that ptsH is cotranscribed with fructose-pts, although major transcripts are generated from its own promoter in C. glutamicum R (Tanaka et al. 2008). The gene encoding ptsI is divergently transcribed from the fructose-pts operon (Fig. 1a). Expression of these general components of PTS increases in the presence of glucose, fructose and sucrose. It is interesting to note that fructose is the most effective-inducing sugar for general components of PTS, which is different from the cases in other bacteria (Tanaka et al. 2008).
Effect of sugR disruption on the expression of pts genes. a Genetic organization of the genes around the fructose-pts operon. Open arrows represent the coding regions. Deduced functions of each gene are indicated below. The promoters are indicated by arrows. b Real-time RT-PCR analysis of pts expression. Total RNAs prepared from wild-type (WT) or sugR disrupted cells grown in BT minimal medium supplemented with casamino acids and 1.0% (w/v) acetate with or without 1.0% (w/v) PTS sugars, were subjected to real-time RT-PCR analysis using primers specific for the ptsI, ptsH, ptsF, ptsG, and ptsS. The ratio against wild type cells grown in the presence of acetate is indicated. The values are the means of three independent experiments and standard deviations are indicated on the bar tops. A Acetate, G glucose, and F fructose
There are two DeoR-type transcriptional regulators which are located close to ptsI and ptsH. We recently found that one of the DeoR-type regulators, FruR, is involved in the regulation of the expression of ptsI and ptsH as well as ptsF. FruR reduces the induction level of these pts genes in the presence of fructose. However, FruR is not responsible for the induction of general component of PTS by PTS sugar, since the induction of pts genes by PTS sugar was still observed in the fruR disrupted cells. Recently, it was reported that SugR, the other DeoR-type regulator, regulates the expression of ptsG, ptsF, and ptsS (Engels and Wendisch, 2007). We also found that SugR, in addition to FruR, bound to the ptsI-fruR intergenic region containing the promoters of the ptsI and fructose-pts operon (Tanaka et al. 2008). However, it was not reported whether expression of general PTS was regulated by SugR. In this study, we examined the involvement of SugR in the regulation of expression of ptsH and ptsI. We also investigated SugR binding on the ptsI-fruR promoter region.
Materials and methods
Media and growth conditions
C. glutamicum R (Yukawa et al. 2007) was grown aerobically at 33°C in BT minimal medium with casamino acids (Kotrba et al. 2001b) supplemented with 1% (w/v) acetate and indicated sugars added at 1% (w/v) as carbon sources. Antibiotics were added at the following final concentrations: kanamycin 50 μg ml-1 and chloramphenicol 5 μg ml−1. Bacterial growth was monitored by determining the optical density at 610 nm.
Bacterial strains and plasmids
C. glutamicum R (Yukawa et al. 2007) was used as a wild-type strain. The sugR gene disrupted strain was constructed by the transposon-mediated mutagenesis method as described previously (Suzuki et al. 2006). sugR gene disruption was confirmed by DNA sequencing. Transposon was inserted at 34 bases downstream from the 5′ end of the sugR ORF. Construction of recombinant plasmid pCRC803 containing sugR gene was carried out as follows. sugR gene was amplified from C. glutamicum R genomic DNA by PCR using primers EcoRI-sugR-23F and SphI-sugR-863R (Table 1). The amplified DNA fragment was digested with EcoRI and SphI, and cloned into the corresponding site on pCRB1 (Nakata et al. 2003) to construct pCRC803. Construction of SugR overexpression plasmid pCRC805 was carried out as follows. sugR gene was amplified from C. glutamicum R genomic DNA by PCR using primers NdeI-sugR-1F and HindIII-sugR-863R (Table 1). The amplified DNA fragment was digested with NdeI and HindIII, and cloned into the corresponding site on pColdI (Takara) to construct pCRC805.
