Abstract
The zygomycete fungus Blakeslea trispora is used commercially as natural source of β-carotene. β-Carotene production is strongly induced during mating of two strains of the opposite sex and results in the production of the pheromone trisporic acid, which in turn stimulates enhanced β-carotene biosynthesis. β-Carotene production is due to the enzymatic activity of phytoene synthase, lycopene cyclase and phytoene dehydrogenase. The corresponding genes, carRA and carB, were isolated from a cosmid library generated from B. trispora strain ATCC14272. The steady state level of carB and carRA mRNA transcripts under different mating conditions was monitored by both northern blot analysis and quantitative real-time PCR. The steady state levels of carRA and carB mRNA of non-mated and mated B. trispora were quantified relative to transcript levels of the translation elongation factor 1α-encoding tef1 gene, since tef1 is transcribed independently of mating. Transcription levels of both carB and carRA were strongly induced only under mating conditions. These data suggest that β-carotene production in B. trispora is due to increased transcription of the biosynthesis genes carB and carRA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carotenoids are natural pigments with yellow, orange and red colours. They are produced by many photosynthetically active organisms, but also by many non-photosynthetic microorganisms including some bacteria and fungi (Britton et al. 1998). Despite their similar backbone of 40 carbon atoms, carotenoids exhibit high structural diversity. Carotenes contain only carbon and hydrogen atoms. The incorporation of oxygen in the form of hydroxyl, carbonyl and carboxyl groups results in the formation of xanthophylls.
Carotenoids have antioxidant properties and protect organisms from reactive oxygen species (Bartley and Scolnik 1995). They are also thought to have anti-tumour effects (Mayne 1996) and have been considered useful in the prevention of chronic diseases (Smith 1998). To date, carotenoids are produced mainly artificially by chemical synthesis, but due to the increasing preference of consumers for biological products, the extraction of carotenoids from natural sources has become an important alternative.
Both the filamentous fungus Blakeslea trispora and the green alga Dunaliella salina have been used for production of β-carotenes (Nelis and De Leenheer 1991). B. trispora belongs to the order Mucorales within the class Zygomycetes. Because of its unique ability to produce high amounts of β-carotene in submerged cultures it is the only species among the Mucorales used for β-carotene production on an industrial scale. In contrast, its close relatives from the genus Phycomyces preferentially produces carotenes on the surface of liquid or solid media.
A characteristic of Zygomycetes is the formation of zygospores during sexual development by mating of mycelia of (+) and (−) strains (Schimek et al. 2003). The accumulation of β-carotene is also linked to the sexual interaction of the fungus, i.e. β-carotene production increases 13- to 15-fold during mating of (−) and (+) strains (Mehta et al. 2003). The induction of carotenoid biosynthesis is based on the diffusion of mating-type-specific pheromones (Sutter et al. 1974), which are degradation products of β-carotene. One of these products is trisporic acid, which acts as the major pheromone triggering the development of zygospores.
Carotenoid biosynthesis in fungi occurs via the mevalonate pathway (Lee and Schmidt-Dannert 2002). The formation of phytoene by condensation of two molecules of geranylgeranyl pyrophosphate is the first and crucial step in the carotenoid pathway. This well conserved step in all carotenogenic organisms is catalysed by the enzyme phytoene synthase. In four subsequent dehydration steps, catalysed by phytoene dehydrogenase, double bonds are introduced and the carotenoid lycopene is synthesised. Lycopene is transformed into β-carotene by introduction of ring structures at both ends of the molecule, catalysed by lycopene cyclase.
Fungi differ from other non-photosynthetic organisms in that they have a bi-functional enzyme that displays both phytoene synthase and lycopene cyclase activity (Verdoes et al. 1999; Velayos et al. 2000; Arrach et al. 2001). In Phycomyces blakesleeanus the carRA and carB gene products are thought to be organised in an enzymatic complex consisting of four units of phytoene dehydrogenase (carB) and two units of lycopene cyclase (carRA) (de la Guardia et al. 1971; Aragón et al. 1976; Ruiz-Hidalgo et al. 1997).
