Abstract
Glycerol formation is vital for reoxidation of nicotinamide adenine dinucleotide (reduced form; NADH) under anaerobic conditions and for the hyperosmotic stress response in the yeast Saccharomyces cerevisiae. However, relatively few studies have been made on hyperosmotic stress under anaerobic conditions. To study the combined effect of salt stress and anaerobic conditions, industrial and laboratory strains of S. cerevisiae were grown anaerobically on glucose in batch-cultures containing 40 g/l NaCl. The time needed for complete glucose conversion increased considerably, and the specific growth rates decreased by 80–90% when the cells were subjected to the hyperosmotic conditions. This was accompanied by an increased yield of glycerol and other by-products and reduced biomass yield in all strains. The slowest fermenting strain doubled its glycerol yield (from 0.072 to 0.148 g/g glucose) and a nearly fivefold increase in acetate formation was seen. In more tolerant strains, a lower increase was seen in the glycerol and in the acetate, succinate and pyruvate yields. Additionally, the NADH-producing pathway from acetaldehyde to acetate was analysed by overexpressing the stress-induced gene ALD3. However, this had no or very marginal effect on the acetate and glycerol yields. In the control experiments, the production of NADH from known sources well matched the glycerol formation. This was not the case for the salt stress experiments in which the production of NADH from known sources was insufficient to explain the formed glycerol.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Both anaerobic and hyperosmotic conditions are known to induce glycerol formation in yeast, thus making these conditions highly pertinent for studies of the regulation of glycerol metabolism (Hohmann 2002). Furthermore, anaerobiosis and hyperosmolarity have been extensively studied in connection to potential large-scale glycerol production by yeasts (reviewed by Wang et al. 2001; Taherzadeh et al. 2002). Currently, the glycerol produced as by-product from biodiesel production has lead to lower prices and higher volumes of glycerol on the market. This may open up for new applications of glycerol as a platform chemical.
When the yeast Saccharomyces cerevisiae grows anaerobically, glycerol is—after ethanol and carbon dioxide—the most plentiful by-product. Glycerol is produced by reduction of the glycolytic intermediate dihydroxyacetone phosphate (DHAP) to glycerol-3-phosphate using nicotinamide adenine dinucleotide (reduced form; NADH) as a reducing agent, followed by dephosphorylation. The fermentative pathway from glucose-6-phosphate to ethanol is per se redox neutral. However, excess NADH is formed in connection with biomass and metabolite synthesis, and this NADH has to be reoxidised (Nordström 1966). As a consequence, the NADH coupled reduction of DHAP to glycerol serves as a central means of maintaining the redox balance during anaerobic growth (Ansell 1997; Bjorkqvist et al. 1997). When S. cerevisiae is exposed to salt stress, the organism responds by increasing the internal concentration of glycerol. The accumulated glycerol functions as an osmolyte, preventing loss of turgor pressure of the cell (Blomberg and Adler 1992). Although several studies have been carried out that elucidate the effects of anaerobicity or salt stress on glycerol production, (Verduyn et al. 1990; Albers et al. 1996; Garcia et al. 1997; Petrovska et al. 1999) the combined effect of both anaerobicity and salt stress has been given very little attention. The exposure of anaerobically growing cells to a hyperosmotic environment leads to a rather delicate problem of regulation for the cell. Glycerol has to be continuously produced and excreted to sustain the redox balance, while at the same time, glycerol needs to be retained inside the cell to balance the osmotic pressure.
