Abstract
In this study di-2-ethylhexyl phthalate (DEHP)-degradation strain CQ0110Y was isolated from activated sludge. According to the biophysical/biochemical characteristics and analysis of 16S rDNA, the strain was identified as Microbacterium sp. The results of this study showed the optimal pH value and optimal temperature which influenced the degradation rate in wastewater: pH 6.5–7.5, 25–35°C. Kinetics of degradation reaction had been performed at different initial concentrations and different time. Analyzed with SPSS10.0 software, the DEHP degradation can be described as the same exponential model when the initial DEHP concentration was lower than 1,350 mg/l. The kinetics equation was ln C = −0.4087t + A, with the degradation half life of DEHP in wastewater (1.59 days). To the best of our knowledge, this is the first reported case of DEHP degradation by Microbacterium sp. strain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phthalic acid esters (PAEs), a class of refractory organic compounds which are widely used in the plastic, coatings, and cosmetics industries, have received extensive attention in recent years. They are distributed in sediment, natural water, wastewater, and soils (IPCS 1992). Release of phthalates into the ecosystem or wastewater effluent occurs during the production phase and via leaching and volatilization from plastic products during their usage and after disposal (Psillakis et al. 2004). Even at very low concentrations, they are suspected of interfering with reproductive systems and behavior in humans and wildlife through disturbance of the endocrine system (Jobling et al. 1995). In addition, some of them are suspected of being teratogenic, mutagenic, and carcinogenic (Huff and Kluwe 1984). Several regulatory bodies, such as the US Environment Protection Agency, the European Union, and the China National Environmental Monitoring Center (Wang et al. 1995), classified phthalate esters as a top-priority environmental pollutant. According to our studies (Tian et al. 2004; Qiu et al. 2003), the PAE pollution in the Yangzi and Jialing rivers of China is widespread; PAEs could be found in every water sample and the concentration arises to 25 μg/l.
di-2-Ethylhexyl phthalate (DEHP) belongs to the family of PAEs. DEHP is used as a plasticizer in polyvinyl chloride (PVC) plastics to impart flexibility, strength, broad-range temperature tolerance, stability during sterilization, and optical clarity to the otherwise hard and brittle unplasticized PVC (ATSDR 2002). It is widely distributed in air, sediment, natural water, waste water, soil, and living organics (Clausen et al. 2004; Suzuki et al. 2001; Cheng et al. 2001; Hu et al. 2003; Koch et al. 2004). Exposure to high concentrations of DEHP produces a wide range of adverse effects in experimental animals, including cancer, liver damage, birth defects, and alterations of the reproductive system, especially for male animals (ATSDR 2002; Gray et al. 2003, 2004; Kavlock et al. 2002).
The metabolic breakdown of DEHP by microorganisms is considered to be one of the major routes of environmental degradation for this widespread pollutant due to its low rate of hydrolysis and photolysis (Wolf et al. 1980). As the application of DEHP-containing PVC plastic films on agriculture dramatically increases and as large quantities of wastewater and municipal and industrial sewage sludge are deposited on agricultural land each year, DEHP is the most identified phthalate ester in agricultural soils (Kampe et al. 1988; Tao et al. 1993; Pang et al. 1995; Meng et al. 1996). In the past few years, considerable attention has been paid to the analysis of the environmental fate, general toxicity, and biological degradabililiy of DEHP (Wang et al. 1997). In recent years, a few researches have reported about DEHP’s degradation by microorganisms (Quan et al. 2005; Kim et al. 2003; Zeng 1999); however, the mechanism of the biodegradation of DEHP is not very clear.
In this study, we aimed to isolate microorganisms capable of degrading DEHP, explore the mechanism of biodegradation, and characterize the environmental factors influencing the degradation process in contaminated water.
Materials and methods
Reagents and chemicals
DEHP, with 99.0% analytical standards, was obtained from Beijing Chemical Reagent Factory (Beijing, China). Chemicals used for dilution and extraction of DEHP were of analytical grade and were redistilled. Other chemicals used in this study were also of analytical grade. Glassware was meticulously cleaned to reduce any background contamination of phthalates. All chromic-acid-washed glassware was placed in a 300°C oven overnight. After cooling, the glassware was rinsed twice with acetone and petroleum ether and air-dried ready for use.
