Abstract
A protein demonstrating laccase activity and potent inhibitory activity towards human immunodeficiency virus (HIV)-1 reverse transcriptase (IC50 1.2 μM) was isolated from fresh fruiting bodies of the medicinal mushroom Ganoderma lucidum. The laccase had a novel N-terminal sequence and a molecular mass of 75 kDa, which is higher than the range (55–56 kDa) reported for most other mushroom laccases. It was isolated by sequential chromatography on DEAE-cellulose and Affi-gel blue gel and adsorption on Con A-Sepharose. Unlike some of the previously isolated laccases, it was adsorbed only on Con A-Sepharose. The enzyme required a pH of 3–5 and a temperature of 70°C to exhibit maximal activity. Minimal activity was detected at pH 6 and 7. Activity was undetectable at pH 8 and 9 and after exposure to 100°C for 10 min.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A variety of proteins have been isolated and characterized from mushrooms and fungi including lectins (Yagi et al. 1997; Wang et al. 1995, 1996, 2004), ribonucleases, ribosome-inactivating proteins (Wang and Ng 2000a, 2001a,b), anti-fungal proteins (Lam and Ng 2001; Wang and Ng 2004d, 2006b), laccases (Wang and Ng 2004b,c,e, 2006b) and ubiquitin-like peptides (Wang and Ng 2000b). Some of these proteins exhibit anti-proliferative/anti-tumour (Wang et al. 1995, 1996), anti-microbial (Lam and Ng 2001; Wang and Ng 2004b,c) and human immunodeficiency virus (HIV)-1 reverse transcriptase (RT) inhibitory (Lam and Ng 2001, Wang and Ng 2001a,b) activities.
From the medicinal fungus Ganoderma lucidum, polysaccharides designated ganoderans A and B, which elicited hypoglycaemia in diabetic mice, have been isolated (Hikino et al. 1985). A polysaccharide peptide with anti-tumour and anti-angiogenic activities has been reported (Cao and Lin 2004). However, little is known about the protein constituents of Ganoderma spp. other than lectins (Tanaka et al. 1989; Kawagishi et al. 1997; Ngai and Ng 2004), an anti-fungal protein (Wang and Ng 2004d,2006a) and a ribonuclease (Wang et al. 2003).
Laccases are ligninolytic enzymes (Evans et al. 1994), but they find applications in biosensors, pulping, textile dyes, detoxification of polluted water and other biotechnological procedures (Palmieri et al. 1993; Brenna and Bianchi 1994; Reid and Paice 1994; Ghindilis et al. 1995; Martirani et al. 1996). The objective of the present study was to isolate a laccase from G. lucidum. The results would add to the existing knowledge about this medicinal mushroom. Hitherto laccases have been isolated from a number of mushrooms (Eggert et al. 1996; Munoz et al. 1997; Cambria et al. 2000; Dedeyan et al. 2000; Shin and Lee 2000; Garzillo et al. 2001; Rogalski et al. 2001; Wang and Ng 2006a,b), mostly from their mycelia, but very few from medicinal mushrooms, e.g. that from Trametes versicolor, also known as Coriolus versicolor (Milsten et al. 1989) and renowned for its polysaccharide peptide (Sakagami et al. 1991; Ng 1998). The present investigation disclosed that the fruiting bodies of the prized medicinal mushroom G. lucidum produce a laccase with some unique characteristics, including a high molecular mass, a novel N-terminal sequence, a fairly high thermostability, a pH optimum ranging from pH 3 to 5 and non-adsorption on various chromatographic media except Con A-Sepharose.
