Abstract
A maltooligosaccharide-forming α-amylase was produced by a new soil isolate Bacillus subtilis KCC103. In contrast to other Bacillus species, the synthesis of α-amylase in KCC103 was not catabolite-repressed. The α-amylase was purified in one step using anion exchange chromatography after concentration of crude enzyme by acetone precipitation. The purified α-amylase had a molecular mass of 53 kDa. It was highly active over a broad pH range from 5 to 7 and stable in a wide pH range between 4 and 9. Though optimum temperature was 65–70 °C, it was rapidly deactivated at 70 °C with a half-life of 7 min and at 50 °C, the half-life was 94 min. The K m and V max for starch hydrolysis were 2.6 mg ml−1 and 909 U mg−1, respectively. Ca2+ did not enhance the activity and stability of the enzyme; however, EDTA (50 mM) abolished 50% of the activity. Hg2+, Ag2+, and p-hydroxymercurybenzoate severely inhibited the activity indicating the role of sulfydryl group in catalysis. The α-amylase displayed endolytic activity and formed maltooligosaccharides on hydrolysis of soluble starch at pH 4 and 7. Small maltooligosaccharides (D2–D4) were formed more predominantly than larger maltooligosaccharides (D5–D7). This maltooligosaccharide forming endo-α-amylase is useful in bread making as an antistaling agent and it can be produced economically using low-cost sugarcane bagasse.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
α-Amylases (E.C. 3.2.1.1) which catalyze the hydrolysis of α-d-(1,4) glycosidic linkages in starch are widely used in industrial applications involving sugar, baking, ethanol, and detergents (Kirk et al. 2002). There is a growing interest in production of amylases that are capable of forming maltooligosaccharides (MOS) for diverse applications like coating agents, viscosity providers, flavor carriers, and crystallization inhibitors (Palacios et al. 2004). Bacterial amylases producing large amount of residual dextrins of degree of polymerization (DP) 3–9 were reported to stop retrogradation of bread (Martin and Hoseney 1991). Duran et al. (2001) found that MOS ranging from maltotriose (G3) to maltopentaose (G5) reduce retrogradation and act as an antifirming agent. It was suggested that G3 and G4 were directly responsible for retardation of retrogradation in bread and these oligomers are of right size to interfere with starch–gluten interactions (Min et al. 1998). They also reported that maltose (G2) is less effective in prevention of bread staling which was suggested to be due to its small size and its ability to diffuse easily. Amylases that are intermediate temperature-stable and active at about 65–70 °C are recommended to be suited for the purpose of retarding staling rather than thermostable enzymes which survive oven temperature and cause overhydrolyzation of starch and result in gumminess of the final products (Gray and Bemiller 2003). Therefore, amylases with the properties of producing MOS with major small oligosaccharides (DP 3–4) and thermostable at intermediate temperature are potential antistaling agents in bread making industry.
Bacillus sp. is well known to produce saccharifying and liquefying amylases. Many Bacillus amylases have been reported to produce specific larger MOS of DP 5–7 those include G7-forming amylase from B. subtilis US116 (Messaoud et al. 2003), G6-forming amylases from Bacillus halodrans LBK (Hashim et al. 2005), and Bacillus stearothermophilus US100 (Ali et al. 2001), G5-forming amylase from Bacillus circulans (Morgan and Priest 1981). A few amylases are known to produce small MOS (DP 3–4) those include G4-forming amylase from Bacillus strains GM8901 (Kim et al. 1995) and a G3-forming amylase from B. subtilis (Takasaki 1985).
A B. circulans amylase has been reported to produce mixed-MOS with major G4 and G5 (Dey et al. 2002). In this study, we report an α-amylase (AmyCR) from the new soil isolate B. subtilis KCC103 which produced various MOS of DP 3–7 and it would be suitable as an antistaling agent. It is interesting to note that in contrast to reported Bacillus amylases, the synthesis of amylase in the new strain was not catabolite repressed by glucose; hence, it would be useful for economical production of this enzyme using low-cost substrates.
