Abstract
To investigate the effect of human pyruvate carboxylase (hPC) on lactate formation in Chinese hamster ovary (CHO) cell lines, FLAG-tagged hPC was introduced into a dihydrofolate-deficient CHO cell line (DG44). Three clones expressing high levels of hPC, determined by Western blotting using an anti-FLAG monoclonal antibody, and a control cell line were established. Immunocytochemistry revealed that a substantial amount of expressed hPC protein was localized in the mitochondria of the cells. hPC expression did not impair cell proliferation. Rather, it improved cell viability at the end of adherent batch cultures with the serum-containing medium probably because of reduced lactate formation. Compared with control cells, specific lactate production rate of the three clones was decreased by 21–39%, which was because of a decreased specific glucose uptake rate and yield of lactate from glucose. Reduced lactate formation by hPC expression was also observed in suspension fed-batch cultures using a serum-free medium. Taken together, these results demonstrate that through the expression of the hPC enzyme, lactate formation in CHO cell culture can be efficiently reduced.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chinese hamster ovary (CHO) cells are the most popular hosts for the commercial production of therapeutic proteins (Jiang et al. 2006; Wong et al. 2006; Yoon et al. 2005). Continuously cultured cell lines including the CHO, however, have a metabolic disadvantage in that they are unable to completely oxidize glucose to CO2 and H2O because of low activities of key enzymes like pyruvate carboxylase (PC), which connects glycolysis to the tricarboxylic acid (TCA) cycle (Bonarius et al. 2001; Neermann and Wagner 1996). This low enzyme activity leads to acidification of the culture medium, given that most of the glycolysis-derived pyruvate is ultimately converted by lactate dehydrogenase (LDH) into lactate. Consequently, to maintain the optimal culture pH at high lactate concentration in a bioreactor, an increased amount of alkali is required and thereby elevates the osmolality of the culture medium, resulting in inhibition of cell growth (Irani et al. 1999) Metabolic pathways such as glycolysis and glutaminolysis can also be influenced by the accumulation of lactate (Lao and Toth 1997).
PC, a member of the biotin-dependent enzyme family, catalyzes the adenosine triphosphate (ATP)-dependent irreversible carboxylation of pyruvate to form oxaloacetate in mitochondria of mammalian cells. PC consists of four identical subunits arranged in a tetrahedron-like structure. Each subunit, approximately 130 kDa, contains three functional domains: the biotin carboxylation, transcarboxylation, and biotin carboxyl carrier domains (Carbone and Robinson 2003; Jitrapakdee and Wallace 1999). In CHO cells, this enzyme was previously shown to be lacking or only present in very small amounts (Neermann and Wagner 1996).
Previously, introduction of cytosolic PC, derived from Saccharomyces cerevisiae, into BHK-21 and CHO-K1, enabled the cells to transfer glycolysis-derived pyruvate into malate, which then entered the TCA cycle for complete oxidation. Cytosolic PC-containing cells were able to survive in batch culture for an extended period of time and showed decreased lactate production levels because of lower glucose consumption rates (Fogolin et al. 2004; Irani et al. 1999).
In this paper, the influence of overexpression of human PC (hPC), as a nuclear-encoded mitochondrial protein derived from mammalian cells, on lactic acid production of CHO cells (DG44) was investigated. DG44 cells, dihydrofolate reductase-deficient (dhfr−) CHO cells, are most widely used hosts for construction of recombinant CHO cells producing therapeutic proteins. To accomplish this, three stable DG44 clones constitutively expressing hPC were established and were cultivated in batch and fed-batch modes.
Materials and methods
Construction of hPC expression vector (p3XFLAG-N1-hPC)
3XFLAG sequence amplified from the p3XFLAG-CMVTM-7.1 (Sigma, St. Louis, MO) was inserted into the BglII site of a multiple cloning site in the pN1 vector (pEGFP-N1, Clontech, Palo Alto, CA) to construct the p3XFLAG-N1. hPC complementary deoxyribonucleic acid (GenBank Accession no. BC011617) including a stop codon was inserted into p3XFLAG-N1 previously digested with EcoRI and BamHI to establish an N-terminal 3XFLAG fusion protein (Fig. 1). For high-level expression, the transcription of hPC was under the control of a cytomegalovirus (CMV) promoter/enhancer.