Materials and methods
Isolation of laccase
Fresh fruiting bodies (800 g) of the mushroom G. lucidum were used. They were extracted with distilled water (3 ml/g) in a Waring blender. The homogenate was centrifuged (13,000×g, 20 min), and the supernatant was saved. Tris–HCl buffer (1 M, pH 7.6) was added to the supernatant until the concentration of Tris attained 10 mM. The supernatant was then passed through a column (5×20 cm) of DEAE-cellulose (Sigma) in 10 mM Tris–HCl buffer (pH 7.4). Unadsorbed proteins eluted with the starting buffer were collected as fraction D1, while adsorbed proteins eluted with 0.8 M NaCl added to the starting buffer were collected as fraction D2. Laccase activity was concentrated in D1. D1 was next subjected to affinity chromatography on an Affi-gel blue gel (Bio-Rad) column (2.5×20 cm). Unadsorbed proteins were washed off the column with 10 mM Tris–HCl buffer (pH 7.4) and collected as fraction B1. Adsorbed proteins were eluted with 10 mM Tris–HCl buffer containing 2 M NaCl and collected as fraction B2. Fraction B1 was applied to a 2.5×20 cm column of Con A-Sepharose (Amersham Biosciences). Unadsorbed proteins were eluted into fraction Con A1 with 50 mM Tris–HCl buffer (pH 7.4) containing 0.5 M NaCl, 10 mM CaCl2 and 10 mM MgCl2. Adsorbed proteins were desorbed with buffer B, i.e. 0.4 M α-methyl-d-glucopyranoside added to the starting buffer and collected as fraction Con A2. Fraction Con A2 was dialyzed, lyophilized and then further fractionated by fast protein liquid chromatography (FPLC) on a gel filtration Superdex 75 HR 10/30 column (Amersham Biosciences) using an AKTA Purifier System (Amersham Biosciences). The first eluted peak represented purified laccase.
Assay of laccase activity
Laccase activity was assayed by measuring the oxidation of 2,7′-azinobis(3-ethylbenzothiazolone-6-sulfonic acid) diammonium salt (ABTS). A modification of the method of Shin and Lee (2000) was used. An aliquot of the enzyme solution was incubated in 1.3 ml of 67 mM sodium acetate buffer (pH 4.5) containing 1.54 mM ABTS at 30°C. One unit of enzyme activity was defined as the amount of enzyme required to produce an absorbance increase at 405 nM of one per minute per millilitre of reaction mixture under the aforementioned condition.
Molecular mass determination by sodium dodecyl sulphate polyacrylamide gel electrophoresis and by FPLC-gel filtration
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) was carried out in accordance with the procedure of Laemmli and Favre (1973), using a 12% resolving gel and a 5% stacking gel. At the end of electrophoresis, the gel was stained with Coomassie brilliant blue. FPLC-gel filtration was carried out using a Superdex 75 column, which had been calibrated with molecular mass standards (Amersham Biosciences).
Analysis of N-terminal amino acid sequence
Amino acid sequence analysis was carried out using an HP G1000A Edman degradation unit and an HP1000 HPLC system (Lam and Ng 2001).
Assay for HIV-1 RT inhibitory activity
The assay for HIV-1 RT inhibitory activity was carried out according to instructions supplied with the assay kit from Boehringer Mannheim (Germany). The assay takes advantage of the ability of RT to synthesize DNA, starting from the template/primer hybrid poly(A) oligo(dT)15. The digoxigenin- and biotin-labelled nucleotides in an optimized ratio are incorporated into one of the same DNA molecule, which is freshly synthesized by the RT. The detection and quantification of synthesized DNA as a parameter for RT activity follows a sandwich ELISA protocol. Biotin-labelled DNA binds to the surface of microtitre plate modules that have been precoated with streptavidin. In the next step, an antibody to digoxigenin, conjugated to peroxidase, binds to the digoxigenin-labelled DNA. In the final step, the peroxidase substrate is added. The peroxidase enzymes catalyze cleavage of the substrate, producing a coloured reaction product. The absorbance of the samples at 405 nm can be determined using a microtitre plate (ELISA) reader and is directly correlated to the level of RT activity. A fixed amount (4–6 ng) of recombinant HIV-1 RT was used. The inhibitory activity of the isolated protein was calculated as percent inhibition as compared to a control without the protein (Lam and Ng 2001; Wang and Ng 2001a,b; Ye and Ng 2002).
