Abstract
The prebiotic effect of a pectic oligosaccharide-rich extract enzymatically derived from bergamot peel was studied using pure and mixed cultures of human faecal bacteria. This was compared to the prebiotic effect of fructo-oligosaccharides (FOS). Individual species of bifidobacteria and lactobacilli responded positively to the addition of the bergamot extract, which contained oligosaccharides in the range of three to seven. Fermentation studies were also carried out in controlled pH batch mixed human faecal cultures and changes in gut bacterial groups were monitored over 24 h by fluorescent in situ hybridisation, a culture-independent microbial assessment. Addition of the bergamot oligosaccharides (BOS) resulted in a high increase in the number of bifidobacteria and lactobacilli, whereas the clostridial population decreased. A prebiotic index (PI) was calculated for both FOS and BOS after 10 and 24 h incubation. Generally, higher PI scores were obtained after 10 h incubation, with BOS showing a greater value (6.90) than FOS (6.12).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidemiological evidence points towards an increased intake of fruits and vegetables as part of a healthier diet (Dauchet et al. 2004). Despite this, uptake of the recommended levels is poor, with current estimates being that less than 10% of consumers eat five or more portions of fruits and vegetables each day. Functional foods are defined as dietary components that may result in physiological effects on the consumer leading to justifiable health claims (Roberfroid 1996). While not adequate substitutes for a balanced healthy diet, they do offer a further choice for the consumer. A prebiotic is defined as “a nondigestible food ingredient which beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon and thus improving host health” (Gibson and Roberfroid 1995). Prebiotics of proven efficacy are able to modulate the gut microbiota by stimulating indigenous beneficial flora components while suppressing, or not affecting, less desirable bacteria, such as proteolytic bacteroides and clostridia (Tuohy et al. 2001). They may contribute to the natural defence against infection in infant health by promoting a proliferation of bifidobacteria, usually correlated with a lower risk of intestinal infection (Vandenplas 2002). Prebiotics have also been reported to indirectly lead to a reduction in serum triglyceride levels (Williams and Jackson 2002). In addition, there is evidence showing that prebiotics may indirectly affect mineral absorption in the large bowel and show beneficial effects against inflammatory bowel diseases by stimulating butyrate production and thus accelerating the mucosal cell proliferation and healing processes (Roberfroid 2000; Bamba et al. 2002). Although any dietary material that enters the large intestine can be considered as potentially prebiotic, currently, the most well known prebiotics are non-digestible oligosaccharides (Gibson et al. 2000). Different oligosaccharides with prebiotic properties are commercially available, such as inulin, fructo-oligosaccharides (FOS), galacto-oligosaccharides and lactulose, but currently there is increasing interest in the identification and development of new prebiotic compounds, perhaps with added functionality (Menne et al. 2000; Rao 2001; Tuohy et al. 2002).
Bergamot (Citrus bergamia Risso) peel is the primary by-product of the processed fruit after the extraction of essential oils and juice and represents about 60% of the dry weight of the fruit. If not processed further, it becomes waste and can give rise to many economic and environmental problems because of its fermentability. However, bergamot peel still contains high-value compounds, such as pectin and polyphenols, e.g. flavonoids (Mandalari et al. 2006). Pectins are complex polysaccharides composed largely of anhydrogalacturonic acid units. The carboxyl groups of galacturonic acid are partially esterified by methyl groups, and some of the hydroxyl groups may be acetylated. The primary chain consists of α-d-galacturonate units linked α-(1→4), with 2–4% of l-rhamnose units linked β-(1→2) and β-(1→4) to the galacturonate units. Pectins are commercially extracted from Citrus peel or apple pomace and they are used in the food industry as natural ingredients due to their gelling capacity (Thakur et al. 1997; May 1990).
