Abstract
A series of high-copy-number Escherichia coli expression vectors equipped with an oxygen-sensitive promoter P vgb of Vitreoscilla hemoglobin (encoded by the vgb gene) were constructed and characterized. Plasmid pKVp containing P vgb was inducible by low oxygen tension, while plasmid pKVpP containing a partition (par) region from plasmid pSC101 ligated to P vgb provided inheritable stability for the vectors in the absence of ampicillin. Plasmid pKVpV had the Vitreoscilla hemoglobin operon vgb ligated to P vgb , while a construct containing P vgb , the vgb operon and a par region constituted plasmid pKVpPV. Shake-flask studies demonstrated that plasmids pKVpV and pKVpPV expressed higher levels of Vitreoscilla hemoglobin under low aeration condition (5% air saturation in water) compared with the levels observed under strong aeration (20% air saturation in water). Introduction of either the enhanced green fluorescent protein (eGFP) gene egfp or the toluene dioxygenase (TDO) gene tod into either pKVpV (P vgb , vgb operon) or pKVpPV (P vgb , vgb operon, par) slightly attenuated (∼30%) the strong expression of VHb under low aeration. However, all displayed approximately a three-fold increase versus that observed for strong aeration. Recombinant E. coli harboring either pKVp-E (P vgb , egfp) or pKVpP-E (P vgb , par, egfp) displayed at least a two-fold increase in eGFP expression under conditions of low aeration and absence of antibiotic, compared with that under strong aeration after 24 h of cultivation. Strong expression of TDO was also observed using low aeration in recombinant E. coli harboring pKVpPV-T (P vgb , vgb operon, par, tod) or pKVpP-T (P vgb , par, tod). Plasmids containing the par region were stable over 100 generations. These results indicate that the novel expression system combining plasmid stability over the cell growth phase and a promoter inducible by low oxygen tension will be very useful for high-density production of foreign proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitreoscilla hemoglobin (VHb) expression is elevated by low oxygen tension via a fumarate and nitrate reductase regulator (FNR)-dependent mechanism (Tsai et al. 1995). When the Vitreoscilla hemoglobin gene (vgb) was introduced into Escherichia coli, Pseudomonas spp and fungi, cell growth and the yield of target protein were increased significantly (DeModena et al. 1993; Dikshit et al. 1990; Khosla and Bailey 1988; Khosravi et al. 1990). When introduced into E. coli along with its promoter, the strong expression of the vgb gene was confirmed by transcriptional analysis (Dikshit et al. 1990). VHb may act as an oxygen trap at low oxygen concentration in vivo to permit microbial strains to grow and survive in microaerophilic environments (Khosla et al. 1990b).
When the dissolved oxygen (DO) concentration was shifted from 20% to 5% air saturation, vgb transcription increased about five times (Khosla and Bailey 1989). Both FNR and cyclic adenosine monophosphate (cAMP) receptor protein (CRP) were needed for the activation of the vgb promoter, with the FNR binding site located at −41.5 nucleotides and the CRP binding site positioned at −97 nucleotides relative to the promoter, respectively (Tsai et al. 1995; Khosla et al. 1990a, b). Khosla et al. (1990a) reported that β-galactosidase under the control of the vgb promoter accounted for up to 10% of the total cellular protein of E. coli in bioreactor cultures. These results indicate that the vgb promoter is regulated by oxygen at the transcriptional level, with low-aeration conditions sufficient to induce VHb gene expression.
There are a number of systems that enable the stable inheritance of plasmids during exponential growth phase. It is well accepted that the par region of plasmid pSC101 is a cis-acting locus that promotes stable plasmid inheritance (Meacock and Cohen 1980). Within plasmid pSC101, a 370-bp segment adjacent to the replication region controls the active distribution of plasmid DNA to the daughter cell (Conley and Cohen 1995). Furthermore, the region is not associated directly with plasmid replication (Miller and Cohen 1993). par-defective plasmids show reduced average copy number in each cell (Biek and Cohen 1992; Manen et al. 1990) and are preferentially lost from cells carrying wild-type pSC101 replicons (Tucker et al. 1984). Since the pSC101 par locus stabilizes the inheritance of a variety of plasmids, such as pACYC184 (Meacock and Cohen 1980), R1 (Gustafsson et al. 1983), oriC, miniF, p15A (Miller et al. 1990) and RK2 (Roberts and Helinski 1992), the mechanism of the segregation of these genetically diverse replicons may be similar (Conley and Cohen 1995). Inserting the par region of pSC101 into plasmid pBR327 (the derivative of pBR322 with ColE1 origin) results in a plasmid, pBR327par, which is more stable than pBR327 (Zurita et al. 1984).