Results
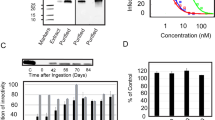
When the fruiting body extract was chromatographed on DEAE-cellulose, laccase activity was confined to fraction D1 (Table 1). D1 was separated on Affi-gel blue gel into an unadsorbed fraction B1 with much higher laccase activity and an adsorbed fraction B2 with much lower activity (Table 1). Con A-Sepharose was capable of adsorbing essentially all of the laccase activity found in B1 (Table 1), although the unbound fraction Con A1 was much larger than the bound fraction designated as Con A2 (Fig. 1). Superdex 75 separated Con A2 into two fractions, S1 and S2. S2 was slightly smaller (Fig. 2). The bulk of laccase activity was retained in S1 (Table 1). S1 appeared as a single band with a molecular mass of 75 kDa in SDS-PAGE (Fig. 3). Its molecular mass as estimated by gel filtration on Superdex 75 was also 75 kDa (Fig. 2). The laccase showed very little resemblance to other mushroom laccases in N-terminal sequence. Some of the other mushroom laccases, e.g. those from Trametes veriscolor, Coriolus hirsutus, Basidiomycete PM1, Cariporiopsis subvermispora, Phlebia radiata and Pycnoporus cinnabarinus exhibited considerable sequence homology to each other (Table 2). The activity of the purified laccase underwent a 80% increase when the temperature was raised from 20 to 70°C. When the temperature was increased to 80°C, there was a small drop in activity (Fig. 4). The activity of the enzyme was totally destroyed after exposure to 100°C for 10 min. It stayed at a high level when the pH was varied from 3 to 5. When the pH was increased to 6, a significant decline in enzyme activity occurred. The activity remained low at pH 7 and further decreased to an undetectable level at pH 8 and 9 (Fig. 5). The enzyme inhibited HIV-1 RT with an IC50 of 1.2 μM.
Affinity chromatography on Con A-Sepharose. Sample: Ganoderma lucidum fruiting body extract unadsorbed successively on DEAE-cellulose, Affi-gel blue gel and then CM-cellulose. Column dimensions: 2.5×20 cm. Starting buffer for eluting fraction Con A1: 50 mM Tris–HCl (pH 7.4) containing 0.5 M NaCl, 10 mM CaCl2 and 10 mM MgCl2. Buffer B for eluting fraction Con A2: 0.4 M α-methyl-d-glucopyranoside in starting buffer
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Left lane: G. lucidum laccase. Right lane: molecular mass standards (Amersham Biosciences). From top downwards: phosphorylase b (94 kDa), bovine serum albumin (67 kDa), ovalbumin (43 kDa), carbonic anhydrase (30 kDa), soybean trypsin inhibitor (20 kDa) and α-lactalbumin (14.4 kDa)
Discussion
In the present study, a laccase was successfully isolated from the fruiting bodies of the medicinal mushroom G. lucidum. Ion-exchange chromatography on DEAE-cellulose, affinity chromatography on Affi-gel blue gel and Con A-Sepharose and gel filtration on Superdex 75 were used successively to remove inactive proteins from the laccase-containing chromatographic fraction. Each of the aforementioned chromatographic media was effective in yielding a laccase-enriched fraction separable from a fraction with little or no laccase activity. In contradistinction to laccases from the mushrooms Pleurotus eryngii (Wang and Ng 2006b), C. hirsutus (Shin and Lee 2000) and Rigidoporus lignosus (Cambria et al. 2000), which are adsorbed on DEAE-Sepharose and Q-Sepharose, G. lucidum laccase is unadsorbed on DEAE-cellulose. G. lucidum laccase is also unadsorbed on Affi-gel blue gel. Con A-Sepharose was thus selected as an adsorption media for the laccase which is presumably a glycoprotein. Superdex 75 was used in the final purification step and also for molecular mass estimation.
Many mushroom laccases demonstrate marked similarity in N-terminal sequence (Table 2). On the other hand, G. lucidum laccase exhibits a dissimilar N-terminal sequence. The molecular mass of G. lucidum laccase (75 kDa) is higher than the range of molecular masses (55–65 kDa) reported for previously isolated laccases (Geiger et al. 1986; Eggert et al. 1996; Munoz et al. 1997; Cambria et al. 2000; Dedeyan et al. 2000; Shin and Lee 2000; Garzillo et al. 2001; Rogalski et al. 2001). It is remarkable that the laccase has a temperature optimum at a high temperature (70°C). The activity of the laccase increases progressively as the ambient temperature is raised from 20 to 70°C. The increase in enzyme activity is about 80%. Nevertheless, the enzyme is susceptible to thermal denaturation at 100°C, and all enzyme activity vanishes after 10 min. On the other hand, C. versicolor laccase manifests a temperature optimum at 45°C (Shin and Lee 2000).