Pectic substances are hydrolysed by the action of pectinases or pectolytic enzymes that are widely distributed in higher plants and microorganisms (Jayani et al. 2006). We have shown that incubating bergamot peel for 2 h with a commercial enzyme preparation from Aspergillus sp. (pectinase 62L) produced a material rich in oligosaccharides, while the flavonoid components were partially deglycosylated by the action of β-glucosidases and α-rhamnosidases. Here, we describe the potential prebiotic properties of an enzyme-solubilised, oligosaccharide-containing extract prepared from the pectinaceous cell wall components of bergamot peel using in vitro fermentations with representative human gut bacteria (pure and mixed cultures).
Materials and methods
Material
Bergamot peel, consisting of a mix of the three major cultivars, Fantastico (90%), Femminello (5%) and Castagnaro (5%), was obtained from a bergamot processing factory in southern Italy, and an alcohol insoluble residue (AIR) was prepared as previously described (Mandalari et al. 2006). Pectinase 62L (endogalacturonase, 1,060 U/ml) was obtained from Biocatalysts (Cefn Coed, Wales, UK). Polygalacturonic acid was purchased from Sigma Chemical (Dorset, UK). All flavone and flavanone glycoside substrates and aglycones were obtained from Extrasynthese (Genay, France). Inulin-type FOS (Raftilose P-95) were acquired from Orafti (Kent, UK).
Bacterial strains
The following bacteria were used for pure culture growth experiments and were obtained from the in-house culture collection of the Institute of Food Research: Lactobacillus casei; Lactobacillus paracasei; Lactobacillus rhamnosus; Bacteroides fragilis; Bacteroides distonis; Clostridium perfringens strains FD00385, FD00389, FD00412, FD00413, 24B730201 and 26B2540598; Bifidobacterium pseudocatenulatum; Bifidobacterium longum; Bifidobacterium infantis; Bifidobacterium breve; Bifidobacterium animalis subsp lactis and B. animalis subsp animalis. Lactobacillus strains were grown aerobically at 37°C on deMan–Rogosa–Sharp broth (Oxoid, Basingstoke, Hampshire, UK), pH 6.2. Bacteroides and Bifidobacterium strains were grown anaerobically at 37°C on brain heart infusion medium (Difco, Augsburg, Germany) pH 7.2. Clostridia strains were grown on reinforced clostridial medium, pH 7.2, containing (per litre) bacteriological peptone (Oxoid) 10 g, Lablemco (Oxoid) 8 g, yeast extract (Oxoid) 2.4 g, cystein.HCl 0.5 g, glucose 5 g, sodium acetate 5 g, soluble starch 1 g and agar 0.5 g. Bacterial growth was followed spectophotometrically at A600 on a Bioscreen C (Labsystems, Helsinki, Finland).
Preparation of bergamot oligosaccharide (BOS)
The oligosaccharide fraction used for prebiotic testing was obtained by enzymatic treatment of bergamot peel (1 g) with 10 U polygalacturonase-equivalent activity of pectinase 62L in 50 mM Na-acetate buffer, pH 5.0, for 2 h in a shaking incubator (37°C, 100 rpm). Polygalacturonase (PGase) activity was determined against 1% (w/v) orange peel pectin (Mandalari et al. 2006). One unit of activity was defined as the amount of enzyme required to release 1 μmol galacturonic acid min−1 at 37°C, pH 5.0. The soluble fraction was analysed for sugars, oligosaccharide profile (molecular weight distribution) and flavonoid composition.
Sugar analysis
Hydrolysed monosaccharides were analysed as their alditol acetates by gas chromatography (Blakeney et al. 1983). Total uronic acid content was determined by the colorimetric method of Blumenkranz and Asboe-Hansen (1973). Free glucose was measured in bergamot oligosaccharide (BOS) by a glucose assay kit (Sigma) following the manufacturer’s instructions.
Flavonoid glycoside and aglycone analysis
The flavonoid glycosides and aglycones released in the fraction after the enzyme treatment were analysed using a Phenomenex Luna C18 reverse-phase column (250×4.6 mm, 5 μm) in combination with a standard quaternary Agilent HP1100 high-performance liquid chromatography with diode-array detector as previously described (Mandalari et al. 2006).