Construction of the p3XFLAG-N1-hPC plasmid for expressing the FLAG-tagged human PC in DG44 cells. PC pyruvate carboxylase, SV40 pA SV40 polyadenylation signal, pCMV cytomegalovirus promoter, HSV TK pA Herpes simplex virus thymidine kinase polyadenylation signal, Kan r/Neor kanamycin/neomycin-resistant gene
Transfection of DG44 cells with hPC expression vector
dhfr− CHO cells (DG44) were transfected with 8 μg of hPC expression vectors using 20 μL of Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. For stable expression, drug selection was carried out for 2 weeks after seeding 1 × 103 cells/well in 96-well tissue culture plates (Nunc, Rokilde, Denmark). Iscove’s modified Dulbecco’s medium (IMDM) supplemented with 10% dialyzed fetal bovine serum (dFBS), 1× hypoxanthine/thymidine (HT) supplement (Gibco, Grand Island, NY), and 550 μg/mL G418 (Invitrogen) was used as a selection medium in a 5% CO2/air mixture, humidified at 37°C. Thirty neomycin-resistant clones were expanded and stored in liquid nitrogen for further study. The control cell line was constructed by transfecting DG44 cells with a negative control pN1 vector.
Western blot analysis
Cell lysates were centrifuged at 12,000 × g for 5 min at 4°C, and supernatants were boiled in sodium dodecyl sulfate (SDS) sample buffer with 14.4 mM 2-mercaptoethanol. Lysates from 1 × 105 cells were electrophoresed on an SDS polyacrylamide gel electrophoresis (PAGE) gel and transferred onto a polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA). Membranes were probed with an anti-FLAG M2 antibody-peroxidase conjugate (Sigma) to detect the hPC enzyme. For detection of β-actin used as a control, anti-β-actin mouse polyclonal antibody (Sigma) was employed, and a horseradish peroxidase-conjugated anti-mouse IgG goat polyclonal antibody (Sigma) was utilized as the secondary antibody. After washing the blots with phosphate-buffered saline (PBS) containing 0.1% Tween-20, bands were visualized by the enhanced chemiluminescent Western blotting system (Amersham, Uppsala, Sweden).
Immunocytochemistry
DG44 cells producing hPC and negative control cells were cultured in four chamber culture slides (BD Bioscience, Bedford, MA) to 50% confluency. For the staining of mitochondria, cells were incubated in a medium containing 200 nM Mitotracker Red CMXRos (Molecular Probes, Eugene, OR) for 45 min. Cells were washed with cold PBS and subsequently fixed with 1% formaldehyde in methanol for 12 h at −20°C. After a cold PBS wash and permeabilization with PBS containing 0.2% Triton X-100 for 30 min at room temperature, cells were further incubated with 10 μg/mL of fluorescein isothiocyanate (FITC)-conjugated anti-FLAG M2 monoclonal antibody (Sigma) for 60 min at room temperature. Confocal images were obtained using an LSM510 confocal microscope (Zeiss, Thornwood, NY).
Batch and fed-batch cultures
For batch culture, exponentially growing cells were inoculated at 5 × 104 cells/mL into T-25 flasks (Nunc) containing 5 mL of IMDM supplemented with 10% dFBS, 1× HT supplement, and 550 μg/mL G418, incubated in a 5% CO2/air mixture, and humidified at 37°C. T-flasks were destroyed daily for the determination of viable cell concentration. Culture supernatants, after centrifugation, were aliquoted and kept frozen at −70°C for later analyses. For fed-batch culture, exponentially growing cells were inoculated at 2 × 105 cells/mL into 125-mL shaking flasks (Corning, Corning, NY) containing 50 mL of a serum-free medium (HyQ SFM4CHO, HyClone, Logan, UT) supplemented with 1 × HT supplement, 4 mM glutamine, and 550 μg/mL G418. After 3 days of cultivation, 2 mM glutamine and 0.5 × HT supplements were added daily to the culture medium. Duplicate cultures were carried out.
Analytical methods
Cell concentrations were estimated using a hemocytometer. Viable cells were distinguished from dead cells by the trypan blue dye exclusion method. Glucose, lactate, and glutamine concentrations were measured using a glucose/lactate analyzer (Model YSI 7100, Yellow Spring Instrument, Yellow Springs, OH).
Evaluation of specific consumption or production rates and apparent yield
The specific consumption or production rates of glucose, lactate, and glutamine were based on the data collected during the exponential growth phase and were evaluated from a plot of the substrate and product concentrations against the time integral values of the growth curve (Renard et al. 1998). Glutamine spontaneously decomposes following the first order kinetics to ammonia and pyrrolidone carboxylate as a function of temperature and pH (Ozturk and Palsson 1990; Tritsch and Moore 1962). The specific glutamine consumption rate (q Gln) was determined by accounting for the degradation of glutamine with the values of the first-order rate constant, k at 0.0031 h−1, as measured experimentally (Ozturk and Palsson 1990). The apparent yield of lactate from glucose was calculated as the mol number of lactate produced divided by the mol number of glucose consumed (Lao and Toth 1997).