Real-time reverse transcriptase-polymerase chain reaction
Total RNA was isolated from exponentially growing cells (OD610 of 1.2) in BT medium supplemented with 1.0% (w/v) carbon source using the RNeasy Kit (QIAGEN). Total RNA of 20 ng was used as template for analysis of the pts genes and 0.4 ng was used for analysis of the 16S rRNA to generate cDNA and for the subsequent PCR reaction. Each real-time reverse transcriptase-polymerase chain reaction (RT-PCR) mixture (20 μl) contained 500 nM of each primer, 10 μl of Power SYBR Green PCR Master Mix, 8 U of RNase Inhibitor and 5 U of MuLV reverse transcriptase (Applied Biosystems). The primers used in these reactions are listed in Table 1. Reactions were performed using the ABI 7500 Fast Real-Time PCR System (Applied Biosystems) with the following cycle parameters: 1 cycle of 50°C for 30 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. The result of 16S rRNA was used as an internal control.
Purification of SugR
Overexpression of His6-SugR was achieved by using a Cold-shock expressing vector pCold I (Takara). E. coli cells harboring pCRC805 were grown at 37°C in 100 ml of LB medium to OD610 of 0.5. Cultures were incubated 30 min at 15°C and then IPTG was added at a final concentration of 0.1 mM and shaken for 24 h at 15°C. Cells were harvested by centrifugation and suspended in 900 μl of His binding buffer (0.5 M NaCl, 20 mM Tris–HCl, 5 mM imidazole pH 7.9) (Novagen), 100 μl of Fast Break Cell Lysis Reagent (Promega), and 0.2 mg of lysozyme. The mixture was incubated for 15 min at room temperature and centrifuged for 5 min at 12,000 ×g and the supernatant was pooled. His6-SugR was purified using His Bind Resin and Buffer Kit (Novagen) according to the procedure specified by the manufacturer.
Electrophoretic mobility-shift assay
Electrophoretic mobility-shift assay (EMSA) was carried out in a total volume of 20 μl of binding buffer (20 mM Tris–HCl, pH 7.9, 100 mM NaCl, 3 mM MgCl2, 0.1 mM EDTA, 0.1 mM DTT, 5% (w/v) glycerol, and 50 μg/ml bovine serum albumin). The ptsI-fruR promote, ptsH promoter and ptsI ORF DNA fragments were amplified by PCR using primers listed in Table 1. The DNA fragments containing nucleotide substitutions at putative SugR binding sites were prepared as follows. First, ptsI promoter DNA fragments listed in Table 1 (ptsIwtF and ptsIwtR, ptsImut1F and ptsImut1R, ptsImut2F and ptsImut2R, and ptsImut3F and ptsImut3R) were annealed and digested by EcoRI and HindIII and cloned into the corresponding site of pCRA1. The DNA fragments containing ptsI promoter region were amplified by PCR using LSVRI and LSVH3 (Table 1).
Two nanomolar of DNA fragments were incubated with the indicated concentration of the purified SugR for 10 min at 33°C. The mixture was fractionated by electrophoresis on a native 5% (w/v) polyacrylamide gel containing 5% (w/v) glycerol in 1/2 TBE (45 mM Tris-borate, pH 8.3, 1 mM EDTA) at room temperature. The DNA bands were stained with ethidium bromide and visualized by UV irradiation.
DNase I footprinting
The ptsI-fruR promoter DNA fragment was amplified by using primer sets listed in Table 1 (fruR and ptsI* and ptsI-P and fruR*). IRD-700 Labeled DNA fragments (2 nM) were mixed with purified SugR in a total volume of 100 μl of a binding buffer (20 mM Tris–HCl, pH 7.9, 100 mM NaCl, 3 mM MgCl2, 0.1 mM EDTA, 0.1 mM DTT, 5% (w/v) glycerol, and 50 μg/ml bovine serum albumin) containing 5 mM CaCl2. The mixtures were incubated for 5 min at 33°C and then placed at 25°C for 5 min. DNase I (Takara) was added at 0.025 U and incubation was continued for 1 min at 25°C. The mixture was treated with phenol and precipitated with ethanol and loaded onto a 5.5% (w/v) polyacrylamide sequence gel next to the sequencing ladder and run in the LI-COR DNA sequencer model 4000 (Aloka).