In this study, a series of high-copy-number plasmids containing the vgb promoter P vgb were constructed. The par region and/or vgb operon were ligated to P vgb to determine whether such a combination of promoter and plasmid stabilization factor could enhance protein production at low oxygen concentration.
Materials and methods
Biochemical reagents
T4 DNA ligase and DNA polymerase were from Takara Co. (Dalian, China). Restriction enzymes were purchased from New England Biolabs (Cambridge, Mass., USA). Ampicillin was from Gibco (Gibco-BRL, France). Calf intestinal alkaline phosphatase was obtained from Promega (Madison, Wis., USA). Other reagents were domestic products of analytical grade.
Strains and plasmids
All strains and plasmids used in this study are listed in Table 1. Conditions for the culture of E. coli strains under different oxygen levels were as described by Dikshit et al. (1990). For shake-flask experiments, 50 ml of culture broth in a 250-ml flask was inoculated at 200 rpm to achieve strong-aeration conditions, whereas 150 ml of cell culture was inoculated into a 250-ml flask shaken at 100 rpm for the low-aeration conditions. Cultures were inoculated with 1% (v/v) seed cultures, which were prepared overnight at 37°C.
Plasmid construction
Plasmid pKVp was constructed by subcloning the 135-bp NdeI–EcoRI fragment containing the vgb promoter from pBR322-vgb (employing primers Pvgb-up, Pvgb-down; shown in Table 2) into the corresponding sites in plasmid pKK223-3 (Brosius and Holy 1984). The 999-bp NdeI–AseI fragment of the vgb operon containing vgb promoter, vgb gene and vgb terminator from pBR322-vgb was inserted into the corresponding sites in pKVp to obtain pKVpV (using primers vgb-up, vgb-down; shown in Table 2). The plasmids pKVpP and pKVpPV were constructed by subcloning the 369-bp NdeI–NdeI fragment containing the par region from plasmid pSC101 (employing primers par-up, par-down; shown in Table 2) into the corresponding sites in plasmids pKVp and pKVpV, respectively. The enhanced green fluorescent protein (eGFP) gene egfp and toluene dioxygenase (TDO) gene todC1C2BA were successively cloned into the vectors pKVp, pKVpP, pKVpV and pKVpPV (using primers eGFP-up or TDO-up with eGFP-down or TDO-down; shown in Table 2) between EcoRI and HindIII or BamHI sites to obtain two groups of detection plasmids. Plasmid pKH-E was constructed by cloning egfp into the EcoRI and HindIII of pKKH sites (using primers eGFP-up, eGFP-down). One group used for eGFP expression included pKVp-E, pKVpP-E, pKVpV-E and pKVpPV-E. Another group used for TDO production contained pKVp-T, pKVpP-T, pKVpV-T and pKVpPV-T. All the constructed vectors were verified by nucleotide sequence determination (BioAsia Life Technologies, Shanghai, China). The primers used were synthesized by BioAsia Life Technologies and are listed in Table 2.
Analysis of VHb concentration using CO-difference spectra
The VHb concentration produced by various recombinant E. coli strains grown under different oxygen conditions (see Strains and plasmids) was calculated using an extinction coefficient of 274 mM−1 cm−1 at 419–436 nm, as described by Dikshit and Webster (1988). The CO-difference spectra were obtained using a UV 8500 spectrophotometer with UV-Vis Analyst software (Biotech, Shanghai, China).