Results
Development of DG44 cells producing high levels of hPC
The expression vector encoding hPC (p3XFLAG-N1-hPC) has an N terminus 3XFLAG epitope (MDYKDHDGDYKDHDIDYKDDDDK) to efficiently select positive clones producing high levels of hPC using an anti-FLAG monoclonal antibody, instead of using the detection system based on the biotin binding site of the PC. Transcription of hPC was regulated by the CMV promoter/enhancer. DG44 cells were transfected with the hPC expression vector, whereas the control cell line was constructed by transfecting DG44 cells with a negative control vector (pN1) as described earlier. For the screening of clones stably expressing a high level of hPC, 30 clones and control cell line were assayed by Western blotting using an anti-FLAG monoclonal antibody. As a result, three clones with a high level of hPC, clone 3 (C3), clone 15 (C15), and clone 24 (C24), were selected for further study and maintained during six passages to confirm the stable expression of hPC (Fig. 2).
Subcellular localization of expressed hPC
To investigate the subcellular localization of the hPC enzyme inside DG44 cells, immunocytochemistry was performed as shown in Fig. 3. The hPC enzyme (green) expressed in C15, as well as the other two clones (data not shown), overlapped in numerous areas with mitochondria (red), whereas only mitochondria were detected in control cell line. This result indicates that a substantial amount of expressed hPC, as nuclear-encoded mitochondrial proteins, could be targeted to mitochondria in DG44 cells.
Batch cultures of cell lines producing high levels of hPC
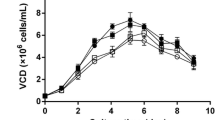
Batch cultures of the three clones and control cell line were carried out to investigate the influence of hPC expression on cell growth and metabolite production and consumption. The maximum cell density and cell viability of the three clones were not decreased compared to the control cell line. This result indicates that expression of hPC did not impair cell proliferation (Fig. 4a and b). A maximum viable cell concentration of the three clones was in the range of 1.71–2.50 × 106 cells/mL, while that of control cell line was 1.66 × 106 cells/mL. A cell viability of the three clones at the end of culture was in the range of 93–96%, while that of control cell line was 85%.
Glucose, lactate, and glutamine concentrations were measured during batch culture (Fig. 5). During the exponential growth phase, days 2 to 5, the specific glucose uptake rates (q Glc) and specific lactate production rates (q Lac) of the three clones were significantly lower than that of the control cell line. The q Glc of the three clones were reduced by 19–40% compared to the control cell line (0.008 < p < 0.047). Similarly, the q Lac values were reduced by 21–39% compared to the control cell line (0.009 < p < 0.048). The apparent yields of lactate from glucose (Y Lac/Glc) of the three clones were decreased by 8–12% compared to the control cell line (0.011 < p < 0.032). The decreased q Glc did not result in increased q Gln for compensating reduced cellular energy. The q Glc, q Lac, q Gln, and Y Lac/Glc were summarized in Table 1.
Fed-batch cultures of cell lines producing high levels of hPC
To mimic the industrial culture environment, C15, chosen to represent the hPC-expressing cells, as well as control cell line were cultivated in fed-batch mode using the serum-free medium. After 3 days of cultivation, 2 mM glutamine and 0.5× HT supplements were added to the culture medium daily. As observed in batch culture using the serum-containing medium, the expression of hPC did not impair cell proliferation in the serum-free suspension culture either. The maximum viable cell density of C15 was very similar to the control cell line and was sustained for a longer period (Fig. 6a). During the exponential growth phase, from days 1 to 5, the glucose consumption and lactate production of C15 were lower than the control cell line (Fig. 6B). The q Glc of C15 was reduced to 80% when compared to the control, and similarly, the q Lac was reduced to 73%. The Y Lac/Glc of C15 was decreased by 15% when compared to the control cell line (p < 0.01; Table 2). Thus, hPC expression in CHO cell culture could reduce lactate formation not only in adherent batch culture with the serum-containing medium but also in the serum-free suspension fed-batch culture.