Isolation of microorganism resistant to DEHP
The activated sludge used for the isolation of bacteria was collected from the Tangjiaqiao sewage treatment factory of Chongqing in China that has been exposed to DEHP wastewater for more than 10 years.
The microorganisms were isolated in basal medium containing 10, 50, 100, 200, 400, 600, 800, 1,000, 1,200, 1,400, 1,600, 1,800, and 2,000 mg/l DEHP. The basal medium contained K2HPO4 1 g/l, NaCl 1 g/l, NH4Cl 0.5 g/l, and MgSO4 0.4 g/l, pH 7.2, and underwent moist heat sterilization for 15 min. DEHP was used as the sole carbon source in this medium. DEHP was added to the triangular flask and the solvent dichloromethane was allowed to evaporate for 30 min before adding the basal medium. Ten grams of sludge was added into a 250-ml triangular flask with 100 ml solid medium. These flasks were incubated at 37°C in a rotary shaker (100 rpm). An aliquot of 1 ml was subcultured to fresh medium every week, the concentration of DEHP increasing from 10 to 2,000 mg/l. After 3 months, some bacteria could grow in the medium with DEHP with 2,000 mg/l; then, 10 μl of medium with bacteria was plated on nutrient agar plates to isolate and purify the bacterium capable of degrading DEHP.
Identification
The primers which were used to amplify the 16S rDNA gene were Pf: 5′-AGAGTTTGATCCTGGCTCAG-3′ and Pr: 5′-ACGGCTACCTTGTTACGACT-3′, corresponding to 8–27 and 1,495–1,514 bases of coli 16S rRNA gene, respectively. PCR amplification conditions were as follows: Each PCR mixture (25 μl) was composed of 10 mM Tris–HCl (pH 8.3), 50 mM KCl, 2.5 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 200 μM, each primer at a concentration of 0.25 μM, template DNA, and 0.45 U of Taq DNA polymerase. The amplification program consisted of one cycle of 94°C for 5 min; 38 cycles of 94°C for 30 s, 55°C for 45 s, and 72°C for 100 s; and finally, one cycle of 72°C for 10 min. The amplification products were subjected to gel electrophoresis in 1% agarose, followed by ethidium bromide staining. All the reagents and service of sequencing were provided by Shanghai Shenbo (Shanghai, China).
The identification of microorganisms with high DEHP biodegradation ability was made on the basis of morphological appearance and physiological characteristics (Krieg and Holt 1984; Microbiology Institute Of CAS 1978). The Vitek-AutoMicrobic System (bioMerieux, SA, Marcy-l’Etoile, France) was also used to characterize it with biochemical tests.
Detection of activity of catechol-dioxygenase
The method for detection of catechol-dioxygenase activity (Haysish 1957; Sala-Trepat and Evams 1971; Sanakis et al. 2003) was adopted. The catechol-dioxygenase activity was assayed spectrophotometrically using a Hitachi U-2000 spectrophotometer. The reaction mixture contained aliquots of the enzyme and 0.5 mM catechol (final volume, 1 ml) in 1.33 mM EDTA/50 mM phosphate (pH = 7.0). The enzymatic reaction was monitored by measuring the formation of product (cis,cis-muconic acid) at 260 nm (= 16.8 mM).
Biodegradation of DEHP by CQ0110Y
DEHP was used as the sole carbon source in basal medium with 2,000 mg/l DEHP. Two hundred microliters of bacterial suspension (OD330 = 0.6) was added into a 250-ml triangular flask with 100 ml basal medium. These triangular flasks were incubated at 30°C in a rotary shaker (100 rpm) and kept from the light to avoid photodegradation of DEHP. By investigating the degradation rate under different pH (4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, and 9.0) and temperature (10, 15, 20, 25, 30, 35, 40, 45, and 50°C) conditions, the optimum degradation conditions were determined.