Results
Effect of sugR disruption on the expression of the general components of PTS
The gene encoding the DeoR-type transcriptional regulator SugR is present in the vicinity of the ptsI gene (Fig. 1a). We hypothesized that SugR is involved in the induction of general pts genes in the presence of PTS sugar. Accordingly, we analyzed the effect of sugR (cgR1761) disruption on the expression of general components of the PTS in the presence or absence of PTS sugars. C. glutamicum R wild-type cells or sugR disrupted cells were grown in minimal medium supplemented with acetate in the presence of or absence of glucose or fructose. Total RNAs were prepared and analyzed by real-time RT-PCR using primers specific for ptsI and ptsH. As we noted previously (Tanaka et al. 2008), the level of ptsI mRNA increased in the presence of PTS sugar in wild type cell (Fig. 1b). Fructose was a more effective inducer than glucose. Then, we examined the ptsI mRNA level in sugR disrupted cells. The levels of ptsI mRNA was higher in sugR disrupted cells than wild type cells in the absence of PTS sugar. Addition of glucose to the culture of sugR disrupted cells slightly reduced the induction of ptsI, but there was still higher expression than in wild type cells grown in the presence of glucose. Additions of fructose further reduced the expression level of ptsI in sugR cells to almost the same level of induction in wild type cells grown in the presence of fructose. Similar results were observed for expression of ptsH, although the effects of PTS sugar and sugR disruption were smaller than those on ptsI expression. In addition, we also analyzed expression of ptsF and ptsG encoding EIIs specific for fructose and glucose, respectively. Similar to ptsI and ptsH, the expressions of ptsF and ptsG showed high levels of expressions in the sugR disrupted cells in the absence of PTS sugar, and the addition of PTS sugar reduced the expressions of ptsF and ptsG.
To confirm that the effect of sugR disruption is caused by inactivation of SugR and not by other gene products, we introduced the plasmid pCRC803, which had the sugR gene, to the sugR disrupted cells and investigated pts gene expression (Fig. 2). The level of ptsI mRNA was higher in the sugR disrupted cells having vector plasmid pCRB1 than wild type cells containing the same plasmid in the absence of fructose, as expected from the results in Fig. 1. In the sugR cells containing pCRC803, expression of ptsI was decreased more than with the sugR cells having vector plasmid in the absence of fructose, and addition of fructose increased the expression to a pattern similar to the wild type cells containing the vector plasmid (Fig. 2). These results indicated that the sugR disruption effect was complemented. Similar results were observed for the expression of ptsH for general PTS, and sugar-specific pts genes, ptsF, and ptsG. We conclude that SugR inhibits the expression of ptsI and ptsH in the absence of PTS sugar.
Complementation of sugR disruption. Total RNAs, prepared from wild type (WT) or sugR disrupted cells with either pCRB1 or pCRA803 grown in BT minimal medium supplemented with casaminoacids and 1.0% (w/v) acetate with or without 1.0% (w/v) fructose, were subjected to real-time RT-PCR analysis using primers specific for the ptsI, ptsH, ptsF, and ptsG. The values are the means of three independent experiments and standard deviations are indicated on the bar tops
Binding of SugR to the promoter regions of the ptsH and ptsI promoter
In C. glutamicum, ptsI is located adjacent to the fruR-ptsF-pfkB-ptsH operon, and is transcribed divergently. Between ptsI and fruR, there are two promoters for ptsI (ptsI-P1 and ptsI-P2) and two promoters for fruR (fruR-P1 and fruR-P2) (Tanaka et al. 2008). ptsH has its own promoter and the majority of the ptsH transcripts are produced from the ptsH promoter (Tanaka et al. 2008). Previously, we observed the binding of SugR to fruR-ptsI promoter DNA by DNA affinity purification analysis (Tanaka et al. 2008). To test whether SugR binds to ptsI and ptsH promoter region, we conducted EMSA using ptsI or ptsH promoter DNA. Hexa-histidine-tagged SugR was overexpressed and purified from E. coli. Then, SugR was mixed with ptsI DNA promoter fragments covering the ptsI-fruR promoter region and the ptsH promoter fragment. We also incubated SugR with the coding region of ptsI which is assumed to not have a SugR binding site. Using ptsI-fruR promoter fragment, addition of SugR resulted in the appearance of three mobility retarded bands, two of which appeared at 7.5-fold molar excess SugR, and slowest mobility band appeared at 15-fold molar excess of SugR (Fig. 3, lanes 1–4).