Soluble protein
Protein concentration in the oligosaccharide fraction after enzyme treatment was determined by the method of Bradford (1976) using the Coomassie Protein Assay Reagent from Pierce (Chester, UK).
Size exclusion chromatography
The molecular weight distribution of the post-enzyme AIR soluble fraction was analysed using size-exclusion chromatography. The fractionation system comprised of a triplet of TSK-GEL stainless steel columns (G3000PW, G4000PW, G6000PW) arranged in series, following a method described by Hizukuri and Takagi (1984). The mobile phase (Na-acetate buffer, pH 5.0) was filtered at source. Samples (30 μl) were loaded onto the column at a flow rate of 0.5 ml/min and peaks monitored by a Gilson 132 RI detector (Anachem, Luton, Bedfordshire, UK) operated in analytical mode, with a cell volume of 8 μl per channel. Data were recorded and analysed using a Hewlett Packard “Chemstation” (Palo Alto, CA, USA). Column calibration was performed with a variety of standards, principally pullulans (Polymer Labs, Church Stretton, Shropshire, UK). A typical run time for each sample was 90 min.
Batch fermentations
Water-jacketed fermenters were filled with 135 ml of pre-sterilised basal growth medium (peptone water 2 g/l, yeast extract 2 g/l, NaCl 0.1 g/l, K2HPO4 0.04 g/l, KH2PO4 0.04 g/l, MgSO4.7H2O 0.01 g/l, CaCl2.6H2O 0.01 g/l, NaHCO3 2 g/l, Tween 80 2 ml, hemin 0.02 g/l, vitamin K1 10 μl, cysteine HCl 0.5 g/l, bile salts 0.5 g/l, pH 7.0) and inoculated with 15 ml of faecal slurry. Before addition of the faecal slurry, prepared by homogenising 10% (w/v) freshly voided faecal material in 0.1 M phosphate-buffered saline (PBS), pH 7.0, the oligosaccharide fraction was added to give a final concentration of 1% (w/v). Each vessel was magnetically stirred and the temperature set at 37°C by a circulating water bath. Culture pH was controlled automatically and maintained at pH 6.8. Anaerobic conditions were maintained by sparging the vessels with oxygen-free nitrogen gas at 15 ml/min. Samples (5 ml) were taken from each vessel at the start (T0) and at intervals over a 24-h incubation period.
Enumeration of bacteria
Bacteria were counted using fluorescent in situ hybridisation (FISH), as described by Rycroft et al. (2001). Samples were diluted four times in 4% (w/v) filtered paraformaldehyde and fixed overnight at 4°C. Samples were then washed twice with filtered PBS and stored at −20°C in PBS/ethanol (1:1, v/v) until further analysis. Hybridisation was carried out at the appropriate temperature using genus-specific 16S rRNA-targeted oligonucleotide probes labelled with the fluorescent dye Cy3 for the different bacterial groups or with 4′,6-diamidino-2-phenylindole for total cell counts. The probes used were Bif 164 specific for Bifidobacterium (Langendijk et al. 1995), Bac 303 specific for Bacteroides (Manz et al. 1996), Lab 158 specific for Lactobacillus/Enterococcus spp. (Harmsen et al. 1999), His 150 specific for Clostridium (C. perfringens/hystolyticum subgroup) (Franks et al. 1998), EREC 482 specific for Eubacterium (Clostridium coccoides–Eubacterium rectale group) (Franks et al. 1998). Hybridised mixture was then filtered using a 0.2-μm membrane filter (Millipore, Edinburgh, UK) and cells were counted using a Nikon Microphot fluorescent microscope. At least 15 random fields were counted on each slide.
Statistical analysis
Differences between bacterial numbers at 0, 10 and 24 h of fermentation for each batch culture were checked for significance by paired t test, assuming equal variances and considering both sides of distribution. Differences were significant at P<0.05.