Recently, the genes for β-carotene biosynthesis in B. trispora were identified (Rodriguez-Saiz et al. 2004). CarB encodes the phytoene dehydrogenase, and carRA encodes a bi-functional enzyme with an R domain for lycopene cyclase activity and an A domain with phytoene synthase activity. As was shown for other Zygomycetes, e.g. P. blakesleeanus (Arrach et al. 2001) or Mucor circinelloides (Velayos et al. 2000), the carB and carRA genes from B. trispora are organised in opposite orientation in a cluster. In this cluster, carB and carRA genes are separated by an intergenic region, which, at least in P. blakesleeanus, mediates independent transcription of carB and carRA (Arrach et al. 2001).
Here, we analysed the transcription of the carotenoid biosynthesis genes carRA and carB in B. trispora under mating and non-mating conditions. For this purpose, carRA and carB genes were isolated from strain ATCC14272 and their DNA sequence determined. After establishment of quantitative real-time PCR, based on the mating-independent translation elongation factor 1 α-encoding gene tef1 as a standard for relative quantification, steady-state levels of mRNA of carRA and carB were analysed during mating. Data were verified by northern blot analysis.
Materials and methods
Strains and growth conditions
Blakeslea trispora wild-type strains ATCC14271 (+) and ATCC14272 (−) were cultivated on MEP medium agar plates [3% (w/v) malt extract, 0.3% (w/v) peptone, 2% (w/v) agar, pH 5.5]. Spores were harvested by rinsing the fully grown agar plates with a solution of 0.9% (w/v) NaCl, 0.1% (v/v) Tween 20. For submerged cultures, 107 spores of the (+) and (−) mating type strains were inoculated together or separately into 50 ml MEP liquid medium with addition of 1% (v/v) Tween 20 in 250-ml Erlenmeyer flasks. The flasks were shaken for 1–3 days at 180 rpm at 27°C without light. The mycelia were subsequently harvested, squeeze dried and frozen in liquid nitrogen.
Standard molecular biological techniques and oligonucleotides
Oligonucleotides are listed in Table 1. Standard techniques for the manipulation of DNA were as described by Sambrook et al. (1989). Vectors and plasmids were propagated in Escherichia coli TOP10F′ (Invitrogen, Carlsbad, Calif.). DNA sequence analysis was performed using an ABI310 sequencer (Applied Biosystems, Foster City, Calif.), utilising an ABI PRISM BigDye Terminator Cycle Sequencing Reaction Kit. E. coli XL1-Blue MRA cells (Stratagene, La Jolla, Calif.) were infected with λ phages to produce cosmids. Cosmid DNA was prepared using the plasmid miniprep kit (Peqlab, Erlangen,Germany) according to the manufacturer’s instructions.
Generation and screening of a B. trispora cosmid library
The generation of a genomic cosmid library of B. trispora strain ATCC14272 was carried out as described in detail by Osiewacz (1994) for Podospora anserina. In brief, plasmid pANsCos1 was used as the cosmid vector. This vector contains two cos sites, which facilitated the efficient construction of a genomic library. Cosmid DNA was cleaved with XbaI and dephosphorylated. The linearised cosmid DNA was then cleaved with BamHI, which yielded two fragments and generated the cloning site for cloning of Sau3AI-digested DNA fragments. Sau3AI DNA fragments were obtained by partial digestion of chromosomal DNA of B. trispora wild-type strain ATCC14272 according to Ballance et al. (1983). The partially digested chromosomal DNA and the two DNA fragments of the cosmid vector obtained by digestion with XbaI and BamHI, were ligated and subjected to in vitro packaging using a Gigapack III XL Packaging Extract (Stratagene) according to the manufacturer’s instructions. E. coli XL1-Blue MRA cells were infected with the λ phages thus generated. Ampicillin-resistant E. coli colonies (n=15,000) were generated by plating appropriate amounts of infected E. coli cells. Twenty colonies were picked at random and cosmid DNA was prepared. The cosmid DNA was cleaved with HindIII. The restriction pattern revealed that all of the recombinant cosmids carried inserts of about 26–42 kb and differed from each other, indicating the generation of different recombinant cosmids.