Materials and methods
Organism
The organism used was B. subtilis strain KCC103 that was isolated from a soil sample collected from the forested area inside the IIT Madras premises. Appropriately, diluted soil samples were spread onto nutrient agar base containing soluble starch (1% w/v). After incubation for 48 h, the plates were flooded with a solution of 0.5% (w/v) I2 and 5.0% (w/v) KI. Colonies exhibiting halo of starch hydrolyzing activity were picked. The strain KCC103 that produced relatively high level of amylase was used for further study. The new isolate was identified based on biochemical tests and 16S rRNA gene sequence. 16S rRNA gene fragment from the strain KCC103 was amplified by polymerase chain reaction and sequenced as described (Pattanapipitpaisal et al. 2001). The gene sequence was submitted to GenBank with the accession number AY973493.
Culture conditions
Seed culture for growth experiments was prepared by transferring a single colony from nutrient agar plate into 5 ml liquid nutrient medium and incubated at 37 °C overnight (16–18 h). A 1.0 ml of the seed culture was diluted to 100 ml with sterile nutrient medium [in g/l: beef extract (1.5), yeast extract (1.5), peptone (5.0), and NaCl (5.0)] or mineral medium [in g/l: Na2HPO4 (6.0), KH2PO4 (3.0), NaCl (5.0), NH4Cl (1.0), and MgSO4 (1.0 ml of 1 M)] containing respective sugars and incubated at 37 °C with shaking (200 rpm). Growth was monitored by measuring absorbance at 600 nm [OD of 1=0.3 (mg dry wt cells) ml−1]. Amylase production was followed by measuring amylase activity in the culture supernatant.
Sugarcane bagasse hydrolysis
To prepare the hydrolysates, 10 g of washed, oven-dried at 60 °C, and milled bagasse was suspended in a solution containing 100 ml of distilled water and 0.5 ml of concentrate H2SO4 and then autoclaved (1 atm) for 20 min. The liquid fraction was separated by filtration on Buchner funnel, using Whatman no. 1 paper, the pH was adjusted to 7.0 using NaOH, and the total sugar concentration was determined using 2,5-dinitrosalicylic acid (DNS) (Analytical grade) reagent. Sugars present in the hydrolysate were identified by high performance liquid chromatography (HPLC) with aminex HPX-87H ion exclusion column (300×7.8 mm; Agilent, USA) at 65 °C using 0.005 M sulfuric acid as mobile phase at a flow rate of 0.6 ml min−1 and the products were detected using refractive index detector maintained at 50 °C. Authentic chromatographic grade glucose, xylose, and arabinose (Sigma) were used as standards for identification and quantification of the sugars in the hydrolysate.
Enzyme assay
A 1.0-ml reaction mixture containing 0.5%(w/v) soluble starch (Sigma) and 50 μl of the enzyme sample in 50 mM sodium phosphate buffer (pH 6) was incubated at 50 °C for 10 min and the amount of reducing sugars released was measured using 2,5-dinitrosalicylic acid (Analytical grade) reagent. One unit of enzyme activity was defined as the amount of enzyme that liberated 1 μmol of reducing sugar as glucose equivalents in 1 min under the assay conditions. Protein was quantified by Lowry’s method using bovine serum albumin as a standard protein.
Purification of α-amylase
To the culture supernatant (25 ml), cold acetone was added (70% v/v) and left at 4 °C for an hour. After centrifugation (16,000 rpm, 4 °C) the precipitate was redissolved in 10 mM sodium phosphate buffer (1/10 volume of supernatant) of pH 6.0. The sample was applied to DEAE-Sephadex A50 (Pharmacia) anion exchange column (1.5×5 cm) preequilibrated with 10 mM sodium phosphate buffer at room temperature (28±1 °C) using preparative low pressure liquid chromatography (BiologicLP, Bio-Rad, USA). The unbound proteins from the column were removed by washing with the same buffer at a flow rate of 0.5 ml min−1. Proteins bound to the column were eluted by passing the same buffer containing NaCl in step gradient [20% (w/v) NaCl] through the column at the same flow rate, and fractions of 2.0 ml were collected with online monitoring of protein elution at 280 nm. Fractions with α-amylase activity showing single protein band on denaturing polyacrylamide gel electrophoresis were pooled, dialyzed against 10 mM sodium phosphate buffer, and used as the purified enzyme.
Electrophoretic analysis
Denaturing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out as described by Laemmli and the zymogram was carried out according to Mitsunaga et al. (2001). The molecular mass of α-amylase was estimated by SDS-PAGE using molecular mass standards [β-galactosidase (116.0 kDa), bovine serum albumin (66.2 kDa), ovalbumin (45 kDa), lactate dehydrogenase (35 kDa), restriction endonuclease Bsp981 (25 kDa), β-lactoglobulin (18.4), and lysozyme (14.4 kDa)] (Fermentas).