Analysis of eGFP fluorescent intensity using fluorescence spectrophotometer
The GFP, encoded by the gap gene of the jellyfish Aequorea victoria (Cubitt et al. 1995; Prasher 1992), requires only oxygen and UV or blue light for the emission of green fluorescence (Andersen et al. 1998). Wild-type GFP was enhanced (eGFP) by modifications affecting the excitation peaks, brightness and protein levels (Yang et al. 1996). Bacteria were transformed with an eGFP-bearing plasmid as reporter. To measure the fluorescent intensity, samples were analyzed with a Hitachi F-450 fluorescence spectrophotometer with a 488 nm excitation source, using FL solution software (Hitachi, Tokyo, Japan). Cells were washed once in phosphate-buffered saline (PBS) and resuspended in 1 ml PBS. The emission maximum was 509 nm, in agreement with published values (Chalfıe et al. 1994). Log-phase cells of eGFP-tagged and non-tagged strains were used as positive and negative controls, respectively.
TDO activity measurement using spectrophotometer
TDO protein converts a wide range of aromatic compounds into their cis-glycol forms, including indole into indigo (Ensely et al. 1983). We modified the assay described by Woo et al. (2000). After determination of the optical density, a 1-ml sample of the culture was immediately transferred to a microcentrifuge tube, centrifuged for 1 min (14,000 rpm at 4°C), washed with PBS (pH 7.2), centrifuged and resuspended in 1 ml PBS. To initiate the reaction, 25 μl indole in N,N-dimethlylformamide was added to the cell suspension and the formation of indigo was monitored spectrophotometrically at 600 nm over 30 min against a control which did not contain indole. The initial rate of indigo formation was determined by plotting the increase in indigo absorbance as a function of time. The enzyme activity was defined as the initial rate of indigo formation normalized to the protein content of the sample (indigo absorbance mg−1 min−1).
Plasmid stability and plasmid copy number assays
Stability assays were carried out according to Easter et al. (1997). In brief, cultures of recombinant E. coli strains were incubated overnight under antibiotic selection at 37°C. An aliquot of each culture was diluted in pre-warmed LB broth and grown with antibiotics to the mid-log phase at 37°C. At time zero, cells were diluted 106-fold into LB broth and allowed to grow without antibiotics overnight to stationary phase. This was defined as 20 generations of log-phase growth. To determine the fraction of cells that retained the plasmid, aliquots were plated onto LB agar; and the resulting colonies were tested for antibiotic resistance by replica-plating onto LB agar with and without antibiotics. Plates were incubated at 37°C for 16–20 h. For statistical analysis, we only considered plates which displayed between 30 and 300 colonies. Plasmid copy numbers were determined as described by Lewington and Day (1986).
Results
Construction of plasmids containing the vgb promoter (Pvgb)
A series of high-copy-number E. coli expression vectors equipped with an oxygen-sensitive promoter P vgb of VHb encoded by the vgb gene were constructed from the high-level expression vector pKK223-3. Plasmid pKVp containing P vgb was generated to selectively express genes under low oxygen tension. Plasmid pKVpP containing a partition (par) region from plasmid pSC101 ligated to P vgb was constructed to provide inheritable stability for the vectors in the absence of ampicillin. Plasmid pKVpV had the VHb operon vgb ligated to P vgb , while P vgb , the vgb operon and the par region constituted plasmid pKVpPV. Both were expected to enhance the expression of the target proteins under low oxygen tension; and pKVpPV was designed to combine this property with increased plasmid stability over time. All four new vectors maintained the basic characteristics of pKK223-3, including the origin of replication and termination, multiple cloning sites and antibiotic selectable marker (Fig. 1).