Northern blot analysis
Primers tef1-for and tef1-rev were designed to amplify a 1,339-bp internal fragment of the B. trispora tef1 gene. Primers carB-exon2-for and carB-exon2-rev were used for amplification of a 520-bp fragment derived from exon 2 of the carB gene. Primers carRA-for and carRA-rev were used to amplify a 560-bp fragment of carRA exon 2. DNA probes for northern hybridisation were labelled with fluorescein-11-dUTP using a Gene Images CDP-Star detection module (Amersham Biosciences, Little Chalfont, UK).
Total RNA was isolated using an RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). For northern analysis, 10 μg RNA was separated on a denaturing formaldehyde agarose gel. The RNA was transferred to Hybond-N+ membrane (Amersham Biosciences). Hybridisation was performed at 60°C overnight in a solution containing 5×SSC, 0.1% (w/v) SDS, 5% (w/w) dextran sulfate and 5% (v/v) liquid block (Amersham Biosciences). The blots were then washed twice at 60°C in 1×SSC 0.1% (w/v) SDS and in 0.5×SSC 0.1% (w/v) SDS for 15 min each. A CDP-Star chemiluminescent detection system (Amersham Biosciences) was used for detection, according to the manufacturer’s instructions.
DNase treatment of isolated RNA
To degrade trace amounts of genomic DNA in RNA preparations, the RNA was treated with DNase I (Roche, Mannheim, Germany). DNase I (1 μl; 10 U/ml) was added to a 20-μl reaction volume containing 5 μg RNA (0.5 μg/μl), 4.4 μl 25 mM MgCl2, 2 μl 10× TaqMan RT Buffer (Applied Biosystems) and 2.6 μl RNase-free water. The reaction mixture was incubated at 37°C for 30 min, followed by heat denaturation at 95°C for 5 min.
Reverse transcription of total RNA
To quantify mRNA steady state levels by real-time PCR, the RNA was reverse transcribed into cDNA, which was used as the template for PCR. Reverse transcription was carried out in a separate reaction (two-step RT qPCR). For this reaction TaqMan reverse transcription reagents (Applied Biosystems) were used. We added 0.5 μg total RNA to 2.5 μl 10× TaqMan RT buffer, 5.5 μl 25 mM MgCl2, 5 μl dNTP mix, 1.25 μl random hexamers, 0.5 μl RNase inhibitor and 31.25 U MultiScribe Reverse Transcriptase. The volume was adjusted to 25 μl with nuclease-free water. The reaction was first incubated at 25°C for 10 min to maximise binding of the primer to RNA, then incubated at 48°C for 30 min. Finally, the enzyme was heat-inactivated at 95°C for 5 min.
Quantitative real-time PCR
Real-time PCR experiments were carried out using the ABI PRISM 7000 Sequence Detection System (Applied Biosystems). Primers with melting temperatures of 58–60°C were designed according to the sequences of the genes carRA, carB and tef1, using Primer Express Software v2.0. (Applied Biosystems) (Table 1). The primers were used at concentrations of 150 nM and the generated amplicon sizes were 129 bp (carB), 138 bp (tef1) and 141 bp (carRA).
The amplification mixture (25 μl) consisted of 13 μl Platinum SYBR-Green qPCR Supermix UDG (Invitrogen) containing Platinum Taq DNA polymerase, uracil-DNA glycosylase (UNG), deoxynucleoside triphosphates with dUTP, ROX (6-carboxy-N,N,N′,N′-tetramethylrhodamine) as passive reference dye and cDNA template (1 μl). After activation of UNG (2 min; 50°C) and Taq polymerase (10 min; 95°C), 45 cycles of denaturation (15 s; 95°C), annealing and elongation (1 min; 60°C) were applied. The use of the ROX passive reference was recommended to correct for concentration inaccuracies in the reporter fluorescence by providing an internal reference to which the reporter fluorescence can be normalised.