Results
Oligosaccharide characterisation
The sugar composition of the oligosaccharide extract derived from bergamot peel AIR after treatment with pectinase 62L is shown in Table 1. While glucose was the main sugar present in the AIR (35% of the total), galacturonic acid represents the major component of the solubilised oligosaccharide fraction (63% of the total), showing the preferential enzyme-catalysed solubilisation of pectinaceous material. Arabinose, galactose, glucose, rhamnose, xylose and mannose were present in smaller quantities in both samples. Only traces of fucose were detected. There was virtually no free glucose present in the soluble BOS fraction.
Previously, it has been shown that the flavonoid content, present as glycosides, in the AIR was very low in the range of 9.5 μg/mg (Mandalari et al. 2006). As expected, the flavonoid content of the BOS fraction derived from the bergamot AIR was negligible (0.5 μg/mg of total flavone glucosides and 5.7 μg/mg of total flavanone glycosides, corresponding to 5% of total flavonoids of the AIR). Only trace amounts of protein (6.2 μg/mg) were detected in the BOS fractions.
The molecular weight distribution of the fraction obtained after enzyme treatment consistently showed the presence of only one peak corresponding to oligomers of molecular weights between 1,400 and 1,700 KDa. This corresponds to potential degree of polymerisation values between 3 and 7.
Pure culture growth experiments
Bacterial strains were initially tested for their ability to ferment the oligosaccharide fraction (Table 2). Generally, bifidobacteria showed higher growth rates than those of the other strains tested. These results agree with those previously obtained by Olano-Martin et al. (2002), where most of the bifidobacteria tested had high growth rates on pectic oligosaccharides (POS) derived from orange peel. Both clostridia and bacteroides showed low growth on the oligosaccharide fraction, while lactobacilli, with the exception of L. rhamnosus, grew better than all the clostridia or bacteroides tested.
Batch culture fermentations
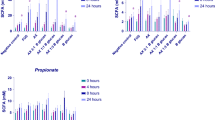
Batch culture fermenters were used to monitor the effect of BOS and FOS addition on the mixed bacterial populations. Samples were removed at intervals and FISH was used to quantify the levels of different bacterial groups (Fig. 1). The results shown in Table 3 indicate that a significant increase in the levels of total bacteria was seen with both FOS and BOS after 10 and 24 h of incubation. Generally, a decrease in clostridia and an increase in bifidobacteria were observed in response to both FOS and BOS oligosaccharides. After 24 h of incubation, the bacteroides population numbers were similar to those at T0. An increase in the number of lactic acid bacteria was observed with both FOS and BOS fractions, the latter showing a greater increase. Both oligosaccharides also significantly increased the numbers of eubacteria, with increases of 0.28 and 0.30 log recorded after 10 h and 0.21 and 0.55 log recorded after 24 h for FOS and BOS addition, respectively (Fig. 1).
Differences in the bacterial population size [black bars fructo-oligosaccharides (FOS); white bars bergamot oligosaccharide fraction (BOS)] compared to the total bacteria counted [(selected bacterial numbers at 24 h/total bacteria counted at 24 h)−(selected bacterial numbers at 0 h/total bacteria counted at 0 h)]
To obtain a general quantitative measure of the prebiotic effect, a prebiotic index (PI) was calculated for the oligosaccharide fractions (Palframan et al. 2003; Sanz et al. 2005). The PI equation is described as follows: PI=(Bif/Total)+(Lac/Total)+(Erec/Total)−(Bac/Total)−(Clos/Total), where Bif is bifidobacterial numbers at sample time/numbers at inoculation, Lac is lactobacilli numbers at sample time/numbers at inoculation, Erec is eubacteria numbers at sample time/numbers at inoculation, Bac is bacteroides numbers at sample time/numbers at inoculation, Clos is clostridia numbers at sample time/numbers at inoculation and Total is the total bacteria numbers at sample time/numbers at inoculation. The PI represents a comparative relationship between the growth of “beneficial” bacteria, such as bifidobacteria, lactobacilli and eubacteria, and that of the “undesirable” ones, such as clostridia and bacteroides, in relation to the changes of the total number of bacteria (Fig. 2). Values at 10 h of incubation were higher than those at 24 h in all of the samples. The BOS fraction produced the greatest PI values of 6.90 and 6.55, followed by FOS with 6.12 and 3.43, at 10 and 24 h of incubation, respectively.