Original plasmid pKK223-3 and derivative plasmids containing vgb promoter. Four different plasmids containing vgb promoter were constructed from pKK223-3. The multiple cloning site (MCS/MSC) region of these four new vectors contains EcoRI, SalI, AccI, BamHI, SmaI, XmaI, PstI and HindIII sites. Ap (R) β-Lactamase gene, par partition region from pSC101, vgb operon VHb operon (vgb promoter, vgb gene, vgb terminator), P vgb vgb promoter, P tac tac promoter, 5S 5S rRNA region, rrnBT 1 T 2 rrnB T1 and T2 terminators, egfp eGFP gene, tod C1C2BA TDO gene C1C2BA
Determination of hemoglobin concentration in vivo
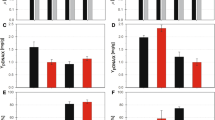
The VHb concentrations of recombinant E. coli JM83 strains carrying various plasmids at 24 h are shown in Fig. 2. Under low aeration (5% air saturation), the hemoglobin concentration of E. coli JM83 harboring pKVpPV (P vgb , par, vgb operon) was 66.53±3.85 nmol g−1 wet cell weight (average of three parallel experiments), approximately the same as the recombinant containing pKVpV (P vgb , vgb operon). When co-expressed with either the eGFP gene or the TDO gene, the VHb produced by different recombinants was reduced approximately 30% from the originals (Fig. 2). In all cases studied, elevated expression was only observed under low aeration conditions (approximately three- to four-fold that of strong aeration). The VHb concentration ranged over 12–18 nmol g−1 wet cell weight for plasmids harboring recombinants grown under strong aeration.
Recombinant E. coli VHb expression level measured by CO-differential spectroscopy. The concentration unit (nmol g−1) was defined as the amount of VHb per constant wet cell weight. Experimental details are given in the section: Analysis of Vitreoscilla hemoglobin concentration using CO_difference spectra. The data shown are the average±SD for three individual observations. Recombinant E. coli JM83 harbored the plasmids: a pKVpV (P vgb , vgb operon), b pKVpPV (P vgb , par, vgb operon), c pKVpV-E (P vgb , vgb operon, egfp), d pKVpPV-E (P vgb , par, vgb operon, egfp), e pKVpV-T (P vgb , vgb operon, tod), f pKVpPV-T (P vgb , par, vgb operon, tod)
eGFP production by recombinant E. coli
The fluorescent intensity of the 24-h culture of E. coli JM83 harboring pKVp-E (P vgb , egfp) grown under strong aeration and in the presence of ampicillin was defined as 1.0. Under strong aeration and with the addition of isopropyl-1-thio-β-d-galactopyranoside (IPTG), the relative fluorescence intensity of E. coli JM83 harboring pKH-E (P tac , egfp) reached its highest level at 18 h and remained stable from then on. Under strong aeration conditions, the relative fluorescent intensity reached 1.69 in E. coli JM83 harboring pKVpP-E (P vgb , par, egfp) over a 24-h cultivation period. Under low aeration, the same recombinant strain emitted the highest relative fluorescent intensity (6.02; Fig. 3b), compared with that of E. coli JM83 harboring pKVp-E (4.6; P vgb , egfp) and pKVpP-E (P vgb , par, egfp; Fig. 3a). Unlike that observed for low aeration growth conditions, the relative fluorescence of the recombinant strains under strong aeration was similar after 6 h (Figs. 3a,b). At 24 h, the presence of the par region in the recombinants improved eGFP production compared with that without the par region. In the presence of the vgb operon, the relative fluorescent intensity observed in E. coli JM83 cells harboring pKVpV-E (P vgb , vgb operon, egfp) and pKVpPV-E (P vgb , par, vgb operon, egfp) was reduced to 1.7 and 2.2, respectively. At the same time, aeration did not affect the relative fluorescent intensity after 12 h of cultivation as much as for the recombinants without the vgb operon, although the par region did slightly increase the relative fluorescent intensity (Figs. 3c,d).
Production of eGFP by recombinant E. coli under the control of the vgb promoter. The intensity of fluorescence emitted by E. coli JM83 containing plasmid pKVp-E (P vgb , egfp) cultured in the presence of ampicillin under strong aeration was considered 1.0. a pKVp-E (P vgb , egfp), b pKVpP-E (P vgb , par, egfp), c pKVpV-E (P vgb , vgb operon, egfp), d pKVpPV-E (P vgb , par, vgb operon, egfp). A Cells cultured under strong aeration, B cells cultured under low aeration, + ampicillin present, − ampicillin absent. Values indicate average±SD of four parallel analyses
Production of TDO by recombinant E. coli
Under low aeration, the TDO activities were approximately two-fold stronger in recombinant E. coli harboring pKVpP-T (P vgb , par, tod), or pKVpPV-T (P vgb , par, vgb operon, tod) than that for the same strains grown under strong aeration (Fig. 4). An earlier expression of TDO was also observed—2 h when cultivated under low aeration conditions compared with 4–6 h under strong aeration.