To confirm that the SYBR Green fluorescence is a direct measure of accumulation of the product of interest subsequent to the PCR reaction cycles, dissociation curve analysis was performed. The amplification results were analysed with Sequence Detection Software (Applied Biosystems). The comparative \( 2^{{ - \Delta \Delta C_{{\text{t}}} }} \) method (Livak and Schmittgen 2001) was used to quantify the results obtained by real-time PCR with tef1 as the endogenous control.
In all experiments, appropriate negative controls containing no template cDNA were subjected to the same procedure to exclude or detect any possible contamination, e.g. by genomic DNA. Each sample was subjected to real-time PCR at least three times. All reaction mixtures were analysed by agarose gel electrophoresis to confirm that only one PCR product was synthesised.
Results and discussion
Library screening and car gene sequence analysis
B. trispora strain ATCC14272 was chosen for several different biotechnologically relevant reasons. Previous experiments showed a good induction of carotenogenesis in ATCC14272(−) when mated with ATCC14271(+), sporulation in both mating types was reproducibly high, and the mycelia grew fast in liquid medium (data not shown).
Due to the variety of B. trispora strains available and their described differences in carotenoid production (Mehta et al. 2003), we decided to isolate and sequence the car gene cluster of strain ATCC14272 (−). A genomic cosmid library of B. trispora strain ATCC14272 was generated as described in Materials and methods. The library was screened for different B. trispora genes, resulting in the isolation of carRA and carB genes (GenBank accession number AY884174).
The car gene region of strain ATCC14272 was sequenced to detect any differences in coding regions, promoter and terminator sequences from the known sequence of strain NRRL2457. The sequence and organisation of the car genes was identical to those of strain NRRL2457 (Rodriguez-Saiz et al. 2004). The carB gene is composed of three exons, the intergenic region is 611 bp in length, and the caRA gene comprises two exons (Fig. 1). Compared with the sequence obtained from strain NRRL2457, a mutation in the carB sequence was found 26 bp upstream of the carB stop codon. An A to C exchange results in a glycine codon instead of a valine codon.
Genomic organisation of the 5.3-kb DNA region encoding the car gene cluster in Blakeslea trispora strain ATCC14272. The exon-intron structure of carB and carRA, the bi-directional promoter, the MOT3 binding motif and the inverted repeat (IR) sequences at the 3′-end of the carRA gene, which form a putative loop structure including the 11-bp insertion, are indicated
Sequencing of the 3′ region of the carRA gene revealed that a 347-bp sequence in the 3′ region of carRA is inversely repeated 231 bp downstream of the carRA stop codon. In addition, an 11-bp insertion (GTGTGTTTTGT) was detected in the 3′ region of the carRA gene 114 bp downstream of the stop codon. This 11-bp insertion was found neither in B. trispora strain NRRL2457 nor in the carotenoid overproducing strain F-744 (Rodriguez-Saiz et al. 2004). Whether this DNA insertion has any function remains to be seen. Remarkably, the insertion is located in the centre of a putative loop region flanked by the 347-bp inverted repeat (IR) sequence (Fig. 1). The function of these IR sequences in regulation of carotenoid biosynthesis remains to be elucidated.
Potential regulatory elements in the bi-directionally orientated car promoter
Besides the Ste11 and APE binding sites published for the NRRL2457 car gene promoter identified by computer analysis using the TRANSFAC 6.0 public database (http://www.gene-regulation.com), an additional binding site (CAGGCA) for the zinc finger protein MOT3, a transcriptional regulator of pheromone signalling in Saccharomyces cerevisiae (Grishin et al. 1998) was found. This binding site is located in the regulatory sequence 79 bp upstream of the carB gene (Fig. 1). The same binding motif can be found in the Mucor circinelloides carB promoter sequence (GenBank accession number AJ238028).