Enzymatic reaction product analysis
A 5.0 U of the purified amylase was incubated with 5% w/v soluble starch in 50 mM sodium phosphate (pH 6) at 30 °C and at different time intervals the reaction products were analyzed by HPLC with a Zorbax-Carbohydrate analysis column (4.6×150 mm; Agilent, USA) at 30 °C using a mixture of acetonitrile to water (75:25, v/v) as mobile phase at a flow rate of 1.4 ml min−1 and the products were detected using refractive index detector maintained at 30 °C. Authentic chromatographic grade glucose and MOS (G2–G7) (Sigma) were used as standards for identification and quantification of the MOS in the reaction mixture. To investigate the effect of pH and temperature on MOS-formation during starch hydrolysis, the above reaction was conducted at three different pH (50 mM sodium acetate, pH 4; 50 mM potassium phosphate, pH 7; and 50 mM sodium carbonate, pH 10) and at two different temperatures (30 and 50 °C).
Degree of hydrolysis of starch
Soluble starch was completely hydrolyzed by treating with hydrochloric acid (5 N) at 121 °C for 20 min in an autoclave. Total reducing sugar as glucose equivalents was estimated by DNS method, which was considered as 100% hydrolysis. Degree of hydrolysis of starch by enzymatic hydrolysis was calculated as relative percent of reducing sugars formed in enzymatic hydrolysis compared to acid hydrolysis.
Effect of pH and temperature on α-amylase
α-Amylase activity of the purified enzyme was measured at 50 °C and at different pH using various buffers (50 mM sodium acetate, pH 4–5.5; 50 mM potassium phosphate, pH 5.5–8; and 50 mM sodium carbonate, pH 8–10). To determine the pH stability, the purified enzyme was incubated in different buffers (pH 4.5 to 9.0) at 4 °C for 24 h and the residual α-amylase activity was measured at 50 °C as described. Effect of temperature was studied by measuring enzyme activity between 30 and 80 °C and at pH 6. For thermal stability, enzyme at pH 6 was incubated between 30 to 70 °C, samples were removed at 10 min intervals, cooled in ice bath, and measured the residual amylase activity.
Effect of metal ions and inhibitors
The α-amylase assay was performed in the presence of various mineral salts (1 mM) (AgNO3, BaCl2, CaCl2, CuSO4, FeCl3, MnCl2, NiCl2, HgCl2, and ZnCl2) at pH 6 and 30 °C. The enzyme was treated with thiol group modifying agents (1 mM) p-hydroxymercurybenzoate, iodoacetic acid, and N-ethylmaleimide at 30 °C for 5 min and the residual α-amylase activity of the chemically modified enzyme was determined.
Estimation of kinetic parameters
The kinetic parameters K m and V max were estimated from Lineweaver–Burk plot. The rate of reaction was expressed as the number of micromoles of reducing sugar as glucose equivalents released in 1 min by 1 mg of total protein in the enzyme sample. The rate constant and doubling time of thermal deactivation of α-amylase was estimated from first order rate equation.
Measurement of optical rotation
The optical rotation of hydrolysate of soluble starch produced by the purified amylase was determined according to Paquet et al. (1991). A reaction mixture consisting of 1% (w/v) soluble starch in 50 mM sodium phosphate buffer (pH 6.0) and purified enzyme (5 U ml−1) was incubated at 30 °C. The optical rotation of the mixture was measured at various times in a polarimeter (Perkin Elmer) using sodium line. The mutarotation of the hydrolysate was determined by adding 5.0 mg of solid sodium carbonate per milliliter of mixture after the optical rotation had become almost constant.
Results
Organism
The soil isolate KCC103 is an aerobic, motile, endospore-forming Gram positive rod. It is positive for catalase, nitrate reduction, Voges–Proskaeur, acid from glucose, starch hydrolysis, gelatin hydrolysis, and growth in NaCl (3–7%) tests; and negative for H2S formation, indole, citrate utilization, methylred, gas from glucose, lipid hydrolysis, urea hydrolysis, and NaCl requirement tests. Phylogenetic comparison of the 16 S rRNA gene sequence of KCC103 and those of known Bacillus sp showed closest (99%) match of KCC103 to that of B. subtilis (GenBank accession number Z99104); hence, this strain was identified as B. subtilis. At pH 4, no growth was found and it grew well over a pH range of 5 to 8.5. It grew and produced amylase in a range of temperatures between 25 and 50 °C with optimum at 37 to 40 °C. It produced α-amylase of various levels in growth media containing various sugars such as arabinose, glucose, galactose, fructose, lactose, maltose, mannose, mannitol, sorbitol, sucrose, xylose, glycerol, and cellulose.