Plasmid stability and copy number assay
Plasmid stability was studied over 100 microbial generations. The results demonstrated that the par region conferred additional stability on the cloning vectors (Fig. 5). After 100 generations of growth, the recombinant harboring pKVpV-E (P vgb , vgb operon, egfp) lost over 90% of its plasmid (the most unstable plasmid in this study), while the recombinant containing pKVp (P vgb ) lost 70% of its plasmids (the most stable plasmid among the non-par-containing plasmids; Fig. 5). In all cases, regardless of the aeration intensity, recombinants harboring the par region displayed complete plasmid stability over 100 growth generations in the absence of ampicillin (Fig. 5). Plasmids were more stable under strong aeration than under low aeration. The plasmids bearing egfp lost more plasmids than their original vectors (without egfp) when cells were grown under the same conditions (Figs. 5c,d). It was also found that the copy number of the vectors constructed in this study was about 300–500, which was around ten times higher than the original plasmid pKK223-3. This increase in plasmid copy number further contributed to enhance protein expression in the host microorganism.
Plasmid stability is supported by par in recombinant E. coli grown in LB medium in the absence of antibiotics. a pKVp (P vgb ), pKVpP (P vgb , par), b pKVpV (P vgb , vgb operon), pKVpPV (P vgb , par, vgb operon), c pKVp-E (P vgb , egfp), pKVpP-E (P vgb , par, egfp), d pKVpV-E (P vgb , vgb operon, egfp), pKVpPV-E (P vgb , par, vgb operon, egfp). A Cells cultured under strong-aeration conditions, B cells cultured under low aeration conditions. Values are the average±SD of three parallel analyses
Discussion
In all aerobic microbial fermentations, oxygen supply is always the most difficult engineering problem, especially when a high cell density is desirable to achieve economic efficiency. For genetically engineered microorganisms, plasmid stability is a factor affecting the cost of the process. With the growing diversity of available genetic engineering tools, it is more feasible to solve problems in the fermentation process using molecular biological techniques.
VHb, which binds to subunit I of cytochrome bo ubiquinol oxidases, effectively utilizes DO in microbial cultures (Park et al. 2002). Heterologous expression of the VHb gene in host bacteria often promotes cell growth, increases biomass yield and enhances the activity of target proteins (Dikshit et al. 1990; Khosla and Bailey 1988). Past studies demonstrated that the regulation of vgb expression under its native promoter was at the transcriptional level in E. coli (Dikshit et al. 1990). Although the vgb promoter is not responsive to nitrosative and oxidative stress in E. coli (Frey et al. 2003), the expression of P vgb is sensitive to the DO concentration (Khosla and Bailey 1989). In cultures of E. coli, the vgb promoter was activated when the DO concentration was lowered from 20% to 5% air saturation (Khosla and Bailey 1989). The vgb promoter offers advantages over other alternatives in that induction is more easily controlled and, therefore, no process temperature shift or the addition of expensive chemicals such as IPTG or galactose is necessary. Thus, P vgb induction is readily stimulated, since reduction of the DO below 20% is facilitated when cells are grown to high density, or by lowering the oxygen supply via reduction in either the air inflow rate or the stirring rate in the fermentor.
It was described previously that the par region of pSC101 is fully capable of stabilizing a related par-plasmid that had an entirely different mode of replication (Gustafsson et al. 1983). The par activity was dependent on its ability to produce supercoiling at the replication origin rather than on the overall superhelical density of the plasmid DNA (Conley and Cohen 1995). The deletion of the rom region of pKK223-3 weakened the binding of RNAI to RNAII, resulting in an increase in plasmid copy number (Jing et al. 1993). The high-copy-number plasmid is more useful for the overproduction of foreign proteins, especially for the production of proteins whose high level expression does not perturb the host cells (Zurita et al. 1984), although it should be noted that plasmid stability decreased with increasing plasmid copy number.