Electro mobility shift assay of binding of SugR to the ptsI-fruR promoter and ptsH promoter. The 432 bp fragment (2 nM) containing ptsI-fruR promoter region, 462 bp fragment (2 nM) containing ptsH promoter, and 415 bp fragment (2 nM) containing ptsI ORF were incubated with indicated amount of SugR. The primers used to generate each DNA fragment are listed in Table 1
By using the ptsH promoter fragment, we observed two shifted bands, one band appeared at 7.5-fold molar excess of SugR and another band appeared at 15-fold molar excess of SugR (Fig. 3, lanes 5–8). DNA fragment of the ptsI coding region showed no shifted bands (Fig. 3, lanes 9–12). We conclude that SugR binds to the ptsI-fruR promoter region and the ptsH promoter region, which suggest that the effect of SugR on the expression of ptsI and ptsH is a direct one. Engels and Wendisch (2007) also showed that purified SugR binds to the fruR-ptsI promoter region by EMSA, which corresponds to our ptsI results. The result of mobility shift assay also suggests that each promoter region contains multiple SugR binding sites.
Determination of the SugR binding sites on ptsI-fruR promoter region
The result of EMSA suggested that several SugR binding sites are present in ptsI-fruR promoter region. To determine the binding sites of SugR, we conducted DNase I footprint analysis on ptsI-fruR promoter region. There are two promoters for ptsI. The initiation site is located at 103 nucleotide and 105 nucleotide upstream of the GTG start codon (ptsI-P1 promoter), and 37 nucleotide upstream of the start codon (ptsI-P2 promoter) (Tanaka et al. 2008). There are also two promoters for fruR. The initiation site is located 294 nucleotide upstream of the ATG start codon (fruR-P1 promoter), and 123 and 126 nucleotide upstream of the ATG start codon of fruR (fruR-P2 promoter). −10 regions of ptsI-P1 and fruR-P1 are overlapping (Tanaka et al. 2008).
The ptsI-fruR promoter DNA fragment was fluorescently labeled at either the ptsI proximal end or the fruR proximal end. These end-labeled DNA fragments were incubated with SugR and challenged by DNase I digestion. Two regions were protected from the nuclease attack by DNase I. One protected region located from ptsI-P2 promoter to ptsI-P1 (fruR-P1) promoter. Another protected region was located downstream of fruR-P2 promoter (Fig. 4). The DNA sequence of ptsI-fruR promoter regions is shown in Fig. 5a. The protected region proximal to ptsI covers the −35 region of ptsI-P2, the −10 and the transcription initiation site of ptsI-P1 and the −10 region of fruR-P1. The length of protected region proximal to ptsI is 67 bp, while the fruR proximal region is 38 bp long. This and the result of EMSA using fruR-ptsI DNA fragment suggest that the DNA region has three SugR binding sites, two of which are located in the protected region proximal to ptsI, and one of which is located downstream of fruR-P2. The putative binding sites have repeats of TG (T) 2–5 G (Fig. 5b) which we previously pointed out is present in all the promoter regions of PTS-sugar inducible pts genes, i.e., ptsH, ptsI, ptsG, ptsF (fruR-ptsF operon), and ptsS (Tanaka et al. 2008). The protected region proximal to ptsI also contains the GTTGCACA sequence which was found to be a motif partly conserved in the SugR binding upstream of ptsG, ptsS, and fruR (Engels and Wendisch 2007). We additionally found that the GTTGCACA motif is also present in the ptsH promoter (Fig. 5b). However, there seems to be no such sequence at the protected region downstream of the fruR-P2 promoter (Fig. 5a).