Analytical methods
The liquid phase and bacterial cell were separated by centrifugation at 8,000×g. Phthalate in the supernatant was extracted with dichloromethane. DEHP contents were analyzed by gas chromatography (SC2000, Chuanyi, Chongqing, China) with a stainless steel column (3 mm × 2 m, OV101 silicon packed). The flame ionization detector was used. The temperatures of the column, injection port, and flame ionization detector were 250, 260, and 280°C, respectively. Nitrogen was used as a carrier gas at a flow rate of 20 ml/min, and the hydrogen and airflow rates were 45 and 230 ml/min, respectively. The biodegradation products of DEHP were analyzed by GC/MS [GC6890/5973MSD, Agilent, Palo Alto, CA, USA, with an HP-5 MS column (Agilent, length 30 m, i.d. 0.25 mm, film thickness 0.25 μm)], in reference to Wu et al. (2005). The conditions were (1) 50°C, 8°C/min, 180°C (2 min), 8°C/min, 250°C (10 min); (2) the scan style was 230°C, 150°C (tetrapolar); (3) the injection port temperature was 270°C, interface temperature was 280°C, pressure was 50.5 kPa, and flow rate was 1.0 ml/min; and (4) sample injection volume was 1 μl. The products were determined by the score matching to the standard library.
Results
Isolation and identification of the DEHP-degrading bacterium
The bacterium capable of utilizing DEHP as the sole source of carbon and energy was isolated from activated sludge. The initial concentration of DEHP was 10 mg/l. After 3 months, one strain that could survive in the concentration of 2,000 mg/l was studied in the following experiments. We designated it as CQ0110Y. The colony showed round morphology, low prominency, smooth and glossy surface, and yellowish coloring. The cell was aerobic and gram-positive.
The complete 16s rDNA sequence of CQ0110Y was determined. The sequence was compared with published 16s rDNA sequences by blasting in GenBank; the results showed that the similarity to Microbacterium sp. was 99.5%. The accession number of CQ0110Y in GenBank is DQ852355. Combined with the analysis of morphology, physio-biochemical character, and genetic specificity, CQ0110Y was identified as a Microbacterium sp. Phylogenetic and molecular evolutionary analyses were conducted using MEGA version 3.1 (Kumar et al. 2004). The phylogeny tree is shown in Fig. 1.
Environmental factors influencing the degradation process in wastewater
The relationship between the degradation rate constants and pH for CQ0110Y is shown in Fig. 2. The rate of DEHP degradation increased quickly when the pH value of the soil was increased from 4.5 to 5.0. A high rate was achieved for CQ0110Y when the pH value was 7.0. When pH value was >7.5, the degradation efficiency decreased. The optimal pH value for CQ0110Y to degrade DEHP was from 6.5 to 7.5.
Effects of pH on DEHP degradation rate. The rate of DEHP degradation increased quickly when the pH value of the soil was increased from 4.5 to 5.0. A high rate was achieved for CQ0110Y when the pH value was 7.0. When the pH value was >7.5, the degradation efficiency decreased. The optimal pH values for CQ0110Y to degrade DEHP were from 6.5 to 7.5
Bacterium growth is sensitive to environmental temperature. In order to determine the optimal temperature for CQ0110Y to degrade DEHP, different temperature conditions (10, 15, 20, 25, 30, 35, 40, 45, and 50°C) were assessed. As is shown in Fig. 3, the degradation rate increased with the increase of temperature between 10 and 30°C. A high rate was achieved for CQ0110Y when the temperature was sustained between 25 and 35°C.
Effects of temperature on DEHP degradation rate. The degradation rate increased with the increase of temperature between 10 and 30°C. A high rate was achieved for CQ0110Y when the temperature was sustained between 25 and 35°C. Higher temperature resulted in the lowering of the degradation rate. The result indicated that the optimum temperature for CQ0110Y to degrade DEHP was 25–35°C, at which temperature the degradation-related enzyme reached the highest activity
Characteristics of DEHP degradation kinetics in wastewater
In order to investigate the effect of initial DEHP concentration on degradation activity, the degradation kinetics character in wastewater was studied. Different initial DEHP concentrations (100, 500, 850, 1,350, 1,600, and 2,000 mg/l) were assessed. As shown in Fig. 4, the time course of degradation rate was recorded.