The vgb promoter P vgb was used with all plasmids in this study. These were constructed to contain genes of the vgb operon, the partition region (par), eGFP (egfp), and TDO (tod), either individually or in combination. The results demonstrate that such combinations can produce advantages for both protein expression and plasmid stability in the recombinant host microorganism.
Under low aeration, the hemoglobin concentration of E. coli JM83 harboring pKVpPV (P vgb , par, vgb operon) was approximately the same as the recombinant containing pKVpV (P vgb , vgb operon). When co-expressed with the eGFP gene or TDO gene, the VHb produced by the host recombinants was reduced about 30% from the original (Fig. 2). In all cases, strong expression was only observed under low-aeration conditions. These results clearly demonstrated that the effect of P vgb was induced under low aeration. The reduction of VHb production was likely due to the simultaneous production of either eGFP or TDO, by lowering the ATP, amino acids and other components required for protein synthesis.
Under strong-aeration conditions, the relative fluorescent intensity reached 1.29 in E. coli JM83 harboring pKVpP-E (P vgb , par, egfp) over 24 h cultivation (Fig. 3b). Under low aeration, the same recombinant strain emitted the highest relative fluorescent intensity (6.02; Fig. 3b), compared with E. coli JM83 harboring pKVp-E (4.6; P vgb , egfp; Fig. 3a). Plasmid stability provided by the par region no doubt accounted for this difference in eGFP production.
Unlike under low aeration, it was observed that the relative fluorescent intensity of E. coli JM83 harboring pKVp-E and pKVpP-E was approximately the same (around 1.0) under strong aeration regardless of growth time (Fig. 3a,b), in agreement with the observation that the P vgb is responsive to low DO concentration. The presence of the par region in the recombinants improved eGFP production compared with that observed for recombinants without the par region (Fig. 3b). In the presence of the vgb operon, reduced relative fluorescent intensity of below 2.5 was observed in E. coli JM83 harboring pKVpV-E (P vgb , vgb operon, egfp) or pKVpPV-E (P vgb , par, vgb operon, egfp) (Figs. 3c,d). Again, such a result is likely due to the competition for energy and substrates between the VHb and eGFP syntheses.
Strong protein expression was also observed in recombinant E. coli harboring pKVpP-T (P vgb , par, tod) or pKVpPV-T (P vgb , par, vgb operon, tod) under low oxygen aeration. TDO activities were approximately two-fold stronger under low oxygen tension than that under strong aeration (Fig. 4), further strengthening the argument that low oxygen is an effective means of inducing P vgb .
In all cases, regardless of aeration intensity, recombinant E. coli cultured in the absence of antibiotics displayed no loss in copy number over 100 generations when harboring plasmids containing the par region (Fig. 5). The plasmid instability rate under oxygen-limiting conditions was higher than that observed under strong-aeration conditions without the par region, as less energy was available to maintain the plasmid under low aeration. The plasmids bearing egfp were more easily lost than their original vectors (without egfp) under the same conditions (Fig. 5c,d), demonstrating again that low oxygen tension and expression of foreign protein were a burden for cell growth and, therefore, the cells easily disposed of these plasmids. It was also found that the copy number of the vectors constructed in this study was about 300–500, which was around ten times that of the original plasmid pKK223-3. This increase in plasmid copy number contributed further to enhance protein expression in the host microorganisms.