Another distinctive feature of G. lucidum laccase is its need of an acidic pH (3–5) for activity. Between pH 5 and 6, there is a sudden drop in activity. At pH 6 and 7, there is only residual activity. At pH 8 and 9, activity is indiscernible. The laccases reported in the literature have an optimal pH of 2–3 (Shin and Lee 2000; Garzillo et al. 2001).
G. lucidum laccase is capable of inhibiting HIV-1 RT, probably by protein–protein interaction. HIV-1 RT inhibitory activity has been reported for some laccases (Wang and Ng 2004a–e), ribosome-inactivating proteins (Lam and Ng 2001; Wang and Ng 2000a, 2001b), ubiquitin-like proteins (Wang and Ng 2000b) and lectins (Wang and Ng 2004a) of mushroom origin. The inhibitory potency of G. lucidum laccase is at the upper end of the range demonstrated by natural products (Ng et al. 1997) and the range demonstrated by mushroom laccases. P. eryngii laccase (Wang and Ng 2006b), Tricholoma giganteum laccase (Wang and Ng 2004e), Hericium erinaceum laccase (Wang and Ng 2004b) and Albatrella dispansus laccase (Wang and Ng 2004c) inhibit the HIV enzyme with an IC50 of 1.2, 2.2, 9.5 and 16 μM, respectively. Some mushroom laccases, e.g. Cantharellus cibarius laccase (Ng and Wang 2004), are devoid of this activity (Wang and Ng 2004a–c,e).
D’Souza et al. (1999) reported that a strain (Karsten FP-58537-Sp) of G. lucidum in high-nitrogen culture and in cultures containing both poplar and pine produced a large amount of laccase. SDS-PAGE revealed two bands with molecular mass of 40 and 66 kDa, respectively, while isoelectric focusing disclosed five bands with pI of 3.0, 4.25, 4.5, 4.8 and 5.1, respectively. Ko et al. (2001) detected three laccase isozymes in the mycelia of G. lucidum ASI 7071-9. These mycelial laccases differ from the fruiting body laccase isolated in the preset study in several aspects. The molecular mass (65–68 kDa), N-terminal sequence (GIGPT), optimum pH (3.5), optimum temperature (20°C) and chromatographic behaviour on DEAE-cellulose (adsorption) of the mycelial laccases (Ko et al. 2001) are different from the fruiting body laccase. This is reminiscent of the differences between fruiting body and mycelial lectins isolated from Tricholoma mongolicum (Wang et al. 1995, 1998).
References
Brenna O, Bianchi E (1994) Immobilized laccase for phenolic removal in must and wine. Biotechnol Lett 16:35–40
Cambria MT, Cambria A, Ragusa S, Rizzarelli E (2000) Production, purification and properties of an extracellular laccase from Rigidoporus lignosus. Protein Expr Purif 18:141–147
Cao QZ, Lin ZB (2004) Antitumor and anti-angiogenic activity of Ganoderma lucidum polysaccharide peptide. Acta Pharmacol Sin 25:833–838
Dedeyan B, Klonowska A, Tagger S, Tron T, Iacazio G, Gill G, Le Petit J (2000) Biochemical and molecular characterization of a laccase from Marasmius quercophilus. Appl Environ Microbiol 66:925–929
D’Souza TM, Meritt CS, Reddy CA (1999) Lignin-modifying enzymes of the white rot basidiomycete Ganoderma lucidum. Appl Microbiol Biotechnol 65:5307–5313
Eggert C, Tempe U, Eriksson KEL (1996) The ligninolytic system of the white-rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccases. Appl Environ Microbiol 62:1151–1158
Evans CS, Dutton MV, Guillen F, Veness RG (1994) Enzymes and small molecular mass agents involved with lignocellulose degradation. FEMS Microbiol Rev 13:235–240
Garzillo AM, Colao MC, Buonocore V, Oliva R, Falcigno L, Saviano M, Santoro AM, Zappala R, Bonomo RP, Bianco C, Giardina P, Palmieri G, Sannia G (2001) Structural and kinetic characterization of native laccases from Pleurotus ostreatus, Rigidoporus lignosus and Trametes trogii. J Protein Chem 20:191–201