Discussion
The present study has demonstrated the prebiotic potential of a POS-rich fraction generated from a by-product of the Citrus-fruit-processing industry treated with a fungal pectinolytic preparation. Recently, it has been demonstrated that POS from orange peel showed prebiotic properties increasing the bifidobacterial and E. rectale numbers (Manderson et al. 2005). Bergamot is mostly used for the extraction of its essential oil, which is widely used in cosmetic and food industries (Salah et al. 1995). Bergamot peel, which consists of 60% of the total weight of the fruit, is the primary by-product of the essential oil extraction and, if not processed further, becomes waste and gives rise to serious environmental pollution.
Modulation of the gut microflora composition using prebiotics is a functional aspect of gut microbiology. It is believed that the colonic microflora plays an important role in host health, and this may be fortified by targeting beneficial colonic bacteria, e.g. a reduced risk of colorectal cancer (Rowland 1988; Olano-Martin et al. 2000). Furthermore, lactic acid bacteria have immunomodulatory effects influencing the interactions between bacterial cell wall components and immune cells, and it is believed that prebiotics may have a similar effect (Schiffrin et al. 1995). There is also evidence that lactic acid bacteria, and hence, prebiotics that increase lactic acid bacterial growth, influence blood lipids leading to a reduction of total and LDL cholesterol levels (Delzenne and Kok 1999). One of the strongest health benefits proposed for prebiotics is the barrier function against invading gastrointestinal pathogens, such as campylobacters, salmonellas and Escherichia coli (Gibson and Roberfroid 1995). Therefore, attempts to identify and develop novel, enhanced prebiotics as functional foods that are able to modulate the composition of human colonic microflora are currently of great interest.
To evaluate the prebiotic activity of new compounds, it is necessary to analyse the evolution of the individual and mixed bacterial population in the presence of the carbohydrate source being tested. The behaviour of different bacteria in the fermentation of a carbohydrate source could vary in pure and mixed cultures due to synergistic, antagonistic and/or competitive effects. The fermentation process in the gut is a complex process whereby many metabolic pathways are carried out by different groups of bacteria. The end products from one group could be metabolised by others that cannot directly metabolise the original source substrate (Gibson and Roberfroid 1995). Therefore, in this study, the effects of the BOS fraction were tested using both pure and pH-controlled mixed cultures to evaluate the potential prebiotic effect of bergamot-derived pectic-oligosaccharides.
When a prebiotic arrives in the colon, it should usually be selectively fermented by probiotic bacterial genera, such as Bifidobacterium and Lactobacillus (Gibson and Roberfroid 1995). Previous studies have demonstrated that POS have a bifidogenic effect, and selected bifidobacteria showed high growth rates on these substrates (Olano-Martin et al. 2002). In this study, the majority of the bifidobacteria grew better on the bergamot oligosasaccharides compared to the other selected gut bacteria. On the other hand, all of the Bacteroides and Clostridium species showed very low growth on the BOS substrate. This clearly indicates that the BOS may allow a prebiotic effect by preferentially enhancing the growth of bifidobacteria and reducing the growth of bacteroides and clostridia. The presence of bergamot extract induced the growth of both bifidobacteria and lactobacilli, while only bifidobacteria growth was enhanced with orange peel substrates (Olano-Martin et al. 2002).