DNase I footprinting analysis of the SugR binding to the ptsI-fruR promoter. The 487 bp ptsI-fruR promoter fragment fluorescently labeled at ptsI terminal or at fruR terminal was incubated with SugR (0, 20, 40, 80, and 160 nM). The mixture was subjected to a partial digestion by DNase I. The products were loaded next to the sequencing product of ptsI-fruR. The protected regions by SugR are shown by vertical lines. Transcriptional start sites of fruR and ptsI are shown by arrows
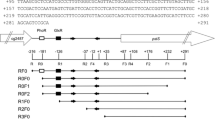
SugR binding regions of pts promoters. a The nucleotide sequence between divergently transcribed ptsI and fruR. Yellow box represents the regions protected by SugR from DNase I digestion. Promoters of ptsI and fruR are depicted as arrows. Possible SugR binding motif (GTTGCACA) suggested by Engels and Wendisch (2007) is underlined by green color. The motif previously found in promoters of pts genes, TG (T) 2–5 G (Tanaka et al. 2008), is underlined by red color. The −10 and the −35 hexamers are underlined by black color. b Comparison of the promoter regions of pts genes. Yellow box represents the regions protected by SugR from DNase I digestion. Possible SugR binding motif suggested by Engels and Wendisch (2007) are boxed by green. TG (T) 2–5 G motifs are boxed by red. Numbers from the transcription initiation sites from ptsI-P1, fruR-P2, ptsG, ptsH-P1, and ptsS are indicated
Effect of nucleotide change at ptsI promoter on SugR binding
The result of DNaseI footprinting suggests that SugR recognizes either or both TG (T) 2–5 G and GTTGCACA sequences. To determine the role of these sequences on SugR binding, we constructed various mutated ptsI promoter DNA fragments that changed the TG (T) 2–5 G or GTTGCACA to random sequences (Fig. 6a). The DNA fragment that covers the −35 region of ptsI-P2 promoter was prepared. Mut1 changed the TG (T) 2–5 G motif, Mut2 changed the GTTGCACA motif and Mut3 changed both motifs. SugR bound to wild type ptsI promoter fragment as expected (Fig. 6b, lanes 1–3). Mutation of the TG (T) 2–5 G motif reduced the binding of SugR to this fragment (Fig. 6b, lanes 4–6). Mutation of the GTTGCACA motif also modestly reduced the SugR binding (Fig. 6b, lanes 7–9). The double mutation (Mut3) showed the most inhibitory effect on SugR binding (Fig. 6b, lanes 10–12). These results suggest that both GTTGCACA and TG (T) 2–5 G sequences are recognized by SugR, and the presence of both motifs enhances the SugR binding to target DNA.
EMSA of binding of SugR to the mutated pts promoter. a DNA fragments used in this study. The ptsI promoter regions are shown. Either or both putative SugR binding motifs (GTTGCACA and TG (T) 2–5 G) were substituted randomly. Putative SugR binding motifs are boxed. Only changed nucleotide sequences are depicted for mutated fragments. b EMSA of SugR binding to ptsI promoter. The ptsI-P2 promoter DNA fragments depicted above (2 nM) were incubated with indicated amount of SugR
Discussion
In our previous paper (Tanaka et al. 2008), we reported that the expression of general components of the PTS (ptsI and ptsH) as well as sugar-specific PTS components (ptsF, ptsG, and ptsS) was increased in the presence of PTS sugar in C. glutamicum. In this study, we revealed that disruption of sugR, which was reported to repress the expression of ptsG, F, and S (Engels and Wendisch 2007) induced the expression of ptsH and ptsI in the absence of PTS sugar. Introduction of sugR in trans resulted in the repression of ptsH and ptsI expression in the absence of PTS sugar, supporting the notion that SugR represses the ptsH and ptsI promoter in the absence of PTS sugar. Therefore, expressions of both cytoplasmic components and transporter complexes of PTS seem to be controlled globally by SugR.