It has been shown possible to increase the anaerobic glycerol yield by overexpressing one of the enzymes Gpd1p or Gpd2p, which catalyzes the reduction of DHAP to glycerol-3-phosphate. The carbon flux distribution in the glycolysis is in that case directly affected. An increased by-product formation in the form of e.g. excreted acetic acid or acetaldehyde results as a consequence of the need for NADH formation. In the classical fermentative process for glycerol production—the bisulphite or Neuberg’s second form of fermentation (Freeman and Donald 1957)—the situation can be described as the opposite. NADH generation is in this case boosted by the chemical capture of acetaldehyde, and the glycerol formation is driven by the need for regeneration of nicotinamide adenine dinucleotide (oxidized form; NAD+). As an alternative to chemically capturing the acetaldehyde, overexpression of genes encoding NAD+-coupled oxidations, e.g. aldehyde dehydrogenases, may yield similar results. One candidate gene in this respect is ALD3, which encodes a NAD+-dependent aldehyde dehydrogenase known to be stress induced (Navarro-Avino et al. 1999; Norbeck and Blomberg 2000).
The aim of the current work was to investigate how growth and metabolite production of cultivated S. cerevisiae were affected by salt stress under anaerobic conditions. Selected industrial and laboratory strains of S. cerevisiae were grown in anaerobic batch-cultures on glucose in the absence or presence of NaCl (40 g/l) in the growth medium. Strains were characterised with respect to glycerol and other metabolite formation and growth characteristics. To investigate possible effects of an artificial NADH-push, an ALD3 overexpressing strain was included in the study.
Materials and methods
Yeast strains and growth medium
The S. cerevisiae strains used in this study are described in Table 1. The growth medium was a defined medium according to Taherzadeh et al. 1996 with an initial glucose concentration of 50 g/l. In the salt stress experiments, 40 g/l NaCl was added to the medium, and in cultures supplemented with any of the auxotrophic markers histidine, methionine, leucine and uracil, a concentration of each marker of 200 mg/l was used. Ergosterol and Tween 80 were not added to the medium in the aerobic cultivations.
Construction of the ALD3 overexpressing strain
Overexpression of ALD3 was achieved by integrating the open reading frame (ORF) behind the TPI promotor in the pYX142 vector (R&D systems) with a LEU2 selection marker. The polymerase chain reaction (PCR) primers used to amplify the ALD3 ORF were 5′-TCACGGAATTCATGCCTACCTTGTAT-3′ and 3′-TCACGGAATTCACGGTATCATACGAT-5′. The resulting 1.6 kb long PCR fragment was cloned using the 2.1-TOPO TA Vector (Invitrogen) and pYX142 vector. The correct orientation was verified using EcoRI and BamHI restriction enzymes.
Reverse transcription and quantitative PCR
Reverse transcription and quantitative PCR experiments were performed at TATAA Biocenter, Göteborg, Sweden. 0.5 μg of total RNA was used for reverse transcription using Bio-Rad iScript First Strand Synthesis kit according to the manufacturer’s instructions. The reaction was scaled down to 10 μl and each sample was reverse transcribed in duplicate. Each sample was controlled for genomic DNA contamination (No-RT control). PCR primers for ALD3 were designed according to forward primer GTAGAGAATCAGGCGACACC and reverse primer GGCCAAGCGCAACATTTC. PCR primers for IPP1 were already available at TATAA Biocenter. Quantitative PCR was performed on the Stratagene Mx3005p, using the Bio-Rad iQ Supermix. cDNA corresponding to 20 ng of RNA was used in each PCR reaction. Three minutes of denaturation at 95°C was followed by 40 cycles of 20 s at each of the temperatures 95, 60 and 72°C. The fluorescence detection was performed at the 72°C step. After amplification dissociation curve analysis was performed to verify the product formed.
Shake flask experiments
Batch fermentation experiments were carried out in 300 ml shake flasks inoculated from fresh agar cultures and placed in a shaker bath running at 170 rpm, kept at a temperature of 30°C. The medium volume was 200 ml with an initial pH of 5.5. Each shake flask was equipped with a loop-trap containing sterile glycerol and two capillaries, one for sample withdrawal and one for the inlet of nitrogen gas. Nitrogen gas was sparged through the flasks initially for 5 min to establish—and while sampling, to maintain—anaerobic conditions.
