Abstract
Nisin production in batch culture and fed-batch cultures (sucrose feeding rates were 6, 7, 8, and 10 g l−1 h−1, respectively) by Lactococcus lactis subsp. lactis ATCC 11454 was investigated. Nisin production showed primary metabolite kinetics, and could be improved apparently by altering the feeding strategy. The nisin titer reached its maximum, 4,185 IU ml−1, by constant addition of sucrose at a feeding rate of 7 g l−1 h−1; an increase in 58% over that of the batch culture (2,658 IU ml−1). Nisin biosynthesis was affected strongly by the residual sucrose concentration during the feeding. Finally, a mathematical model was developed to simulate the cell growth, sucrose consumption, lactic acid production and nisin production. The model was able to describe the fermentation process in all cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nisin is a heat-stable peptide and is produced by some strains of Lactococcus lactis. It is composed of 34 amino acids and has a molecular mass of 3,500 Da (Gross and Morell 1971). Nisin is widely used as a natural food preservative since it shows antimicrobial activity against most Gram-positive bacteria. It has been accepted as a safe and natural preservative in >50 countries (Hiroshi et al. 1999).
For the commercial application of large amounts of nisin, enhancement of its production is necessary. Optimal nisin production usually requires complex media and well-controlled factors such as temperature and pH (De Vuyst et al. 1996). Carbon-source regulation affects cell growth and nisin biosynthesis (Van’t Hul and Gibbons 1997). A higher initial sucrose concentration leads to the repression of catabolism and inhibits nisin biosynthesis in batch culture (De Vuyst and Vandamme 1992). To maximize the productivity of nisin, it is necessary to determine the most appropriate sucrose concentration.
Many mathematical models have been reported to describe the kinetics of cell growth and lactic acid production in lactic acid fermentation (Yeh et al. 1991; Mercier et al. 1992; Mallika et al. 2003). However, there are only a few models which describe bacteriocin production (Parente et al. 1994, 1997), especially for nisin production. These models were developed with a term for growth-associated bacteriocin production and a term for bacteriocin degradation or adsorption.
Fed-batch culture can eliminate substrate inhibition and control the substrate concentration at the appropriate level. However, there are few reports on nisin production in fed-batch culture. In this study, nisin production was investigated by using the fed-batch culture of L. lactis in order to improve its production, and the results were compared with those of batch culture. A mathematic model was then developed to express the fermentation process.
Materials and methods
Experimental conditions
Bacterial strains and media
Lactococcus lactis subsp. lactis ATCC 11454, a nisin-producing strain, was grown on complex medium (CM medium) containing 30 g sucrose, 10 g yeast extract (Oxoid), 10 g soy peptone, 10 g KH2PO4, 2 g NaCl, 0.2 g MgSO4·7H2O per litre. To prepare the inoculum, a similar medium (CM inoculation medium) was used which contained 10 g l−1 sucrose and lacked soy peptone. Micrococcus flavus NCIB 8166, which was used as the indicator strain in nisin bioactivity assay, was grown on SI medium containing 8 g tryptone, 5 g glucose, 5 g yeast extract (Oxoid), 5 g NaCl, 2 g NaH2PO4, 12 g agar per litre.
Batch and fed-batch culture
Batch and fed-batch culture were carried out in a 1-l jar fermenter, which was equipped with a pH, and temperature electrode and magnetic stirrer. Before the fermentation was performed, L. lactis was subcultured in 10 ml CM inoculation medium, and statically incubated at 30°C for 12 h. An inoculum comprising 1% (v/v) of the seed culture was transferred to the 1-l jar fermenter which contained 500 ml CM medium for primary culture. The fermenter was operated at 30°C and the pH was controlled at 6.8±0.05 by automatically adding 5 N NaOH solution with a peristaltic pump. The flow rate of aseptic N2 was 100 ml min−1 to maintain the anaerobic condition, and a slow agitation (100 r.p.m.) was maintained to keep the fermentation broth homogeneous. For the fed-batch culture, concentrated sucrose (400 g l−1) was added at different feeding rates after 5 h of batch culture. Samples were withdrawn at regular intervals, and the volume of fermentation broth was kept constant.
Analysis of biomass, sucrose, lactic acid and nisin titer
Biomass was measured as optical density at 600 nm. Values were converted to cell dry weight (CDW) from a standard curve. Residual sucrose levels were measured using the Roe method (Oardini et al. 1955). Lactic acid was determined using a rapid colorimetric method (Steinsholt and Calbert 1960).
The Nisin titer was determined by the a modified Tramer and Fowler method (1964). The samples were adjusted to pH 2.0 with a few drops of 10 N HCl, heated in boiling water in a water bath for 5 min and cooled to room temperature, then centrifuged (8,000 r.p.m.) for 10 min. The supernatant was appropriately diluted with 0.02 N HCl and the nisin assay was performed by using an agar well diffusion assay. A standard curve (1–10 IU ml−1) was plotted using a stock solution of 1,000 IU ml−1 nisin (1×105 IU g−1 solid; nisin content, 2.5% by weight; Sigma). Each assay was performed in triplicate and the average results are shown.
Model development
Batch culture
For cell growth, a simple, modified, logistic model (Mercier et al. 1992) was adequate to describe the growth of L. lactis ATCC 1454:
where X is the cell dry mass concentration (g l−1), μmax is the maximum specific growth rate (h−1), Xmax is the maximum attainable cell dry mass concentration (g l−1), and K x is specific rate of cell death (h−1).