Northern analysis of the carRA and carB genes
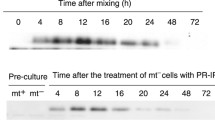
Although an increase in carotenoid production during mating of Zygomycetes has been shown (Mehta et al. 2003; Lopez-Nieto et al. 2004), no data were available on the influence of mating of (+) and (−) strains on the regulation of car gene transcription. It was therefore unclear whether the induction of carotenoid production was due to increased mRNA steady state levels of the biosynthetic genes. Transcription of carB and carRA is most likely mediated by the bi-directional promoter located in the intergenic region. Transcription of both carB and carRA was analysed by northern hybridisation using RNA samples obtained from B. trispora grown for 48 h after spore inoculation in liquid medium. Strains of (+) and (−) mating type were grown under either mating or non-mating conditions. The results are shown in Fig. 2.
A significant influence of mating conditions on car gene transcription was evident. Only in mated cultures was a strong increase in the amount of carRA and carB transcript observed. No signal for car gene transcripts was obtained in single cultures.
These findings led to the conclusion that mating significantly influences car gene transcription. Furthermore, the increased mRNA steady state levels of car genes during mating correlated well with an increase in β-carotene production in mated cultures (Mehta et al. 2003). Because trisporic acid synthesis is stimulated by reciprocal diffusion of mating-type-specific pheromones (Caglioti et al. 1967), carotenoid synthesis barely occurs in hyphae when mating is not triggered.
Quantitative real-time PCR
Real-time PCR was performed to confirm the data obtained by northern analyses and to compare transcript levels of carB and carRA genes directly. To analyse and quantify mating-dependent carB and carRA gene transcription, quantitative real-time PCR was established.
The associated software for quantitative real-time PCR used the exponential phase of PCR for quantification. The cycle threshold (Ct) was determined for each sample and used for analysis. Ct is the point (cycle number) at which the signal is detected above the background and the amplification reaction is in an exponential phase. The more abundant the template, the earlier this point is reached. For sample analysis, relative quantification was used. An endogenous control gene, i.e. the tef1 gene, was used to normalise the results of a variable target gene and to correct for sample-to-sample variations.
Tef1 transcription analysis
The endogenous control should have a constant level of transcription throughout the experiment and should not be affected by the experimental conditions. The control gene should also be transcribed at a similar level as the gene of interest and the range of linear amplification should be known (Bustin 2000). Therefore, the tef1 gene encoding the translation elongation factor 1 α was cloned.
Tef1 is one of the most abundant proteins in eukaryotic cells (Slobin 1980). Therefore, tef1 is a good candidate for an endogenous control transcript in quantitative real-time PCR. The steady-state level of tef1 mRNA was first monitored by northern blot analysis to verify that tef1 transcription occurs independently of the developmental status of B. trispora mycelia in submerged cultures and independently of the mating situation (Fig. 2c).
Quantitative analysis of the relative transcript levels of carRA and carB genes
Results obtained for carB and carRA transcripts by real-time PCR were normalised to the tef1 gene. For this purpose, the comparative \(2^{{ - \Delta \Delta C_{t} }} \) method for calculation (Livak and Schmittgen 2001) was employed. The \(2^{{ - \Delta \Delta C_{t} }} \) method determines relative expression levels compared to a control. The amount of target template is normalised to tef1 and relative to the calibrator. As the calibrator (value=1), the transcript level of the carRA gene in a single culture of the (+) strain 24 h after spore inoculation was used.
Consistent with the results obtained by northern analyses, induction of car genes is induced only in hyphae under mating conditions (Fig. 3).