Fermentor experiments
Controlled anaerobic and aerobic batch cultivations were carried out in Belach BR 1.1 fermentors (Belach Bioteknik AB, Solna, Sweden) at 30°C with a stirrer speed of 600 rpm. The initial working volume was 500 ml, and the pH was kept constant at 5.0 by addition of 0.75 NaOH. Nitrogen (containing less than 5 ppm O2), controlled by a mass flow metre (Bronkhurst Hi-Tec, Ruurlo, The Netherlands) was continuously supplied to the reactor at 0.3 l/min in the anaerobic cultivations. In the aerobic cultivations, the nitrogen was replaced by air at the same flow rate. All controlled cultivations were inoculated with 5 ml of a preculture grown in 300 ml shake flasks in a rotary shaker at 160 rpm and 30°C for 24 h. The liquid volume in the precultures was 100 ml with a glucose concentration of 15 g/l. The carbon dioxide evolution rate (CER) was monitored online by measuring the concentrations of carbon dioxide and oxygen in the outgoing gas from the reactor with a CP460 gas analyser (Belach Bioteknik). Calibration of the gas analyser was performed using a gas containing 20.0% oxygen and 5.0% carbon dioxide.
Biomass and metabolite analyses
Cell concentrations were determined from absorbance measurements at 610 with samples diluted to give an absorbance less than 0.4. Final cell concentrations of the cultivations were also determined by dry-weight measurements from duplicate 10 ml samples, which were centrifuged, washed with distilled water and dried for 24 h at 105°C. Metabolite samples were immediately centrifuged and stored at −20°C until analysis. The amounts of glucose, ethanol, glycerol, pyruvate, succinate and acetate were determined by liquid chromatography. The high-performance liquid chromatography set up consisted of an Aminex HPX-87H (Bio-Rad) column and a refractive index detector (Waters 410, Millipore, Milford USA) eluted with 5 mM H2SO4 prepared in ultra pure water. Evaporated ethanol during the controlled cultivations was estimated based on measured gas flow leaving the reactor and the ethanol concentration in the fermentation broth (Duboc and von Stockar 1998).
NADH balance
The amount of NADH formed in connection with synthesis of biomass and the metabolites acetate, succinate and pyruvate was compared to the obtained glycerol yield. Biomass was assumed to give a net formation of 13.4 mmol NADH/g biomass (taken as the upper limit calculated by Albers et al. 1996) for cells growing anaerobically on glucose with ammonium as nitrogen source. The metabolites acetate, succinate and pyruvate yield a net production of 1–2, 1–5 and 1 mol NADH/mol metabolite produced, respectively (Gancedo and Serrano 1989; Albers et al. 1996; Camarasa et al. 2003). The net contribution of NADH from each source was used to calculate a minimum and maximum ratio of mol NADH produced/mol glycerol produced.
Results
Growth under salt stress
The response to hyperosmotic stress was studied by adding 40 g/l of NaCl to the medium of anaerobic cultures with 50 g/l glucose. Biomass and product concentrations were measured during the batch cultivation until complete glucose conversion. Baker’s yeast, Saf yeast and CBS 8066 were all found to require prolonged fermentation times to reach complete glucose conversion when exposed to salt in the medium (Table 2). The shortest cultivation time in hyperosmotic medium was observed for Baker’s yeast (100 h), whereas CBS 8066 needed 160 h for complete glucose consumption. However, there was no significant difference in fermentation time between the strains in the control experiments. In agreement with the increased fermentation time, the specific growth rates, μ, decreased in hyperosmotic medium. The specific growth rates for CBS 8066, Baker’s yeast and Saf yeast were decreased by 89, 83 and 78%, respectively (cf. Table 2).