DEHP-degradation kinetics character in wastewater by CQ0110Y. The time course of degradation rate was recorded in different initial DEHP concentrations (100, 500, 850, 1,350, 1,600, and 2,000 mg/l). When DEHP initial concentration is <1,350 mg/l, DEHP biodegradation reaction fit with the first-order kinetics. The half life of DEHP in soil was 1.59 days
Detection of activity of catechol-dioxygenase
As shown in Table 1, the activity of catechol 1,2-dioxygenase other than catechol 2,3-dioxygenase could be detected in the crude enzyme of CQ0110Y strain. In addition, the higher activity of catechol 1,2-dioxygenase was detected only after DEHP inducing.
Degradation products of DEHP by CQ0110Y
In this study, the degradation products of DEHP by CQ0110Y were determined at the half life time (t 1/2) to clearly understand the degradation pathway. The determined products are shown in Table 2 when the degradation process was at 1.5 days, the half life time of DEHP by CQ0110Y.
Discussion
We isolated a DEHP-degrading bacterium utilizing DEHP as sole carbon and energy sources from activated sludge, which is designated as CQ0110Y. According to the analysis of genetic specificity, morphology, and physio-biochemical character, the strain CQ0110Y belongs to Microbacterium sp. To the best of our knowledge, this is the first reported case of DEHP degradation by Microbacterium sp. strains. Metabolic breakdown of PAEs by a microorganism is considered to be one of the major routes of environmental degradation. It is well known that PAEs with longer alkyl chains (i.e., di-octyl phthalate, DEHP) are more difficult to biodegrade than those with shorter alkyl chains (i.e., di-ethyl phthalate, di-butyl phthalate), and it is also confirmed that a correlation exists between increasing length of the ester side-chain and decreasing biodegradability (O’Grady et al. 1985).
The DEHP biodegradation data we collected fit well with the exponential model, C = b 0*e (b1*t). A first-order kinetics model, ln C = −kt + A, could be constructed by logarithmic transformation, where C is the initial concentration (mg/l), k is the biodegradation rate constant, t is the time period, A is the constant, and t 1/2 = ln 2/k is the half life. Table 3 shows the different DEHP degradation kinetics equations at different DEHP initial concentrations. The results showed that when DEHP initial concentration <1,350 mg/l, DEHP biodegradation reaction fit with the first-order kinetics. Analyzed by SPSS10.0, the DEHP degradation kinetics equation in wastewater by CQ0110Y was ln C = −0.4087t + A, and the half life of DEHP was 1.59 days. When the initial concentration of DEHP is higher than 1,600 mg/l, the half life will increase due to the inhibition by the higher concentration of DEHP.
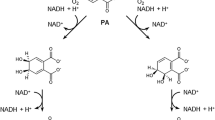
Catechol is the intermediate product of aromatic hydrocarbon compounds, and the cleaving benzene ring is the common pathway of aromatic hydrocarbon metabolism. Ring opening reaction could be catalyzed by catechol 1,2-dioxygenase and catechol 2,3-dioxygenase (Strachan et al. 1998). Because the activity of catechol 1,2-dioxygenase is higher than that of catechol 2,3-dioxygenase in the crude enzyme of the CQ0110Y strain, catechol was suspected to be an intermediate product of DEHP and cleaving the benzene ring was catalyzed by catechol 1,2-dioxygenase. The later detection of degradation products confirmed the occurrence of catechol during the degradation process. For the degradation pathway of phthalates, it is generally supposed that hydrolysis of the ester side-chain of the di-alkyl phthalate through mono-alkyl phthalate occurs, leaving phthalic acid and alkyl alcohols available for further conversion. According to the degradation products (the supposed degradation pathway of DEHP by strain CQ0110Y is shown in Fig. 5), DEHP was hydrolyzed to monoethylhexylphthalate and phthalic acid by the action of esterase, then benzenecarboxvlic acid would form with the decarboxylation of phthalic acid; through the hydroxylation at the consecutive position of benzenecarboxvlic acid, orthohydroxybenzoic acid would be produced, and then to pyrocatechin and muconic acid; after the tricarboxylic acid cycle, the terminal degradation products were CO2 and H2O. Quan et al. (2005) proposed the pathways for DEHP degradation by strain number 66 as follows: one ester bond of DEHP was hydrolyzed by the strain to form mono-alkylphthalate and alcohol, and then the mono-alkylphthalate was hydrolyzed to protocatechuic acid and alcohol. Although the process from DEHP to phthalic acid is the same, the hydroxylation and decarboxylation is different. The activities of catechol-dioxygenase in CQ0110Y also confirm the ring cleavage beginning with the break at the consecutive position; Table 1 shows the activity of catechol 1,2-dioxygenase higher than that of catechol 2,3-dioxygenase; with the oxidization by catechol 1,2-dioxygenase, the muconic acid is produced.