α-Amylase production
When B. subtilis KCC103 was grown with soluble starch and or glucose as the carbon sources, α-amylase production began at early exponential phase of growth and reached maximum level at stationary phase (Fig. 1). Addition of glucose to starch medium showed α-amylase production similar to that in starch-grown culture and glucose as a sole carbon source produced α-amylase of about 42% of that in starch medium. Similar trend of α-amylase synthesis was found when cultured in mineral medium containing appropriate sugars but with low growth rate and a maximum activity of only 6.0 U ml−1 (data not shown). This indicates that glucose has not repressed the synthesis of amylase. Other small-MOS G2 and G3, which are the major products of starch hydrolysis, induced amylase production equivalent to starch.
Because amylase production was not inhibited by glucose indicating resistance of the strain to catabolite repression, the amylase production in media containing sugarcane bagasse hydrolysate, which consists of simple sugars was investigated. When the sugarcane hydrolysate was added to growth medium as carbon source, the amylase production was 110 U ml−1 which was equivalent to that in starch medium. It is evident that economical production of amylase is possible using the low-cost agro-by-product sugarcane bagasse, which is rich in lignocellulosics and not starch.
Purification of AmyCR
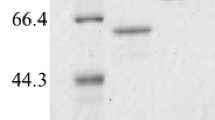
The summary of purification of α-amylase is given in Table 1. α-Amylase was purified by combination of acetone precipitation and anion-exchange column chromatography. α-Amylase was eluted from the DEAE-Sephadex column with buffer containing 20% (w/v) NaCl. The purified α-amylase was obtained with a specific activity of 483 U mg−1 and a yield of 45%. SDS-PAGE analysis of the purified amylase showed single protein band corresponding to 53 kDa and it coincided with the α-amylase activity band found in the zymogram of culture filtrate (Fig. 2).
Properties of α-amylase
The α-amylase was active in a wide pH range with optimum activity at pH 6–7 and was stable over a broad pH range (5 to 9.5) with retention of more than 98% of initial activity. The α-amylase was optimally active at 65 to 70 °C with retention of 80% of the maximum activity at 75 °C. Addition of CaCl2 (1 mM) to the enzyme did not enhance the activity. The amylase was stable at 50 °C with a half-life of 94 min. Though 70 °C was found to be the optimal temperature for activity, the amylase lost 80% of the initial activity after 10 min of incubation with half-life of 7 min (Fig. 3). Similar to activity, stability of the enzyme was also not improved by the addition of CaCl2 to the enzyme which suggested that the α-amylase was not dependent on Ca2+ for its activity and stability. Amylase activity was about 120% for Ca2+ and Fe3+ while 80–90% for Ba2+, Cu2+, Mn2+, Ni2+, and Zn2+. However, the chelating agent EDTA at high concentration (50 mM) abolished 50% of α-amylase activity. The sulfydryl oxidant metals Ag+ and Hg2+ and the sulfydryl modifying reagents p-hydroxymercurybenzoic acid and iodoacetic acid showed severe loss (70–90%) of the α-amylase activity while N-ethylmaleimide did not affect the activity. These results indicate the possible role of cystein residue in catalytic function of the α-amylase. The optical rotation of the starch hydrolysate was measured during hydrolysis of starch. From the mutarotation study, the downward shift of optical rotation on the addition of sodium carbonate to the starch hydrolysate indicated that the hydrolysis products had an α-anomeric configuration and, hence, the enzyme belongs to α-amylase. The apparent K m and V max values, calculated by fitting the rate of hydrolysis into Lineweaver–Burk plot, were 2.6 mg starch ml−1 and 909 U mg protein−1, respectively.