Metabolite formation
Biomass and product formation are summarised in Table 2. The glycerol yield almost doubled in medium containing 40 g/l NaCl compared to the anaerobic control experiments. At the same time, a reduction of the ethanol and biomass yield was seen. In addition, final concentrations of acetate increased when cells were subjected to salt stress. Even if the qualitative pattern was similar for all strains, there were clear quantitative differences between the strains in terms of product formation. CBS 8066, the strain having the lowest growth rate and longest fermentation time, showed the highest product yields of glycerol and acetate, 0.15 and 0.022 g/g glucose, respectively. In contrast, the most salt-tolerant yeast strain (in terms of fermentation time), Baker’s yeast showed the lowest product yields of glycerol (0.12 g/g glucose) and acetate (0.009 g/g glucose). The carbon balance ranged from 0.95 to 0.99 and 0.91 to 0.94 in the control and salt stress experiments, respectively (Table 2). Long fermentation times, mainly in the salt stress experiments, reduced the carbon recovery to some extent, probably due to ethanol evaporation.
NADH balance
To determine how well the theoretical NADH formation from biomass and metabolites correlated to the glycerol production, a calculation based on the product yields was made. As the net production of NADH from metabolites in some cases depends on the active pathway, the calculated NADH formation will vary according to what pathways are assumed to be used. Only biomass formation and the formation of metabolites given in Table 2 were considered in this calculation. In Table 3, the ratios between NADH formed and glycerol produced are given with ranges corresponding to the estimated minimum and maximum NADH formation (see Materials and methods). The range contains, or is very near the value 1, i.e. the NADH balance closed satisfactorily, in all control experiments.
The situation was different during osmotic stress. When the glycerol production increased under salt stress, a larger interval was obtained in the calculations of the NADH balance. This was due to decreased biomass formation and increased levels of the by-products acetate and succinate, which to a larger extent influenced the NADH formation as an uncertainty of co-factor utilisation was allowed. If a minimised NADH formation was assumed, the ratio between NADH formation and consumption was approximately 0.5 (Table 3) for all strains in the salt stress experiments. Even if a maximised NADH formation was assumed in the calculations of the NADH balance, the ratio was still clearly below 1 for Baker’s yeast (0.71) and Saf yeast (0.66). For CBS 8066, it was slightly higher (0.85), due to a higher acetate yield.
Overexpression of ALD3
A possible route of synthesis of acetate in S. cerevisiae is by oxidising acetaldehyde to acetate using a NAD+-dependent aldehyde dehydrogenase encoded by ALD3. ALD3 is reported to be induced under various stress conditions, primarily salt stress (Navarro-Avino et al. 1999; Norbeck and Blomberg 2000), and has been shown to contribute to the increased acetate formation in high gravity fermentations (Pigeau and Inglis 2005). Furthermore, the NADH balance suggested that acetate is generated by an NADH generating reaction in the salt stress experiments. To investigate the effect of Ald3p activity on product formation and salt tolerance we therefore overexpressed ALD3 in the strain BY4741. Reverse transcription and quantitative PCR showed a relative mRNA expression of ALD3 that was 497 (±80) higher in the ALD3 overexpression strain, BY4741 pALD3, compared to the BY4741 strain carrying the empty plasmid. BY4741 pALD3 was, as for previous strains, evaluated under normal conditions and under salt stress (40 g/l NaCl). The genetic modification did not result in any significant changes in acetate and glycerol yields in neither control nor salt stress experiments (Table 4). Furthermore, practically no changes in growth and fermentation times were seen for the modified strain compared to the control with the same genetic background (Table 4).
Effect of auxotrophic markers on salt tolerance
To be able to compare the strains derived from BY4741 with the prototrophic strains in the study, 200 mg/l of histidine, methionine and uracil was added to the previously described salt stress experiments. This procedure also provided experimental data on how the auxotrophic markers concurrently affected salt tolerance. Furthermore, these experiments were repeated with, in addition to the previously stated markers, 200 mg/l of leucine.