Relative quantification by real-time PCR of a carRA and b carB gene transcripts in non-mated and mated cultures over a 72-h period in submerged cultures of B. trispora. All results were standardised to tef1 mRNA steady-state levels. CarRA transcripts at 24 h in single culture of the (+) strain was used as the calibrator (value=1) for relative quantification of the mRNA levels of all samples. c A direct comparison of mating-induced mRNA transcription levels of carB and carRA
For carRA, a 36-fold increase in mRNA steady state level was observed 24 h after spore inoculation, with a 41-fold increase for carB in mated cultures (Fig. 3a, b). In cultures containing a single strain, only weak transcription of car genes was detected at this time point. Maximum mating-dependent induction of both carRA (128-fold) and carB (148-fold) occurred 48 h after spore inoculation. At the 72-h time point, the induction of carB and carRA decreased to 103- and 87-fold, respectively. A slight induction of car gene transcription, up to 10-fold after 48 h and 15-fold after 72 h, also occurred in cultures containing a single strain.
Besides binding sites for Ste11, other transcription factor binding sites, e.g. for APE, are present in the bi-directional promoter of the car genes. Therefore, stimuli other than mating might also play a role in gene regulation. This notion might explain the increase of carB and carRA transcription in later stages of fermentation.
Furthermore, it has been shown in B. trispora that carotenogenesis was induced not only by mating-type-specific pheromones but also by other factors, e.g. cAMP (Dandekar and Modi 1980), hydrogen peroxide (Jeong et al. 1999), or Span 20 (Kim et al. 1997). The car genes of Mucor circinelloides showed coordinated regulation of their transcription by light, suggesting a complex mode of transcriptional control (Velayos et al. 2000). A light-dependent carotenoid accumulation has also been described for P. blakesleeanus (Avalos et al. 1993). In contrast, in B. trispora carotenoid biosynthesis was suggested to occur independently of light (Sutter 1970). Under all conditions applied here, both carRA and carB mRNA steady state levels increased at comparable levels (Fig. 3c). Based on the most likely assumption that this increase was due to enhanced transcriptional initiation, there is no preferred direction for transcription mediated by the bi-directional promoter. Due to the fact that the extent of car gene induction in non-mated cultures differed by at least one order of magnitude to the induction achieved in mated cultures, we concluded that regulation of car genes in B. trispora is regulated mainly by mating-dependent signals.
These results will be important for future improvement of strains with industrial importance because they form the basis for manipulation of carotenoid biosynthesis. For example, overexpression of carB and carRA genes under the control of mating-independent promoters (e.g. tef1) would circumvent the necessity for co-cultivation of strains of different mating types and, therefore, result in the optimisation of biotechnological production of carotenoids.
References
Aragón CMG, Murillo FJ, De la Guardia MD, Cerdá-Olmedo E (1976) An enzyme complex for the dehydrogenation of phytoene in Phycomyces. Eur J Biochem 63:71–75
Arrach N, Fernández-Martin R, Cerdá-Olmedo E, Avalos J (2001) A single gene for lycopene cyclase, phytoene synthase and regulation of carotene biosynthesis in Phycomyces. Proc Natl Acad Sci USA 98:1687–1692
Avalos J, Bejarano ER, Cerdá-Olmedo E (1993) Photoinduction of carotenoid biosynthesis. Methods Enzymol 214:283–294
Ballance DJ, Buxton FP, Turner G (1983) Transformation of Aspergillus nidulans by the orotidine-5′-phosphate decarboxylase gene of Neurospora crassa. Biochem Biophys Res Commun 112:284–289
Bartley GE, Scolnik PA (1995) Plant carotenoids: pigments for photoprotection, visual attraction and human health. Plant Cell 7:1027–1038
Britton G, Liaaen-Jensen S, Pfander H (1998) Carotinoids. Birkhäuser, Basel