Mode of action of the α-amylase
The degradation of starch by α-amylase was monitored by measuring the reduction in blue color value and production of reducing sugars as a function of time. The profile of reduction in blue value was in great coincidence with the formation of reducing sugars. At the point where the blue value was almost zero, the proportion of reducing sugars in the hydrolysate was about 24%. The shape of curves of starch degradation and product formation suggested that the α-amylase acts on starch by endolytic mode. At pH 7 and at 50 °C, the hydrolysis of starch formed MOS ranging from G3 to G7 along with G2 and glucose (G1) (Fig. 4). The kinetics of MOS formed from starch hydrolysis is shown in Fig. 5. At the early stage (1 to 2 h) of hydrolysis, the proportional level of MOS (G3 to G7) formed was higher than G2 and negligible level of G1 was observed. On further incubation, there was a linear increase in G1 to G4 and slow decrease in the level of G6 and G7 while G5 remained almost constant. After 24 h of hydrolysis the MOS accumulated indicating that they were not further hydrolyzed. The proportional amounts of MOS formed after 24 h of hydrolysis were 44% of G3–G4 as main oligosaccharides with 39% of G1–G2 and 17% of G5 to G7. The trend and proportion of products formed were investigated at acidic and alkaline pH conditions. At acidic pH 4, the product profile was similar to that at pH 7 (Table 2). However, the degree of hydrolysis was about 68% at pH 7 and it was lower at pH 4 (47 to 52%). At alkaline pH 10 hydrolysis of starch was not observed. These results indicate that this MOS-forming amylase can be used in a wide range of pH in acidic side.
Discussion
Zymogram analysis of culture filtrate from all the growth phases and culture media containing starch and or glucose showed a single α-amylase activity band. These results strongly suggested that B. subtilis KCC103 synthesized one α-amylase and its synthesis was not catabolite repressed. Furthermore, we investigated the production of α-amylase using sugarcane bagasse hydrolysate, which consists of simple sugars such as glucose, xylose, and arabinose. The sugarcane bagasse hydrolysate supported amylase production equivalent to starch medium which shows the feasibility of amylase production using low-cost substrate sugarcane bagasse. Despite reports on using wheat bran as the carbon source for production of amylase by Bacillus strains (Ikram-ul-Haq et al. 2003), use of sugarcane bagasse as carbon source has not been reported. In the case of other Bacillus strains, use of bagasse as carbon source could have not been possible because the glucose in the bagasse hydrolysate repress amylase synthesis in those strains. Catabolite repression of amylase synthesis in Bacillus amylases is very well documented (Dahl 2002). Therefore, it is clear that the tolerance to catabolite repression in the present strain confers capacity to coproduce amylase during catabolism of simple sugars and mechanism of amylase synthesis differs from other Bacillus strains. These results show that component sugars in the bagasse hydrolysate are not inhibitory for cell growth and amylase production that this strain would be used for economical production of amylase using sugarcane bagasse.
The AmyCR was active in the range of pH and temperature as reported for B. subtilis saccharifying α-amylases (Messaoud et al. 2003; Ali et al. 2001). Though it is stable over a wide pH range, it has moderate thermostability up to 50 °C. Addition of CaCl2 to the enzyme was not necessary for the optimum activity and thermostability, while most of the Bacillus amylases require Ca2+ for maximum activity. However, EDTA at high concentration (50 mM) partially abolished the activity of AmyCR. Like AmyCR, some calcium independent amylases have also been reported (Hagihara et al. 2001). AmyCR was severely inhibited by sulfydryl oxidant metal ions Ag+ and Hg2+ showing the possible role of cystein residues in the catalysis. This is supported by the inhibition of the α-amylase activity by p-hydroxymercurybenzoate, which is highly specific thiol group modifier. Similar type of inhibition by thiol group specific agents has been reported for Bacillus amylases.
Action pattern of AmyCR on starch showed predominant formation of small MOS (G3 and G4). The proportion of the hydrolytic products formed differs from other MOS-forming amylases from Bacillus sp. MOS-forming amylase of B. circulans was reported to produce high level of G5 (31%), G4 (25%), and G2 (30%) with little G3 (Dey et al. 2002). It suggests that the mechanism of action of the amylase of KCC103 is different from reported amylases. Predominant formation of G3 and G4 by the present amylase with stability at moderate temperature makes it useful as an antistaling agent in bread making industry. Furthermore, the addition of Ca2+ for application of the present α-amylase is not necessary. Production of this amylase is economical because its synthesis is not hindered by biomass-based sugars and sugarcane bagasse hydrolysate-based medium was found to support high level of amylase synthesis in the new strain.