In general for all strains, addition of growth markers had the expected positive effect on fermentation times and specific growth rates (Table 4). The fermentation time of the strain CBS 8066 was decreased by almost 50% (down to 85 h) with a doubled specific growth rate (increased to 0.10 h−1) in the presence of 200 mg/l of the growth factors in the medium. Notably, the auxotrophic BY4741 strain was able to ferment all glucose in 40 h under salt stress. Furthermore, Baker’s yeast almost restored its maximum specific growth rate in marker enriched salt medium (μ = 0.25 h−1 in comparison to 0.35 h−1 under normal conditions). Final concentrations of glycerol decreased and the biomass concentration increased in all experiments compared to the salt experiments with no addition of growth markers. The tendency of decreased fermentation times and increased growth rates was even more pronounced after addition also of leucine in the medium (Table 4).
Anaerobic and aerobic salt stress cultivations of CBS 8066 in fermentor
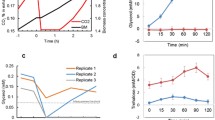
Additional studies of the strain CBS 8066, which had the highest glycerol yield in the study, were made using fermentor experiments. Figure 1 shows the CER during anaerobic and aerobic cultivation on 50 g/l glucose with 40 g/l NaCl in the medium. Yields of biomass and products were determined after the respiratory phase in the aerobic cultivations, and after complete glucose consumption in the anaerobic cultivations (Table 5). Aerobic control experiments showed a respirofermentative phase of 20 h, which equaled the fermentation time in the anaerobic control experiments. Surprisingly, aerobic conditions did not decrease the time for complete glucose consumption in hyperosmotic environment (Fig. 1). On the contrary, longer cultivation time, (110 h of respirofermentative growth) and also a slightly higher glycerol yield (0.21 g/g) were obtained compared to the anaerobic case (Table 5). The characteristic phase of respiration after glucose depletion (first steep decrease in CER), where organic acids, glycerol and ethanol are consumed, was also seen in the aerobic salt stress experiments (Fig. 1).
The results of the controlled anaerobic salt stress experiments agreed qualitatively well with those of the shake flask experiments (Tables 2 and 5). The glycerol yield approximately doubled from 0.094 to 0.177 g/g, and the biomass yield halved from 0.087 to 0.042 g/g. The ratios between NADH formed and glycerol produced (see Materials and methods) for the control and salt stress experiments, given with lower and upper limits, were 1.29–1.50 and 0.56–0.86, respectively. Clearly, sufficient amounts of NADH were provided by the measured metabolites in the control. However, in line with the shake flask experiments, the NADH balance was below 1 in the controlled salt stress experiments.
Discussion
Glycerol formation is central for both anaerobic growth and osmoregulation. A mutant impaired in the glycerol formation pathway exhibits very poor, or no, growth in an anaerobic or a hyperosmotic environment (Ansell 1997). A combination of anaerobic conditions and hyperosmotic conditions, as studied here, represents an interesting challenge with respect to regulation of glycerol production and transport. The cell needs to keep the internal glycerol concentration sufficiently high to maintain turgor pressure, while at the same time produce and excrete a sufficient amount of glycerol to regenerate the amount of NAD+ necessary to sustain growth. All strains of S. cerevisiae in the current study responded qualitatively similarly to high osmolarity (40 g/l NaCl) in the sense that the glycerol yield was doubled compared to anaerobic growth in the absence of added NaCl. The increase of the glycerol yield is in line with previously reported data on glycerol production in respiro-fermentatively growing S. cerevisiae cultures (Garcia et al. 1997; Petrovska et al. 1999). Furthermore, the yields of acetate, succinate and pyruvate all increased and the biomass yield was reduced for all investigated strains. The industrial strains, i.e. Baker’s yeast and Saf yeast, were least affected, whereas the strain CBS 8066 was most sensitive to the divergence from an isotonic medium composition.