The supposed degradation pathway of DEHP by strain CQ0110Y. By detecting the degradation products of DEHP at half life time, we supposed the degradation pathway of DEHP by strain CQ0110Y: DEHP was hydrolyzed to monoethylhexylphthalate and phthalic acid by the action of esterase, then benzenecarboxvlic acid would form with the decarboxylation of phthalic acid; through the hydroxylation at consecutive position of benzenecarboxvlic acid, orthohydroxybenzoic acid would be produced, and then to pyrocatechin and muconic acid; after the tricarboxylic acid cycle, the terminal degradation products were CO2 and H2O
References
ATSDR (2002) Toxicological profile for di(2-ethylhexyl)phthalate (DEHP). Agency for Toxic Substances and Disease Registry. http://www.atsdr.cdc.gov/toxprofiles/tp9.html Cited 26 July 2004
Cheng HF, Chen SY, Lin JG (2001) Hazardous organic matters in municipal sewage sludge in Taiwan. Water Sci Technol 10:65–70
Clausen PA, Hansen V, Gunnarsen L, Afshari A, Wolkoff P (2004) Emission of di-2-ethylhexyl phthalate from PVC flooring into air and uptake in dust: emission and sorption experiments in FLEC and CLIMPAQ. Environ Sci Technol 9:2531–2537
Gray LE, Barlow NJ, Furr JR, Brock J, Silva MJ, Barr DB, Ostby JS (2003) Transgenerational effects of di(2-ethylhexyl) phthalate in the male rat. Toxicol Sci 72:283
Gray LE, Furr J, Lambright C, Ostby J (2004) Chronic exposure to diethyl hexyl phthalate (DEHP) delays puberty and reduces androgen-dependent tissue weights in the male rat. Biol Reprod 113:166 (meeting abstract)
Haysish O (1957) Studies on oxygenases. J Biol Chem 229:905–920
Hu XY, Wen B, Shan XQ (2003) Survey of phthalate pollution in arable soils in China. J Environ Monit 4:649–653
Huff JE, Kluwe WM (1984) Phthalate esters carcinogenicity in F344/N rates and B6C3 F mice. Prog Clin Biol Res 141:137–154
International Programme on Chemical Safety (IPCS) (1992) Environmental Health Criteria 131. Diethylhexyl phthalate. World Health Organization, Geneva
Jobling S, Reynolds T, White R, Parker MG, Sumper JP (1995) A variety of environmentally persistent chemicals, including some phthalate plasticizers, are weakly estrogenic. Environ Health Perspect 103:582–587
Kampe W, Zurcher C, Jobst H (1988) Harmful organic substances in soils and plants after intensive sewage sludge applications. VDLUFA-Schriftenreihe 23:507–532
Kavlock R, Boekelheide K, Chapin R, Cunningham M, Faustman E, Foster P, Golub M, Henderson R, Hinberg I, Little R, Seed J, Shea K, Tabacova S, Tyl R,Williams P, Zacharewski T (2002) NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di(2-ethylhexyl) phthalate. Reprod Toxicol 16:529–653
Kim YH, Lee J, Moon SH (2003) Degradation of an endocrine disrupting chemical, DEHP[di-(2-ethylhexyl)-phthalate], by Fusarium oxysporum f. sp. pisi cutinase. Appl Microbiol Biotechnol 63:75–80
Koch HM, Drexler H, Angerer J (2004) Internal exposure of nursery-school children and their parents and teachers to di(2-ethylhexyl)phthalate (DEHP). Int J Hyg Environ Health 1:15–22
Krieg NR, Holt JG (1984) Bergey’s manual of systematic bacteriology. Williams & Wilkins, Baltimore
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Meng PR, Wang XK, Xu GT (1996) Determination and distribution of phthalate alkyl esters in soil in Jinan. Environ Chem 15:427–432
Microbiology Institute Of CAS (China Academy of Sciences) (1978) Identification manual of systematic bacteriology. Science Publication, Beijing