When co-expressed with other foreign proteins, such as eGFP or TDO, VHb showed contrasting effects. The maximal TDO enzyme activity was obtained by co-expression of TDO and VHb via pKVpPV-T (P vgb , vgb operon, par, tod; Fig. 4). Yet, the strongest relative fluorescent intensity was emitted by E. coli JM83 containing plasmid pKVpP-E (P vgb , par, egfp) without VHb expression (Fig. 3). It appeared that the expression of VHb not only played an active role in cell metabolism and target protein expression, but also served as a burden when expressed in large amount, especially when VHb and target protein were transcribed under the same vgb promoter P vgb . The difference between eGFP and TDO activities may be due to the functions of the expressed proteins. Thus, when co-expressed with enzymes such as TDO which requires oxygen as a substrate, VHb showed a positive effect in promoting TDO activity (Fig. 4). In contrast, the activity of eGFP does not rely so much on oxygen (Chalfıe et al. 1994). Therefore, in the latter case, the expression of VHb was a burden, resulting in reduced expression of eGFP and reduced fluorescent intensity (Fig. 3c,d).
In conclusion, plasmids constructed in this study will be very useful for fermentative production of foreign proteins in E. coli hosts. For example, the vgb promoter allowed the transcription of a target gene to be activated by a shift in oxygen concentration, while the expression of VHb could lead to enhanced expression of a foreign protein by the same host. The presence of par stabilized the plasmids in E. coli and could lead to elevated production of target proteins, by minimizing loss of the exogenous gene over time. In addition, removal of the restriction sites for BamHI, SalI and AccI from outside the multiple cloning sites provides the new vectors with more flexibility for genetic manipulation. Finally, increased expression of foreign genes in E. coli was also facilitated by the high copy number of the vectors carrying the gene of interest. These advantages were demonstrated by the enhanced production of eGFP and TDO by E. coli harboring those plasmids.
References
Andersen JB, Sternberg C, Poulsen LK, Bjorn SP, Molin S (1998) New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Appl Environ Microbiol 64:2240–2246
Biek DP, Cohen SN (1992) Propagation of pSC101 plasmids defective in binding of integration host factor. J Bacteriol 174:785–792
Brosius J, Holy A (1984) Regulation of ribosomal RNA promoters with a synthetic lac operator. Proc Natl Acad Sci USA 81:6929–6933
Chalfıe M, Tu Y, Euskierchen G, Ward WW, Prasher DC (1994) Green fluorescent protein as a marker for gene expression. Science 263:802–805
Conley DL, Cohen SN (1995) Isolation and characterization of plasmid mutations that enable partitioning of pSC101 replicons lacking the partition (par) locus. J Bacteriol 177:1086–1089
Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien RY (1995) Understanding, improving and using green fluorescent proteins. Trends Biochem Sci 20:448–455
DeModena JA, Gutierrez S, Velasco J, Fernandez F, Fachini RA, Galazzo JL, Hughes DE, Matin JF (1993) The production of cephalosporin C by Acremonium chrysogenum is improved by the intracellular expression of a bacterial hemoglobin. Biotechnology 11:926–929
Dikshit KL, Webster DA (1988) Cloning, characterization and expression of the bacteria globin gene from Vitreoscilla in Escherichia coli. Gene 70:377–386
Dikshit KL, Dikshit RP, Webster DA (1990) Study of Vitreoscilla globin gene expression and promoter activity in E. coli through transcriptional fusion. Nucleic Acids Res 18:4149–4155
Easter CL, Sobecky PA, Helinski DR (1997) Contribution of different segment of the par region to stable maintenance of the broad-host-range plasmid RK2. J Bacteriol 179:6472–6479
Ensely BD, Ratzkin BJ, Osslund TD, Simon MJ, Wackett LP, Gibson DT (1983) Expression of naphthalene oxidation genes in Escherichia coli: results in the biosynthesis of indigo. Science 222:167–169
Frey AD, Koskenkorva TJ, Kallio PT (2003) Vitreoscilla hemoglobin promoter is not responsive to nitrosative and oxidative stress in Escherichia coli. FEMS Microbiol Lett 224:127–132