Bustin SA (2000) Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 25:169–193
Caglioti L, Cainelli G, Camerino B, Mondelli R, Prieto A, Quilico A, Salvatore T, Selva A (1967) The structure of trisporic-C acid. Tetrahedron 7:175
Dandekar S, Modi VV (1980) Involvement of cyclic AMP in carotenogenesis and cell differentiation in Blakeslea trispora. Biochim Biophys Acta 628:398–406
Grishin AV, Rothenberg M, Downs MA, Blumer KJ (1998) Mot3, a Zn finger transcription factor that modulates gene expression and attenuates mating pheromone signaling in Saccharomyces cerevisiae. Genetics 149:879–892
Guardia MD de la, Aragón CMG, Murillo FJ, Cerdá-Olmedo E (1971) A carotenogenic enzyme aggregate in Phycomyces: evidence from quantitative complementation. Proc Natl Acad Sci USA 68:2012–2015
Jeong JC, Lee IY, Kim SW (1999) Stimulation of beta-carotene synthesis by hydrogen peroxide in Blakeslea trispora. Biotechnol Lett 21:683–686
Kim SW, Seo WT, Park YH (1997) Enhanced production of beta-carotene from Blakeslea trispora with Span 20. Biotechnol Lett 19:561–562
Lee PC, Schmidt-Dannert C (2002) Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl Microbiol Biotechnol 60:1–11
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the \(2^{{ - \Delta \Delta C_{t} }} \) method. Methods 25:402–408
Lopez-Nieto MJ, Costa J, Peiro E, Mendez E, Rodriguez-Saiz M, de la Fuente JL, Cabri W, Barredo JL (2004) Biotechnological lycopene production by mated fermentation of Blakeslea trispora. Appl Microbiol Biotechnol 66:153–159
Mayne ST (1996) Beta-carotene, carotenoids and disease prevention in humans. FASEB J 10:690–701
Mehta BJ, Obraztsova IN, Cerda-Olmedo E (2003) Mutants and intersexual heterokaryons of Blakeslea trispora for production of beta-carotene and lycopene. Appl Environ Microbiol 69:4043–4048
Nelis HJ, De Leenheer AP (1991) Microbial sources of carotenoid pigments used in foods and feeds. J Appl Bacteriol 70:181–191
Osiewacz HD (1994) A versatile shuttle cosmid vector for the efficient construction of genomic libraries and for the cloning of fungal genes. Curr Genet 26:87–90
Rodriguez-Saiz M, Paz B, De La Fuente JL, Lopez-Nieto MJ, Cabri W, Barredo JL (2004) Blakeslea trispora genes for carotene biosynthesis. Appl Environ Microbiol 70:5589–5594
Ruiz-Hidalgo MJ, Benito EP, Sandmann G, Eslava AP (1997) The phytoene dehydrogenase gene of Phycomyces: regulation of its expression by blue light and vitamin A. Mol Gen Genet 253:734–744
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Schimek C, Kleppe K, Saleem AR, Voigt K, Burmester A, Wostemeyer J (2003) Sexual reactions in Mortierellales are mediated by the trisporic acid system. Mycol Res 107:736–747
Slobin LI (1980) The role of eucaryotic factor Tu in protein synthesis. The measurement of the elongation factor Tu content of rabbit reticulocytes and other mammalian cells by a sensitive radioimmunoassay. Eur J Biochem 110:555–563
Smith TAD (1998) Carotinoids and cancer: prevention and potential therapy. Br J Biomed Sci 55:268–275
Sutter RP (1970) Effect of light on beta-carotene accumulation in Blakeslea trispora. J Gen Microbiol 64:215–221
Sutter RP, Harrison TL, Galasko G (1974) Trisporic acid biosynthesis in Blakeslea trispora via mating type specific precursors. J Biol Chem 249:2282–2284
Velayos A, Eslava AP, Iturriaga EA (2000) A bifunctional enzyme with lycopene cyclase and phytoene synthase activities is encoded by the carRP gene of Mucor circinelloides. Eur J Biochem 267:5509–5519
Verdoes JC, Krubasik KP, Sandmann G, van Ooyen AJ (1999) Isolation and functional characterisation of a novel type of carotenoid biosynthetic gene from Xanthophyllomyces dendrorhous. Mol Gen Genet 262:453–461
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmidt, A.D., Heinekamp, T., Matuschek, M. et al. Analysis of mating-dependent transcription of Blakeslea trispora carotenoid biosynthesis genes carB and carRA by quantitative real-time PCR. Appl Microbiol Biotechnol 67, 549–555 (2005). https://doi.org/10.1007/s00253-005-1941-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-1941-2