Engels and Wendisch (2007) reported that SugR binds to the ptsG promoter region, and this binding is inhibited by fructose-6-phosphate. They suggested that expression of ptsG is repressed by SugR in the absence of PTS sugar and inhibition of SugR activity by fructose-6-phosphate results in the induction of ptsG. The regulation of general PTS expression may be also controlled by such a mechanism. However, we observed that expressions of ptsH, ptsI, ptsF, and ptsS are more induced in the presence of fructose than in the presence of glucose. Since it was reported that intracellular concentrations of fructose-6-phosphate during growth on glucose is higher than during growth on fructose (Georgi et al. 2005), it is difficult to explain the expression pattern of pts genes simply by the action of SugR and fructose-6-phosphate.
It is interesting to note that addition of PTS sugar reduced the expressions of ptsH, ptsI, ptsG, and ptsF genes in sugR disrupted cells. These results suggest the existence of another transcriptional regulator(s) that decreases pts gene expression in the presence of PTS sugar. One candidate is FruR which is located upstream of ptsF gene (Fig. 1a). Previously, we have demonstrated that FruR reduces the induction of ptsF-operon, ptsI, and ptsH induction in the presence of fructose (Tanaka et al. 2008). However, other regulator(s) should also exist, since FruR does not regulate ptsG expression. Expression of C. glutamicum pts genes may be controlled in a complicated manner, as in the case of other bacteria. In E. coli, expression of ptsG is negatively regulated by Mlc and positively regulated by CRP-cAMP. In the presence of glucose, activity of Mlc is inhibited and results in the induction of the ptsG, while the activity of CRP-cAMP is reduced. Hence, full induction of ptsG is not observed in wild-type cell grown in glucose. The transcriptional regulators and mechanisms involved in the regulation of C. glutamicum PTS have many differences from that of other bacteria. Further investigation is required to clear the mechanism of the regulation of pts gene expression.
By EMSA and DNase I footprinting analysis, we investigated SugR binding to the ptsI promoter region. Between ptsI and fruR, there are two promoters for ptsI (ptsI-P1 and ptsI-P2) and two promoters for fruR (fruR-P1 and fruR-P2) (Fig. 5a). The −10 regions of ptsI-P1 and fruR-P1 are overlapping. There are several TG (T) 2–5 G sequences which are found in the promoters of pts genes upregulated in the presence of PTS sugar (Fig. 5b). Two regions were protected from the nuclease attack by DNase I. One protected region was located between the ptsI-P2 promoter to the ptsI-P1 fruR-P1 promoter. Another protected region was located downstream of the fruR-P2 promoter (Fig. 5a). We found that the TG (T) 2–5 G sequence is present in these regions. Engels and Wendisch examined the SugR binding site by EMSA using several deletion sets of the ptsG promoter. They suggested that the GTCGGACA or GTTGCACA motif is present at the upstream regions of ptsG, ptsS, and the fruR-ptsF operon. We found that this motif is also present at the ptsH promoter region, and showed the binding of SugR to the region by EMSA. However, the GTCGGACA or GTTGCACA sequence is not present in the fruR-P2 promoter region that was protected by SugR against DNase I attack, although GTTGCACA is present at the pts-P1 region that was protected by SugR. These results suggest that the TG (T) 2–5 G sequence in addition to GTTGCACA is involved in SugR binding to DNA. We tested this hypothesis by examining the effect of mutation at these putative SugR binding motifs. We found that both sequences contribute to the binding of SugR to target DNA. These motifs are present in all the pts upstream regions. However, the locations of these motifs are different for each pts promoter. Especially, there is no GTCGGACA or GTTGCACA in the fruR-P2 promoter region. It is interesting how these differences affect the action of SugR.