As mentioned, the NADH balance (in terms of the ratio of net production of NADH/produced glycerol) is clearly affected by the activity of alternative pathways. The NADH balance was found satisfactory in the control experiments without salt addition, which is in accordance with the studies of Albers et al. 1996 and Taherzadeh et al. 1996. However, the increased glycerol yield in the salt stress experiments could not fully be explained by the NADH production from measured by-product formation, even when assuming the most generous NADH formation from by-products possible. For Baker’s yeast and Saf yeast, the NADH formation was at least 30% too low, and for CBS 8066 at least 15% remained unexplained, even if a maximised NADH formation was assumed for all synthetic pathways. A maximised NADH formation requires acetate production to be catalysed by an NAD+-dependent aldehyde dehydrogenase. This is supported by previous studies where the gene ALD3 was found to be induced by osmotic stress (Norbeck and Blomberg 2000; Krantz et al. 2004), and that its gene product contributed to the increased levels of acetate in fermentations with high sugar concentrations (Pigeau and Inglis 2005). In addition, the net NADH production from succinate has to be assumed to be oxidatively produced to improve the NADH balance. However, Camarasa et al. (2003) showed that 75% of the succinate produced in wine fermentation was reductively formed, and Arikawa et al. (1998) found that a mutant deleted for the enzyme fumarate reductase, catalysing the reaction from fumarate to succinate, was not able to grow under anaerobic conditions. This indicates that rather than assuming a fully active oxidative pathway, calculations of the theoretical glycerol yield should be based on a net NADH production from succinate less than the maximum. The deviation between known NADH production and glycerol formation would in such case be even larger than calculated here.
The metabolic role of the gene ALD3 is not yet entirely clear, but it has been suggested to be involved in redox metabolism under hyperosmotic conditions and/or in ethanol oxidation (Navarro-Avino et al. 1999). Saint-Prix et al. (2004) showed that Ald3p is not required for acetate production during anaerobic growth on glucose and that the NADP+ dependent isoenzymes Ald6p and Ald5p are mainly responsible for the formed acetate. In contrast, under hyperosmotic stress Ald3p has been reported to be responsible for part of the increased acetate production (Pigeau and Inglis 2005). However, overexpression of ALD3 in the current study did not significantly change the glycerol and acetate formation or growth characteristics in either control or salt stress experiments. It has previously been shown that the ALD2 and ALD3 are a requisite for pantothenic acid metabolism in the step of β-alanine biosynthesis from 3-aminopropanal (White et al. 2003). This suggests that the physiological substrate of ALD3 is in fact 3-aminopropanal rather than acetaldehyde. Furthermore, the metabolite and growth pattern from an ald3Δ mutant included in the study could not be separated from the wild type in neither experiments with nor without salt (results not shown). The possibility of the homolog ALD2 compensating for the disrupted ALD3 was investigated by Navarro-Avino et al. 1999, who found that a ald2Δ ald3Δ double mutant did not show any increased sensitivity to osmotic stress.
The addition of a mixture of amino acids has been shown to increase growth rate and decrease glycerol formation by anaerobically growing cells (Albers et al. 1996). This was also seen in the present study for anaerobically growing cells in hyperosmotic medium. The addition of a mixture of growth factors, mainly amino acids, to the medium considerably lowered fermentation times and glycerol yields. Apart from the expected direct redox effect, one should keep in mind that there might also be less obvious indirect effects caused by the addition of auxotrophic markers (Pronk 2002).