Geiger JP, Rio B, Nandris D, Nicole M (1986) Laccases of Rigidoporus lignosus and Phellinus noxius: I. Purification and some physico-chemical properties. Appl Biochem Biotechnol 12:121–133
Ghindilis AL, Makower A, Bier FF, Scheller FW (1995) A laccase–glucose dehydrogenase recycling—enzyme electrode based on potentiometric mediatorless electrocatalytic detection. Anal Methods Instrum 2:129–132
Hikino H, Konno C, Mirin Y, Hayashi T (1985) Isolation and hypoglycemic activity of ganoderans A and B, glycans of Ganoderma lucidum fruiting bodies. Planta Med 4:339–340
Kawagishi H, Mitsunaga SI, Yamawaki M, Ido M, Shimada A, Kinoshita T, Murata T, Usui T, Kimura A, Chiba S (1997) A lectin from mycelia of the fungus Ganoderma lucidum. Phytochemistry 44:7–10
Ko EM, Leem YE, Choi HT (2001) Purification and characterization of laccase isozymes from the white-rot basidiomycete Ganoderma lucidum. Appl Microbiol Biotechnol 57:98–102
Kobayashi H, Inokuchi N, Koyama T, Watenabe H, Iwami M, Ohgi K, Irie M (1992) Primary structure of the nonspecific and adenylic acid preferential ribonuclease from the fruit bodies of Lentinus edodes. Biosci Biotechnol Biochem 55:2003–2010
Laemmli UK, Favre M (1973) Gel electrophoresis of proteins. J Mol Biol 80:575–599
Lam SK, Ng TB (2001) First simultaneous isolation of a ribosome inactivating protein and an antifungal protein from a mushroom (Lyophyllum shimeiji) together with evidence for synergism of their antifungal effects. Arch Biochem Biophys 3939:271–280
Martirani L, Giardina P, Marzullo L, Sannia G (1996) Reduction of phenol content and toxicity in olive oil mill waste waters with the ligninolytic fungus Pleurotus ostreatus. Water Res 30:1914–1918
Milstein O, Nicklas B, Huttermann A (1989) Oxidation of aromatic compounds in organic solvents with laccase from Trametes versicolor. Appl Microbiol Biotechnol 31:70–74
Munoz C, Guillan F, Martinez AT, Martinez MJ (1997) Induction and characterization of laccase in the ligninolytic fungus Pleurotus eryngii. Curr Microbiol 34:1–5
Ng TB (1998) A review of research on the protein-bound polysaccharide (polysaccharopeptide, PSP) from the mushroom Coriolus versicolor (Basidiomycetes: Polyporaceae). Gen Pharmacol 30:1–4
Ng TB, Wang HX (2004) A homodimeric laccase with unique characteristics from the yellow mushroom Cantharellus ciborius. Biochem Biophys Res Commun 313:37–41
Ng TB, Huang B, Fong WP, Yeung HW (1997) Anti-HIV natural products with special emphasis on HIV-1 reverse transcriptase inhibitors. Life Sci 61:933–949
Ngai PHK, Ng TB (2004) Isolation of a mushroom (Ganoderma carpense) lectin with spectacular thermostability, potent mitogenic activity on splenocytes and antiproliferative activity on tumor cells. Biochem Biophys Res Commun 314:988–993
Palmieri G, Giardina P, Desiderio B, Marzullo L, Giamberini M, Sannia G (1993) A new enzyme immobilization procedure using copper alginate gel: application to a fungal phenol oxidase. Enzyme Microb Technol 16:151–158
Reid ID, Paice MG (1994) Biological bleaching of kraft pulps by white-rot fungi and their enzymes. FEMS Microbiol Rev 13:369–376
Rogalski J, Fiedurek J, Leonowicz A (2001) Production of ligninolytic and feed-back type enzymes by Phlebia radiata on different media. Acta Biol Hung 52:149–160
Sakagami H, Aoki T, Simpson A, Tanuma SI (1991) Induction of immunopotentiation activity by a protein-bound polysaccharide, PSK. Anticancer Res 11:993–1000
Shin KS, Lee YJ (2000) Purification and characterization of a new member of the laccase family from the white-rot basidiomycete Coriolus hirsutus. Arch Biochem Biophys 384:109–115
Tanaka S, Ko K, Kino K, Tsuchiya K, Yamashita A, Murasugi A, Sakuam S, Tsuno H (1989) An immunomodulator from a fungus Ganoderma lucidum having similarity to immunoglobin variable region. J Biol Chem 264:16372–16377