a, b Profiles of serum-free fed-batch cultures. a Viable cell concentration. Empty circles, control cell line; filled squares, clone 15. b Glucose and lactate. Empty circles, glucose of control cell line; filled circles, glucose of clone 15; empty squares, lactate of control cell line; filled squares, lactate of clone 15. Error bars, standard deviation determined in duplicate experiments
Discussion
It is well known that the accumulation of lactate converted from glucose causes acidification of the culture medium. Acidification increases the amount of alkali required to control the culture pH, which in turn elevates the osmolality of the medium, ultimately resulting in termination of the culture. Accordingly, lactate accumulation is a concern in achieving extended culture longevity. In this study, cell cultures were carried out in T-flasks and/or shaking flasks. It was observed that the pH value of PC-transfected clones was higher than that of the control cell line over the cell growth period, which was probably due to decreased lactate production.
In an effort to alleviate lactate accumulation, several genetic approaches to change metabolic pathways, including introduction of yeast PC into BHK-21 or CHO cells (Irani et al. 1999), disruption of the LDH-A gene by homologous recombination in hybridoma cells (Chen et al. 2001), and reduction in LDH-A activity through antisense messenger ribonucleic acid (RNA) expression (Jeong et al. 2001) or small interfering RNA-targeting vector (Kim and Lee 2007) in CHO cells, have been attempted.
Previously, it was reported that when hybridoma cells were cultured in a chemostat bioreactor, which can maintain the steady-state phase, about 23% of the glucose-derived pyruvate pool entered the TCA cycle through the action of pyruvate dehydrogenase, 68% was used to produce lactate by LDH, and 7% was used to produce nonessential amino acids. Only a relatively small portion (<2%) was moved to the TCA cycle by the PC (Bonarius et al. 2001). This result suggests that additional hPC expression can be a possible way to reduce lactate production, as it shunts pyruvate directly into the TCA cycle, thereby decreasing LDH flux.
In this study, the expression of hPC, as a nuclear-encoded mitochondrial enzyme, was used to suppress lactate production. To date, mammalian PC has not been investigated in CHO cells probably because of the fact that mammalian PC is strictly dependent on the presence of acetyl-coenzyme A in mitochondria, whereas microbial PC, such as the cytosolic PC of yeast, is active even without the presence of this molecule (Jitrapakdee and Wallace 1999). Immunocytochemisty using a FITC-conjugated antibody against a FLAG-tagged fusion protein revealed that a substantial amount of hPC was colocalized with mitochondria, indicating that the primary location of mammalian PC in DG44 cells is mitochondria.
CHO cells are considered heterogeneous even after a very short period of cultivation (Kim et al. 1998; Kim et al. 2001; Kim and Lee 1999). Accordingly, clonal variations should be considered when evaluating the efficacy of introduced foreign proteins using the genetic manipulation method. In this respect, three different clones with high levels of hPC expression rather than a single clone were evaluated. As shown in Fig. 4, hPC expression did not impair cell proliferation. Rather, it improved cell viability at the end of batch culture probably because of reduced formation of lactate, which is known to be toxic to cells. Compared with control cells, q Lac of the three clones was decreased by 21–39%.
Reduced lactate formation caused by the expression of hPC is likely to be mainly due to the decreased glucose uptake rate in the glycolytic pathway and the decreased yield of lactate from glucose. It was previously reported that the introduction of yeast cytosolic PC into BHK-21 and CHO-K1 resulted in decreased glucose consumption rate, which might be due to increased intracellular ATP content by increased flux of glucose into the TCA (Irani et al. 1999). This mechanism was directly proved by flux analysis using [6–14C]-glucose. Introduced hPC probably decreased the pyruvate concentration by downregulating the glucose–pyruvate flux and upregulating the flux rate of glucose carbon to CO2, which may in turn have reduced the lactate production rate. Compared to the control cell line, the q Glc and Y Lac/Glc of the three clones in batch cultures were decreased by 19–40% and by 8–12%, respectively. Furthermore, similar effects of hPC on lactate formation were also observed in the serum-free suspension fed-batch culture, which is favored for large-scale commercial production of therapeutic proteins.
In conclusion, the stable expression of hPC in DG44 cells significantly reduced lactate formation by decreasing the glucose uptake rate and Y Lac/Glc. This effect of hPC expression was observed not only in adherent batch culture using the serum-containing medium but in the serum-free suspension fed-batch culture as well, demonstrating its potential use to extend the culture longevity of CHO cell culture, which often shows a significant accumulation of lactate.