Although the strains responded qualitatively similar to the addition of salt, there were quantitative differences in fermentation times and the glycerol yields obtained. Clearly, osmoprotective retention of glycerol inside the cell is not necessarily correlated with glycerol production rate because strong production was associated with low salt tolerance in the present work. Because active uptake of glycerol is not seen in cells grown on glucose under either normal or high osmolarity conditions (Holst et al. 2000), the differences in glycerol retention at the level of the plasma membrane may be one of the explanations for the poor correlation of salt tolerance and glycerol production. The expression of STL1, encoding a glycerol proton symporter in S. cerevisiae (Ferreira et al. 2005), is lower for osmostressed cells cultured under anaerobic than aerobic conditions (Krantz et al. 2004). Gori et al. 2005 showed that difference in maximum levels of intracellular glycerol was due to the ability to retain the glycerol and that high intracellular glycerol levels are beneficial for salt tolerance. Studies also show that retention of glycerol is incomplete because glycerol is overproduced under hyperosmolarity conditions even when oxygen is present (Olz et al. 1993), which is in agreement with the current study. The channel protein Fps1p controls the release of glycerol through the plasma membrane (Tamas et al. 1999). Differences in expression, topology and/or control of Fps1p, will affect the glycerol excretion rate, and thereby (indirectly), rate of NADH regeneration and specific growth rate. However, an interesting question that still remains to be answered is how the cells in fact generate the NADH needed for glycerol synthesis during combined hyperosmotic and anaerobic conditions.
References
Albers E, Larsson C, Liden G, Niklasson C, Gustafsson L (1996) Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl Environ Microbiol 62:3187–3195
Ansell R (1997) Redox—and osmoregulation in Saccharomyces cerevisiae: the role of the two isogenes encoding NAD+ dependent glycerol 3-phosphate dehydrogenase. Ph.D. thesis Göteborg University, Sweden
Arikawa Y, Enomoto K, Muratsubaki H, Okazaki M (1998) Soluble fumarate reductase isoenzymes from Saccharomyces cerevisiae are required for anaerobic growth. FEMS Microbiol Lett 165:111–116
Bjorkqvist S, Ansell R, Adler L, Liden G (1997) Physiological response to anaerobicity of glycerol-3-phosphate dehydrogenase mutants of Saccharomyces cerevisiae. Appl Environ Microbiol 63:128–132
Blomberg A, Adler L (1992) Physiology of osmotolerance in fungi. Adv Microb Physiol 33:145–212
Camarasa C, Grivet JP, Dequin S (2003) Investigation by 13C-NMR and tricarboxylic acid (TCA) deletion mutant analysis of pathways for succinate formation in Saccharomyces cerevisiae during anaerobic fermentation. Microbiology-Sgm 149:2669–2678
Duboc P, von Stockar U (1998) Systematic errors in data evaluation due to ethanol stripping and water vaporization. Biotechnol Bioeng 58:426–439
Ferreira C, van Voorst F, Martins A, Neves L, Oliveira R, Kielland-Brandt MC, Lucas C, Brandt A (2005) A member of the sugar transporter family, Stl1p is the glycerol/H+ symporter in Saccharomyces cerevisiae. Mol Biol Cell 16:2068–2076
Freeman GG, Donald GMS (1957) Fermentation processes leading to glycerol. 1. The influence of certain variables on glycerol formation in the presence of sulfites. Appl Microbiol 5:197–210
Gancedo C, Serrano R (1989) Energy-yielding metabolism. In: Yeasts, vol 3, 2nd edn. Academic, New York, pp 205–257
Garcia MJ, Rios G, Ali R, Belles JM, Serrano R (1997) Comparative physiology of salt tolerance in Candida tropicalis and Saccharomyces cerevisiae. Microbiology-UK 143:1125–1131
Gori K, Mortensen HD, Arneborg N, Jespersen L (2005) Expression of the GPD1 and GPP2 orthologues and glycerol retention during growth of Debaryomyces hansenii at high NaCl concentrations. Yeast 22:1213–1222
Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev 66:300–372
Holst B, Lunde C, Lages F, Oliveira R, Lucas C, Kielland-Brandt MC (2000) GUP1 and its close homologue GUP2, encoding multimembrane-spanning proteins involved in active glycerol uptake in Saccharomyces cerevisiae. Mol Microbiol 37:108–124
Krantz M, Nordlander B, Valadi H, Johansson M, Gustafsson L, Hohmann S (2004) Anaerobicity prepares Saccharomyces cerevisiae cells for faster adaptation to osmotic shock. Eukaryot Cell 3:1381–1390
Navarro-Avino JP, Prasad R, Miralles VJ, Benito RM, Serrano R (1999) A proposal for nomenclature of aldehyde dehydrogenases in Saccharomyces cerevisiae and characterization of the stress-inducible ALD2 and ALD3 genes. Yeast 15:829–842
Norbeck J, Blomberg A (2000) The level of cAMP-dependent protein kinase A activity strongly affects osmotolerance and osmo-instigated gene expression changes in Saccharomyces cerevisiae. Yeast 16:121–137
Nordström K (1966) Yeast growth and glycerol formation. Acta Chem Scand 20:1016–1025
Olz R, Larsson K, Adler L, Gustafsson L (1993) Energy flux and osmoregulation of saccharomyces-cerevisiae grown in chemostats under NaCl stress. J Bacteriol 175:2205–2213
Petrovska B, Winkelhausen E, Kuzmanova S (1999) Glycerol production by yeasts under osmotic and sulfite stress. Can J Microbiol 45:695–699
Pigeau GM, Inglis DL (2005) Upregulation of ALD3 and GPD1 in Saccharomyces cerevisiae during Icewine fermentation. J Appl Microbiol 99:112–125
Pronk JT (2002) Auxotrophic yeast strains in fundamental and applied research. Appl Environ Microbiol 68:2095–2100
Saint-Prix F, Bonquist L, Dequin S (2004) Functional analysis of the ALD gene family of Saccharomyces cerevisiae during anaerobic growth on glucose: the NADP(+)-dependent Ald6p and Ald5p isoforms play a major role in acetate formation. Microbiology-Sgm 150:2209–2220
Taherzadeh MJ, Liden G, Gustafsson L, Niklasson C (1996) The effects of pantothenate deficiency and acetate addition on anaerobic batch fermentation of glucose by Saccharomyces cerevisiae. Appl Microbiol Biotechnol 46:176–182
Taherzadeh MJ, Adler L, Liden G (2002) Strategies for enhancing fermentative production of glycerol—A review. Enzyme Microb Technol 31:53–66
Tamas MJ, Luyten K, Sutherland FCW, Hernandez A, Albertyn J, Valadi H, Li H, Prior BA, Kilian SG, Ramos J, Gustafsson L, Thevelein JM, Hohmann S (1999) Fps1p controls the accumulation and release of the compatible solute glycerol in yeast osmoregulation. Mol Microbiol 31:1087–1104
Verduyn C, Postma E, Scheffers WA, van Dijken JH (1990) Physiology of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J Gen Microbiol 136:395–403
Wang ZX, Zhuge J, Fang HY, Prior BA (2001) Glycerol production by microbial fermentation: a review. Biotechnol Adv 19:201–223
White WH, Skatrud PL, Xue ZX, Toyn JH (2003) Specialization of function among aldehyde dehydrogenases: The ALD2 and ALD3 genes are required for beta-alanine biosynthesis in Saccharomyces cerevisiae. Genetics 163:69–77
Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, Chu AM, Connelly C, Davis K, Dietrich F, Dow SW, El Bakkoury M, Foury F, Friend SH, Gentalen E, Giaever G, Hegemann JH, Jones T, Laub M, Liao H, Davis RW et al (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285:901–906
Acknowledgment
The authors would like to thank A. Forsmark for her assistance in the mRNA analyses. This work was supported by grants from the Swedish Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Modig, T., Granath, K., Adler, L. et al. Anaerobic glycerol production by Saccharomyces cerevisiae strains under hyperosmotic stress. Appl Microbiol Biotechnol 75, 289–296 (2007). https://doi.org/10.1007/s00253-006-0821-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0821-8