References
Ali MB, Mhiri S, Mezghani M, Bejar S (2001) Purification and sequence analysis of the atypical maltohexaose-forming α-amylase of the B. stearothermophilus US100. Enzyme Microb Technol 28:537–542
Dahl MK (2002) CcpA-independent carbon catabolite repression in Bacillus subtilis. J Mol Microbiol Biotechnol 4:315–321
Dey G, Palit S, Banerjee R, Maiti BR (2002) Purification and characterization of maltooligosaccharide-forming amylase from Bacillus circulans GRS313. J Ind Microbiol Biotechnol 28:193–200
Duran E, Leon A, Barber B, de Barber CB (2001) Effect of low molecular weight dextrins on gelatinization and retrogradation of starch. Eur Food Res Technol 212:203–207
Gray JA, Bemiller JN (2003) Bread staling: molecular basis and control. Comp Rev Food Sci Food Safety 2:1–21
Hagihara H, Igarashi K, Hayashi Y, Endo K, Ikawa-Kitayama K, Ozaki K, Kawai S, Ito S (2001) Novel alpha-amylase that is highly resistant to chelating reagents and chemical oxidants from the alkaliphilic Bacillus isolate KSM-K38. Appl Environ Microbiol 67:1744–1750
Hashim SO, Delgado OD, Martínez MA, Kaul RH, Mulaa FJ, Mattiasson B (2005) Alkaline active maltohexaose-forming α-amylase from Bacillus halodurans LBK 34. Enzyme Microb Technol 36:139–146
Ikram-ul-Haq, Ashraf H, Iqbal J, Qadeer MA (2003) Production of alpha amylase by Bacillus licheniformis using an economical medium. Biores Technol 87:57–61
Kim TU, Gu BG, Jeong JY, Byun SM, Shin YC (1995) Purification and characterization of a maltotetraose-forming alkaline α-amylase from an alkalophilic Bacillus strain GM8901. Appl Environ Microbiol 61:3105–3112
Kirk O, Borchert TV, Fugslang CC (2002) Industrial enzyme application. Curr Opin Biotechnol 13:345–351
Martin ML, Hoseney RC (1991) A mechanism of bread firming II role of starch hydrolyzing enzymes. Cereal Chem 68:503–507
Messaoud EB, Ali MB, Elleuch N, Masmoudi NF, Bejar S (2003) Purification and properties of a maltoheptaose-and maltohexaose-forming amylase produced by Bacillus subtilis US116. Enzyme Microb Technol 34:662–666
Min BC, Yoon SH, Kim JW, Lee YW, Kim YB, Park KH (1998) Cloning of novel maltooligosaccharide-producing amylases as antistaling agents for bread. J Agric Food Chem 46:779–782
Mitsunaga S, Kawakami O, Numata T, Yamaguchi J, Fukui K, Mitsui T (2001) Polymorphism in rice amylases at an early stage of seed germination. Biosci Biotechnol Biochem 65:625–662
Morgan FJ, Priest FG (1981) Characterization of a thermostable α-amylase from Bacillus licheniformis NCIB6346. J Appl Bacteriol 50:107–114
Palacios HR, Schwarz PB, D’Appolonia LD (2004) Effect of α-amylases from different sources on the retrogradation and recyclization of concentrated wheat starch gels: relationship to bread staling. J Agric Food Chem 52:5978–5986
Paquet V, Croux C, Goma G, Soucaille P (1991) Purification and characterization of the extracellular alpha-amylase from Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 57:212–218
Pattanapipitpaisal P, Brown NL, Macaskie E (2001) Chromate reduction and 16S rRNA identification of bacteria isolated from a Cr(VI)-contaminated site. Appl Microbiol Biotechnol 57:257–261
Takasaki Y (1985) An amylase producing maltotriose from Bacillus subtilis. Agric Biol Chem 49:1091–1097
Acknowledgements
This work was supported by SERC, Department of Science and Technology, New Delhi. Ms. Dilli Rani and Mr. Gopinath are awarded with Junior Research Fellow by the Indian Institute of Technology Madras.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagarajan, D.R., Rajagopalan, G. & Krishnan, C. Purification and characterization of a maltooligosaccharide-forming α-amylase from a new Bacillus subtilis KCC103. Appl Microbiol Biotechnol 73, 591–597 (2006). https://doi.org/10.1007/s00253-006-0513-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0513-4