Wang HX, Ng TB (2000a) Flammulin: a novel ribosome-inactivating protein from fruiting bodies of the winter mushroom Flammulina velutipies. Biochem Cell Biol 78:1–4
Wang HX, Ng TB (2000b) Isolation of a novel ubiquitin-like protein from Pleurotus ostreatus mushroom with anti-human immunodeficiency virus, translation-inhibitory, and ribonuclease activities. Biochem Biophys Res Commun 276:587–593
Wang HX, Ng TB (2001a) Examination of lectins, polysaccharopeptide, polysaccharide, alkaloid, coumarin and trypsin inhibitors for inhibitory activity against human immunodeficiency virus transcriptase and glycohydrolase. Planta Med 67:669–672
Wang HX, Ng TB (2001b) Isolation and characterization of velutin, a novel low-molecular-weight ribosome-inactivating protein from winter mushroom (Flammulina velutipes) fruiting bodies. Life Sci 68:2151–2158
Wang HX, Ng TB (2003) Isolation of a ribonuclease from fruiting bodies of the wild mushroom Termitomyces globulus. Peptides 24:973–977
Wang HX, Ng TB (2004a) Isolation of a novel lectin from the mushroom Xerocomus spadiceus. Peptides 25:7–10
Wang HX, Ng TB (2004b) A new laccase from dried fruiting bodies of the monkey head mushroom Hericium erinaceum. Biochem Biophys Res Commun 322:17–21
Wang HX, Ng TB (2004c) A novel laccase with fair thermostability from the edible wild mushroom (Albatrella dispansus). Biochem Biophys Res Commun 310:381–385
Wang HX, Ng TB (2004d) Eryngin, a novel antifungal peptide from fruiting bodies of the mushroom Pleurotus eryngii. Peptides 25:1–5
Wang HX, Ng TB (2004e) Purification of a novel low-molecular-mass laccase with HIV-1 reverse transcriptase inhibitory activity from the mushroom Tricholoma giganteum. Biochem Biophys Res Commun 315:450–454
Wang HX, Ng TB (2006a) Ganodermin, an antifungal protein from fruiting bodies of the medicinal mushroom Ganoderma lucidum. Peptides 27:27–30
Wang HX, Ng TB (2006b) Purification of a laccase from fruiting bodies of the mushroom Pleurotus eryngii. Appl Microbiol Biotechnol 69:521–525
Wang HX, Ng TB, Liu WK, Ooi VEC, Chang ST (1995) Isolation and characterization of two distinct lectins with antiproliferative activity from the cultured mycelium of the edible mushroom Tricholoma mongolicum. Int J Pept Protein Res 46:508–513
Wang HX, Liu WK, Ng TB, Ooi VEC, Chang ST (1996) The immunomodulatory and antitumor activities of lectins from the mushroom Tricholoma mongolicum. Immunopharmacology 31:205–211
Wang HX, Ng TB, Ooi VEC (1998) Lectin activity in fruiting bodies of the edible mushroom Tricholoma mongolicum. Biochem Mol Biol Int 44:135–141
Wang HX, Ngai PHK, Ng TB (2003) A ubiquitin-like peptide with ribonuclease activity against various polyhomoribonucleotides from the yellow mushroom Cantharellus cibarius. Peptides 24:509–513
Wang HX, Ng TB, Chiu SW (2004) A distinctive ribonuclease from fresh fruiting bodies of the medicinal mushroom Ganoderma lucidum. Biochem Biophys Res Commun 314:519–522
Yagi F, Miyamoto M, Abc T, Minami T, Tadera K, Goldstein IJ (1997) Purification and carbohydrate-binding specificity of Agrocybe cylindracea lectin. Glycoconj J 14:281–288
Ye XY, Ng TB (2002) Isolation of a cyclophilin-like protein from chickpeas with mitogenic, antifungal and anti-HIV-1 reverse transcriptase activities. Life Sci 70:1129–1138
Acknowledgement
We thank Miss Fion Yung for her skilled secretarial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H.X., Ng, T.B. A laccase from the medicinal mushroom Ganoderma lucidum . Appl Microbiol Biotechnol 72, 508–513 (2006). https://doi.org/10.1007/s00253-006-0314-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0314-9