Gustafsson PH, Wolf-Watz H, Lind L, Johansson KE, Nordstrom K (1983) Binding between the par region of plasmids R1 and pSC101 and the outer membrane fraction of the host bacteria. EMBO J 2:27–32
Jing GZ, Huang Z, Liu ZG, Zou Q (1993) Plasmid pKKH: an improved vector with higher copy number for expression of foreign genes in Escherichia coli. Biotechnol Lett 15:439–442
Khosla C, Bailey JE (1988) Heterologous expression of a bacterial hemoglobin improves the properties of recombinant Escherichia coli. Nature 331:633–635
Khosla C, Bailey JE (1989) Characterization of the oxygen dependent promoter of the Vitreoscilla hemoglobin gene in Escherichia coli. J Bacteriol 171:5990–6004
Khosla C, Curtis JE, Bydalek P, Swartz JR, Bailey JE (1990a) Expression of recombinant proteins in Escherichia coli using an oxygen-responsive promoter. Bio/Technology 8:554–558
Khosla C, Curtis JE, Demodena J, Rinas U, Bailey JE (1990b) Expression of intracellular hemoglobin improves protein synthesis in oxygen-limited Escherichia coli. Bio/Technology 8:849–853
Khosravi M, Webster DA, Stark BC (1990) Presence of the bacterial hemoglobin gene improves α-amylase production of a recombinant E. coli strain. Plasmid 24:190–194
Lewington J, Day MJ (1986) A rapid electrophoretic method for the measurement of plasmid copy number. Lett Appl Microbiol 3:109–112
Manen DT, Goebel T, Caro L (1990) The par region of pSC101 affects plasmid copy number as well as stability. Mol Microbiol 4:1839–1846
Meacock PA, Cohen SN (1980) Partitioning of bacteria plasmid during cell division: a cis-acting locus that accomplishes stable plasmid inheritance. Cell 20:529–542
Miller CA, Cohen SN (1993) The partition (par) locus of pSC101 is an enhancer of plasmid incompatibility. Mol Microbiol 9:695–702
Miller CA, Beaucage SL, Cohen SN (1990) Role of DNA superhelicity in partitioning of the pSC101 plasmid. Cell 62:127–133
Park KM, Kim KJ, Howard AJ, Stark BC, Webster DA (2002) Vitreoscilla hemoglobin binds to subunit I of cytochrome bo ubiquinol oxidases. J Biol Chem 227:33334–33337
Prasher DC (1992) Primary structure of Aequorea victoria green-fluorescent protein. Gene 111:229–233
Roberts RC, Helinski DR (1992) Definition of a minimal plasmid stabilization system from the broad-host-range plasmid RK2. J Bacteriol 174:8119–8132
Tsai PS, Kallio PT, Bailey JE (1995) Fnr, a global transcriptional regulator of Escherichia coli, activates the Vitreoscilla hemoglobin promoter and intracellular VHb expression increases cytochrome d promoter activity. Biotechnol Prog 11:288–293
Tucker WT, Miller CA, Cohen SN (1984) Structural and functional analysis of the par region of the pSC101 plasmid. Cell 38:191–201
Woo HJ, Sanseverino J, Cox CD, Robinson KG, Sayler GS (2000) The measurement of toluene dioxygenase activity in biofilm culture of Psedomonas putida F1. J Microbiol Methods 40:181–191
Yang TT, Cheng L, Kain SR (1996) Optimized codon usage and chromophore mutations provide enhanced sensitivity with the green fluorescent protein. Nucleic Acids Res 24:4592–4593
Zurita M, Bolivar F, Soberon X (1984) Construction of plasmid pBR327par, a completely sequenced, stable derivative of pBR327 containing the par locus of pSC101. Gene 28:119–122
Acknowledgements
This research was supported by the National Nature Science Foundation of China for Distinguished Young Scholars (No. 30225001) and Key Items (No. 20334040). The State 863 Funds (No. 2002AA333030) also provided a financial contribution for this study. We are grateful for Prof. Z.Y. Shen (Dept. of Chemical Engineering, Tsinghua University) for the generous donation of plasmid pBR322-vgb and to Dr. S. Yasuda (Dept. of Microbial Genetics, National Institute of Genetics, Japan) for kindly providing us with plasmid pSC101.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, T., Chen, Jy., Zheng, Z. et al. Construction of highly efficient E. coli expression systems containing low oxygen induced promoter and partition region. Appl Microbiol Biotechnol 68, 346–354 (2005). https://doi.org/10.1007/s00253-005-1913-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-1913-6