O’Grady DP, Howard PH, Warner AF (1985) Activated sludge biodegradation of 12 commercial phthalate-esters. Appl Environ Microbiol 49:443–445
Pang JM, Duan YL, Chi BL (1995) Residue and toxicity of DEHP in the soil and chinise cabbage. Environ Chem 14:239–242
Psillakis E, Mantzavinos D, Kalogerakis N (2004) Monitoring the sonochemical degradation of phthalate esters in water using solid-phase microextraction. Chemosphere 54:849–857
Qiu Z, Shu W, Tian H (2003) An analysis of the organic extracts from source water and drinking water in city C and the effects on DNA damage in primary hepatocytes in rats. Acad Med Militaris Tertiae 5:423–426
Quan CS, Liu Q, Tian WJ, Kikuchi J, Fan SD (2005) Biodegradation of an endocrine-disrupting chemical, di-2-ethylhexyl phthalate, by Bacillus subtilis No. 66. Appl Microbiol Biotechnol 66:702–710
Sala-Trepat JM, Evams WC (1971) The meta cleavage of catechol by azobacter species. Eur J Chem 20:400–413
Sanakis Y, Mamma D, Christakopoulos P, Stamatis H (2003) Catechol 1,2-dioxygenase from Pseudomonas putida in organic media—an electron paramagnetic resonance study. Int J Biol Macromol 33:101–106
Strachan PD, Freer AA, Feson CA (1998) Purification and characterization of catechol,2-dioxygenase from Rhodococcus rhodochrous NCIMB 13259 and cloning and sequencing of its catA gene. Biochemistry 333:741–747
Suzuki T, Yaguchi K, Suzuki S, Suga T (2001) Monitoring of phthalic acid monoesters in river water by solid-phase extraction and GC-MS determination. Environ Sci Technol 18:3757–3763
Tao S, Deng BS, Hermosin B, Saiz-Jimenez C (1993) Identification of organic pollutants in agricultural soils from Tianjin, China. Fresenius Environ Bull 11:677–682
Tian H, Shu W, Qiu Z, Chen Ji-an, Zhao Q (2004) Primary study of estrogenic pollutants in drinking water in a city on the Yangtze Rive. Acad Med Militaris Tertiae 19:1751–1754
Wang JL, Liu P, Qian Y (1995) Microbial degradation of di-n-butyl phthalate. Chemosphere 31:4051–4056
Wang JL, Liu P, Shi H, Qian Y (1997) Kinetics of phthalic acid ester degradation by acclimated activated sludge. Process Biochem 32:567–571
Wolf NL, Burns LA, Steen WC (1980) Phthalate ester hydrolysis: linear free energy relationship. Chemosphere 9:939–942
Wu SP, Tao S, Xu FL, Dawson R, Lan T, Li BG, Cao J (2005) Polycyclic aromatic hydrocarbons in dustfall in Tianjin, China. Sci Total Environ 345:115–126
Zeng F, FU JM, Sheng GY (1999) Study progress on biodegradation of PAEs organic pollutants. Prog Environ Sci 7(4):1–13
Acknowledgement
This work was supported by the National Natural Science Foundation of China (grant no. 50408027), the Youth Foundation of Sichuan Province Education Department of China (grant no. 2004B004), and the Key Project of Ministry of Science and Technology of China (grant no. 2003BA869C and no. 2003BA903B03-02). We thank Mr. Xuekui Zhang of Chongqing Municipal Center of Disease Control and Prevention for helping detect the degradation products of DEHP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiang Li and Ji-an Chen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, Ja., Li, X., Li, J. et al. Degradation of environmental endocrine disruptor di-2-ethylhexyl phthalate by a newly discovered bacterium, Microbacterium sp. strain CQ0110Y. Appl Microbiol Biotechnol 74, 676–682 (2007). https://doi.org/10.1007/s00253-006-0700-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0700-3