In summary, we demonstrated that SugR controls ptsH and ptsI expression that means SugR regulates expressions of both general cytoplasmic components and transporter complexes of PTS. We also found that the TG (T) 2–5 G sequence was involved in the SugR binding in addition to the GTTGCACA sequence.
During the submission of this manuscript, Gaigalat et al (2007) also reported that SugR regulates the expression of ptsH and ptsI. They showed that sugR gene disruption resulted in the increased expression of ptsH and ptsI mRNA in the absence of PTS sugar. They determined a 21 bp sequence in the ptsI-fruR promoter region as the SugR binding site containing the TG (T) 2–5 G motif determined in this study. They also showed that fructose-1-phosphate, fructose-1,6-bis-phosphate and glucose-6-phosphate can act as negative effectors of the SugR repressor. The highly efficient effector, fructose 1-phosphate, which is generated during the transport of fructose into the cell by fructose-PTS, may cause higher expression of pts genes in cells grown on fructose than in those grown on glucose.
References
Dominguez H, Lindley ND (1996) Complete sucrose metabolism requires fructose phosphotransferase activity in Corynebacterium glutamicum to ensure phosphorylation of liberated fructose. Appl Environ Microbiol 62:3878–3880
Engels V, Wendisch VF (2007) The DeoR-type regulator SugR represses expression of ptsG in Corynebacterium glutamicum. J Bacteriol 189:2955–2966
Gaigalat L, Schlüter JP, Hartmann M, Mormann S, Tauch A, Pühler A, Kalinowski J (2007) The DeoR-type transcriptional regulator SugR acts as a repressor for genes encoding the phosphoenolpyruvate: sugar phosphotransferase system (PTS) in Corynebacterium glutamicum. BMC Mol Biol 8:104
Georgi T, Rittmann D, Wendisch VF (2005) Lysine and glutamate production by Corynebacterium glutamicum on glucose, fructose and sucrose: roles of malic enzyme and fructose-1,6-bisphosphatase. Metab Eng 7:291–301
Ikeda M (2003) Amino acid production processes. Adv Biochem Eng Biotechnol 79:1–35
Inui M, Murakami S, Okino S, Kawaguchi H, Vertès AA, Yukawa H (2004) Metabolic analysis of Corynebacterium glutamicum during lactate and succinate productions under oxygen deprivation conditions. J Mol Microbiol Biotechnol 7:182–196
Kelle R, Hermann T, Bathe B (2005) L-Lysine production. In: Eggeling L, Bott M (eds) Handbook of Corynebacterium glutamicum. CRC Press, Boca Raton, Fla, pp 465–488
Kim SY, Nam TW, Shin D, Koo BM, Seok YJ, Ryu S (1999) Purification of Mlc and analysis of its effects on the pts expression in Escherichia coli. J Biol Chem 274:25398–25402
Kinoshita S, Udaka S, Shimono M (1957) Studies on the amino acid fermentation. I. Production of l-glutamic acid by various microorganisms. J Gen Appl Microbiol 3:193–205
Kotrba P, Inui M, Yukawa H (2001a) Bacterial phosphotransferase system (PTS) in carbohydrate uptake and control of carbon metabolism. J Biosci Bioeng 92:502–517
Kotrba P, Inui M, Yukawa H (2001b) The ptsI gene encoding enzyme I of the phosphotransferase system of Corynebacterium glutamicum. Biochem Biophys Res Commun 289:1307–1313
Lee SJ, Boos W, Bouche JP, Plumbridge J (2000) Signal transduction between a membrane-bound transporter, PtsG, and a soluble transcription factor, Mlc, of Escherichia coli. EMBO J 19:5353–5361