References
Bonarius HPJ, Ozemre A, Timmerarends B, Skrabal P, Tramper J, Schmid G, Heinzle E (2001) Metabolic-flux analysis of continuously cultured hybridoma cells using 13CO2 mass spectrometry in combination with 13C-lactate nuclear magnetic resonance spectroscopy and metabolite balancing. Biotechnol Bioeng 74:528–538
Carbone MA, Robinson BH (2003) Expression and characterization of a human pyruvate carboxylase variant by retroviral gene transfer. Biochem J 370:275–282
Chen K, Liu Q, Xie L, Sharp PA, Wang DIC (2001) Engineering of a mammalian cell line for reduction of lactate formation and high monoclonal antibody production. Biotechnol Bioeng 72:55–61
Fogolin MB, Wagner R, Etcheverrigaray M, Kratje R (2004) Impact of temperature reduction and expression of yeast pyruvate carboxylase on hGM-CSF-producing CHO cells. J Biotechnol 109:179–191
Irani N, Wirth M, Heuvel JD, Wagner R (1999) Improvement of the primary metabolism of cell cultures by introducing a new cytoplasmic pyruvate carboxylase reaction. Biotechnol Bioeng 66:238–246
Jeong DW, Kim TS, Lee JW, Kim KT, Kim HJ, Kim IH, Kim IY (2001) Blocking of acidosis-mediated apoptosis by a reduction of lactate dehydrogenase activity through antisense mRNA expression. Biochem Biophys Res Commun 289:1141–1149
Jiang Z, Huang Y, Sharfstein ST (2006) Regulation of recombinant monoclonal antibody production in Chinese hamster ovary cells: a comparative study of gene copy number, mRNA level, and protein expression. Biotechnol Prog 22:313–318
Jitrapakdee S, Wallace JC (1999) Structure, function and regulation of pyruvate carboxylase. Biochem J 340:1–16
Kim SJ, Lee GM (1999) Cytogenetic analysis of chimeric antibody producing CHO cells in the course of dihydrofolate reductase-mediated gene amplification and their stability in the absence of selective pressure. Biotechnol Bioeng 64:741–749
Kim SH, Lee GM (2007) Down-regulation of lactate dehydrogenase-A by siRNA for reduced lactic acid formation of Chinese hamster ovary cells producing thrombopoietin.. Appl Microbiol Biotechnol 74::152–159
Kim NS, Kim SJ, Lee GM (1998) Clonal variability within dihydrofolate reductase-mediated gene amplified Chinese hamster ovary cells: stability in the absence of selective pressure. Biotechnol Bioeng 60:679–688
Kim NS, Byun TH, Lee GM (2001) Key determinants in the occurrence of clonal variation in humanized antibody expression of CHO cells during dihydrofolate reductase mediated gene amplification. Biotechnol Prog 17:69–75
Lao MS, Toth D (1997) Effects of ammonium and lactate on growth and metabolism of a recombinant Chinese hamster ovary cell culture. Biotechnol Prog 13:688–691
Neermann J, Wagner R (1996) Comparative analysis of glucose and glutamine metabolism in transformed mammalian cell lines, insect and primary liver cells. J Cell Physiol 166:152–169
Ozturk SS, Palsson BO (1990) Chemical decomposition of glutamine in cell culture media: effect of media type, pH, and serum concentration. Biotechnol Prog 6:121–128
Renard JM, Spagnoli R, Mazier C, Salles MF, Mandine E (1998) Evidence that monoclonal antibody production kinetics is related to the integral of viable cells in batch systems. Biotechnol Lett 10:91–96
Tritsch GL, Moore GE (1962) Spontaneous decomposition of glutamine in cell culture media. Exp Cell Res 28:360–364
Wong NSC, Yap MGS, Wang DIC (2006) Enhancing recombinant glycoprotein sialylation through CMP-sialic acid transporter over expression in Chinese hamster ovary cells. Biotechnol Bioeng 93:1005–1016
Yoon SK, Choi SL, Song JY, Lee GM (2005) Effect of culture pH on erythropoietin production by Chinese hamster ovary cells grown in suspension at 32.5 and 37.0°C. Biotechnol Bioeng 89:345–356
Acknowledgment
This research was supported in part by grants from the Ministry of Commerce, Industry, and Energy (ATC program) and Daejeon city (Bio/RIS program) and the Ministry of Education (Brain Korea 21 Program).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, S.H., Lee, G.M. Functional expression of human pyruvate carboxylase for reduced lactic acid formation of Chinese hamster ovary cells (DG44). Appl Microbiol Biotechnol 76, 659–665 (2007). https://doi.org/10.1007/s00253-007-1041-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1041-6