In mixed culture the behaviour of BOS was similar. Bifidobacteria and lactobacilli grew well and their numbers significantly increased by 1.06 and 0.75 log compared to T0, whereas clostridia numbers decreased by 0.28 log and bacteroides levels were not significantly altered. At both 10 and 24 h the PI values were higher than FOS, for which prebiotic effects have consistently been demonstrated in different human volunteer studies (Gibson et al. 1995; Roberfroid et al. 1998). After 24 h, the PI score of BOS slightly decreased, while the decrease in the PI value with FOS was even greater. This is probably due to a different composition of the two materials; FOS being utilised quicker than BOS. A different pattern in PI scores was reported by Manderson et al. (2005), where the PI of an orange peel POS increased between 10 and 24 h. This is probably the reflection of differences in the structural composition of the two oligosaccharide materials and also variation in the bacterial composition of the faecal material used. In contrast to BOS, the PI values of POS were reported to be lower than those of FOS.
On the basis of the data obtained through in vitro fermentations, oligosaccharides from bergamot exhibited a potential to be used as an effective source of prebiotics, increasing the populations of bifidobacteria, lactobacilli and eubacteria and decreasing the numbers of clostridia. Although these in vitro studies show promising prebiotic function for BOS to be effective in vivo, more detailed studies on the digestibility of BOS need to be performed using human volunteers.
References
Bamba T, Kanauchi O, Andoh A, Fujiyama Y (2002) A new prebiotic from germinated barley for nutraceutical treatment of ulcerative colitis. J Gastroenterol Hepatol 17:818–824
Blakeney AB, Harris PJ, Henry RJ, Stone BA (1983) A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr Res 113:291–299
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Bradford M (1976) A rapid and sensitive method for the quantisation of microgram quantities of protein utilising the principle of protein-dyebinding. Anal Biochem 72:248–254
Dauchet L, Ferrières J, Arveiler D, Yarnell JW, Gey F, Ducimetière P, Ruidavets J-B, Haas B, Evans A, Bingham A, Amouyel P, Dallongeville J (2004) Frequency of fruit and vegetable consumption and coronary heart disease in France and Northern Ireland: the PRIME study. Br J Nutr 92:963–972
Delzenne NM, Kok NN (1999) Biochemical basis of oligofructose-induced hypolipidemia in animal models. J Nutr 129:1467S–1470S
Franks AH, Harmsen HJM, Raangs GC, Jansen GJ, Schut F, Welling GW (1998) Variations of bacterial populations in human faeces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol 64:3336–3345
Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concepts of prebiotics. J Nutr 125:1401–1412
Gibson GR, Beatty ER, Wang X, Cummings JH (1995) Selected stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108:975–982
Gibson GR, Ottaway PB, Rastall RA (2000) Prebiotics: new developments in functional foods. Chandos, Oxford, UK
Harmsen HJM, Elferrich P, Schut F, Welling GW (1999) A 16S rRNA-targeted probe for detection of lactobacilli and enterococci in faecal samples by fluorescent in situ hybridization. Microb Ecol Health Dis 11:3–12
Hizukuri S, Takagi T (1984) Estimation of the distribution of molecular weight for amylase by the low-angle laser-light-scattering technique combined with high-performance gel chromatography. Carbohydr Res 134:1–10
Jayani RS, Saxena S, Gupta R (2005) Microbial pectinolytic enzymes: a review. Process Biochem 40:2931–2944
Langendijk PS, Schut F, Jansen GJ, Raangs GC, Kamphuis GR, Wilkinson MHF, Welling GW (1995) Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in faecal samples. Appl Environ Microbiol 61:3069–3075
Mandalari G, Bennett RN, Bisignano G, Saija A, Dugo G, Lo Curto R, Faulds CB, Waldron KW (2006) Characterization of flavonoids and pectins from bergamot (Citrus bergamia Risso) peel, a major by-product of essential oil extraction. J Agric Food Chem 54:197–203