Moon MW, Kim HJ, Oh TK, Shin CS, Lee JS, Kim SJ, Lee JK (2005) Analyses of enzyme II gene mutants for sugar transport and heterologous expression of fructokinase gene in Corynebacterium glutamicum ATCC 13032. FEMS Microbiol Lett 244:259–266
Mori M, Shiio I (1987) Phosphoenolpyruvate, sugar phosphotransferase systems and sugar metabolism in Brevibacterium flavum. Agric. Biol. Chem. 51:2671–2678
Nakata K, Inui M, Kos PB, Vertès AA, Yukawa H (2003) Vectors for the genetics engineering of corynebacteria. In: Saha BC (ed) Symposium Series No 855: Fermentation Biotechnology. American Chemical Society, Washington, pp 145–154
Nam TW, Cho SH, Shin D, Kim JH, Jeong JY, Lee JH, Roe JH, Peterkofsky A, Kang SO, Ryu S, Seok YJ (2001) The Escherichia coli glucose transporter enzyme IICB (Glc) recruits the global repressor Mlc. EMBO J 1:491–498
Okino S, Inui M, Yukawa H (2005) Production of organic acids by Corynebacterium glutamicum under oxygen deprivation. Appl Microbiol Biotechnol 68:475–480
Parche S, Burkovski A, Sprenger GA, Weil B, Krämer R, Titgemeyer F (2001) Corynebacterium glutamicum, a dissection of the PTS. J Mol Microbiol Biotechnol 3:423–428
Plumbridge J (1999) Expression of the phosphotransferase system both mediates and is mediated by Mlc regulation in Escherichia coli. Mol Microbiol 33:260–273
Postma PW, Lengeler JW, Jacobson GR (1993) Phosphoenolpyruvate, carbohydrate phosphotransferase systems of bacteria. Microbiol Rev 57:543–594
Stülke J, Martin-Verstraete I, Zagorec M, Rose M, Klier A, Rapoport G (1997) Induction of the Bacillus subtilis ptsGHI operon by glucose is controlled by a novel antiterminator, GlcT. Mol Microbiol 25:65–78
Suzuki N, Okai N, Nonaka H, Tsuge Y, Inui M, Yukawa H (2006) High-throughput transposon mutagenesis of Corynebacterium glutamicum and construction of a single-gene disruptant mutant library. Appl Environ Microbiol 72:3750–3755
Tanaka Y, Kimata K, Inada T, Tagami H, Aiba H (1999) Negative regulation of the pts operon by Mlc, mechanism underlying glucose induction in Escherichia coli. Genes Cells 4:391–399
Tanaka Y, Kimata K, Aiba H (2000) A novel regulatory role of glucose transporter of Escherichia coli: membrane sequestration of a global repressor Mlc. EMBO J. 16:5344–5352
Tanaka Y, Okai N, Teramoto H, Inui M, Yukawa H (2008) Regulation of the expression of phosphoenolpyruvate, carbohydrate phosphotransferase system (PTS) genes in Corynebacterium glutamicum R. Microbiology 154:264–274
Yukawa H, Omumasaba CA, Nonaka H, Kos P, Okai N, Suzuki N, Suda M, Tsuge Y, Watanabe J, Ikeda Y, Vertès A, Inui M (2007) Comparative analysis of the Corynebacterium glutamicum group and complete genome sequence of strain R. Microbiology 153:1042–1058
Acknowledgements
We thank Roy H. Doi (University of California at Davis) for his critical reading of the manuscript. This work was supported by a grant from the New Energy and Technology Development Organization (NEDO).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, Y., Teramoto, H., Inui, M. et al. Regulation of expression of general components of the phosphoenolpyruvate: carbohydrate phosphotransferase system (PTS) by the global regulator SugR in Corynebacterium glutamicum . Appl Microbiol Biotechnol 78, 309–318 (2008). https://doi.org/10.1007/s00253-007-1313-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1313-1