Manderson K, Pinart M, Tuohy KM, Grace WE, Hotchkiss AT, Widmer W, Yadhav MP, Gibson GR, Rastall RA (2005) In vitro determination of prebiotic properties of oligosaccharides derived from an orange juice manufacturing by-product stream. Appl Environ Microbiol 71:8383–8389
Manz W, Amann R, Ludwig W, Vancanneyt M, Schleifer KH (1996) Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum cytophaga-flavobacter-bacteroides in the natural environment. Microbiology 142:1097–1106
May CD (1990) Industrial pectins: Sources, production and applications. Carbohydr Polym 12:79–99
Menne E, Guggenbuhl N, Roberfroid M (2000) Fn-type chicory inulin hydrolysate has a prebiotic effect in humans. J Nutr 130:1197–1199
Olano-Martin E, Mountzouris KC, Gibson GR, Rastall RA (2000) In vitro fermentability of dextran, oligodextran and maltodextrin by human gut bacteria. Br J Nutr 83:247–255
Olano-Martin E, Gibson GR, Rastall RA (2002) Comparison of the in vitro bifidogenic properties of pectins and pectic-oligosaccharides. J Appl Microbiol 93:505–511
Palframan R, Gibson GR, Rastall RA (2003) Development of a quantitative tool for the comparison of the prebiotic effect of dietary oligosaccharides. Lett Appl Microbiol 37:281–284
Rao VA (2001) The prebiotic properties of oligofructose at low intake levels. Nutr Res 6:843–848
Roberfroid MB (1996) Functional effects of food components and the gastrointestinal system: chicory fructooligosaccharides. Nutr Rev 54:S38–S42
Roberfroid MB (2000) Prebiotics and probiotics: are they functional food? Am J Clin Nutr 71:1682s–1687s
Roberfroid MB, Van Loo JAE, Gibson GR (1998) The bifidogenic nature of chicory inulin and its hydrolysis products. J Nutr 128:11–19
Rowland IR (1988) Role of the gut microflora in toxicity and cancer. Academic, London
Rycroft CE, Jones MR, Gibson GR, Rastall RA (2001) A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J Appl Microbiol 91:878–887
Salah N, Miller NJ, Paganga G, Tijburg L, Bolwell GP, Rice-Evans C (1995) Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch Biochem Biophys 2:339–346
Sanz ML, Gibson GR, Rastall RA (2005) Influence of disaccharide structure on prebiotic selectivity in vitro. J Agric Food Chem 53:5192–5199
Schiffrin EJ, Rochat F, Link-Amster H, Aeschlimann JM, Donnet-Hugues A (1995) Immune system stimulation by probiotics. J Dairy Sci 78:1597–1606
Thakur BR, Rakesh KS, Handa AK (1997) Chemistry and uses of pectin—a review. Crit Rev Food Sci Nutr 37:47–73
Tuohy KM, Kolida S, Lustenberger AM, Gibson GR (2001) The prebiotic effects of biscuits containing partially hydrolysed guar gum and fructo-oligosaccharides—a human volunteer study. Br J Nutr 86:341–348
Tuohy KM, Ziemer CJ, Klinder A, Knöbel Y, Pool-Zobel BL, Gibson GR (2002) A human volunteer study to determine the prebiotic effects of lactulose powder on human colonic microbiota. Microb Ecol Health Dis 14:165–173
Vandenplas Y (2002) Oligosaccharides in infant formula. Br J Nutr 87:S293–S296
Williams CM, Jackson KG (2002) Inulin and oligofructose: effects on lipid metabolism from human studies. Br J Nutr 87:S261–S264
Acknowledgements
We would like to thank Grant Colder (John Innes Centre, Norwich, UK) for his help in microscopy. We gratefully acknowledge the help provided by Yvan Lemarc (Institute of Food Research) with the statistic analyses. This research was funded by the Biotechnology and Biological Research Sciences Council (UK), by the University of Messina (Italy) and by Ministero dell’Universita’ e della Ricerca, Italy (project no. 12930).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mandalari, G., Nueno Palop, C., Tuohy, K. et al. In vitro evaluation of the prebiotic activity of a pectic oligosaccharide-rich extract enzymatically derived from bergamot peel. Appl Microbiol Biotechnol 73, 1173–1179 (2007). https://doi.org/10.1007/s00253-006-0561-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0561-9