Since L. lactis ATCC 1454 is a homofermentative lactic acid bacteria, sucrose was mainly converted to lactic acid. Sucrose consumption can be described by the equation:
where S is the residual sucrose concentration (g l−1),YL/S is the lactic acid yield based on the sucrose consumption (g lactic acid g−1 sucrose), and ms is the maintenance constant (g sucrose g−1 CDW h−1).
Lactic acid production can be simulated by the Luedeking–Piret model (Luedeking and Piret 1959):
where L is the lactic acid concentration (g l−1), α is the constant for growth-associated kinetics of lactic acid production (g lactic acid g−1 CDW) and β is the non-growth-associated constant (g lactic acid g−1 CDW h−1).
Nisin was mainly produced in the exponential growth phase, and the nisin titer decreased after the biomass reached its maximum (see Results). So the nisin production can be described by:
where N is the nisin titer (mg l−1), KN is the specific nisin production rate (mg g−1 CDW), and KD is the rate of nisin inactivation (mg g−1 CDW h−1).
Fed-batch culture
The fed-batch culture profile can be simulated based on the kinetic model developed for the batch culture. The model equations are derived from the batch model Eqs. 1, 2, 3, and 4:
where V is the volume of the fermentation broth (l), FV is the feeding rate (l h−1), Fin and Fout are the inflow and outflow, respectively (l h−1), and Sin is the sucrose concentration in feeding solution (g l−1). In this paper, because the feeding volume was approximately equal to the out volume for sampling, the variety of the fermentation broth volume could be ignored (dV/dt=0).
Model parameter estimation
Modelling of fermentation data was carried out with the VENSIM DSS 1.62 (Ventana Systems) optimization program for non-linear regression. The model equations were integrated by the fourth-order Runge–Kutta method, and the optimization used was the Powell method to get the minimum total residual sum of squares in order to achieve the best fit of the experimental data.
Results
Batch culture
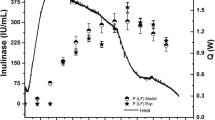
The typical time course of biomass and nisin production with 30 g sucrose l−1 at controlled pH 6.8 is shown in Fig. 1. A maximum biomass of 3.5 g CDW l−1 was obtained after 8 h fermentation when sucrose was completely consumed. The nisin titer increased with cell growth and reached its maximum, 2,658 IU ml−1, at 8 h; nisin biosynthesis showed primary metabolite kinetics (De Vuyst and Vandamme 1992; De Vuyst et al. 1996; Parente and Ricciardi 1994). Both biomass and nisin titer dropped after reaching their maximum.
Fed-batch culture
In the fed-batch cultures, the feeding solution (400 g sucrose l−1) was added at different constant feeding rates when the residual sucrose concentration was reduced to almost 4 g l−1 (at 5 h). The initial sucrose concentration was 10 g l−1 and the results are shown in Figs. 2, 3, 4, and 5.
When sucrose was added at the constant feeding rate of 6 g l−1 h−1 from 5 to 12 h, the maximum biomass of 3.9 g CDW l−1 was obtained at 10 h. The nisin titer increased slowly during the feeding and reached its maximum of 2,926 IU ml−1 (Fig. 2). The residual sucrose in the fermentation broth remained close to zero during feeding, which restricted the cell growth and nisin production.
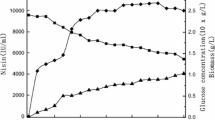
The profiles in response to the constant addition of sucrose at the feeding rates of 7 and 8 g l−1 h−1 were similar (Figs. 3, 4). A final maximum biomass of 4.1 g CDW l−1 and 4.0 g CDW l−1 was reached, respectively. The nisin titer increased quickly during feeding and reached its maximum of 4,185 and 3,754 IU ml−1, respectively.
When sucrose was added at the constant feeding rate of 10 g l−1 h−1, the residual sucrose in the fermentation broth increased from 3.8 to 15.2 g l−1 when the feeding ended at 9 h (Fig. 5). The cell growth was similar to that under other conditions (Figs. 3, 4), but maintaining a higher amount of residual sucrose during feeding did not favour nisin biosynthesis—the maximum nisin titer obtained at 9 h was only 3,410 IU ml−1.
Kinetic parameter estimation
Experimental data in batch and fed-batch cultures with different feeding rate were used to obtain the kinetic parameter values in Eqs. 1, 2, 3, 4, 5, 6, 7, and 8. The model prediction for the biomass, residual sucrose, lactic acid and nisin production are shown in Figs. 1, 2, 3, 4, and 5, and the parameter values were calculated from the experimental data (Table 1). It can be clearly seen that the model fits the experimental data very well in all conditions.
Discussion
The kinetics of L. lactis ATCC 11454 for cell growth, sucrose consumption, lactic acid and nisin production were investigated in batch and different fed-batch cultures. A simple model describing the fermentation process was successfully developed. Nisin production showed primary metabolite kinetics in batch and fed-batch cultures; the nisin titer increased with cell growth and reached its maximum when cell growth ceased, and then decreased. This result was consistent with some other researchers’ work (De Vuyst and Vandamme 1992; Parente et al. 1997). The decrease in the nisin titer may be caused by cell adsorption or destruction by non-specific proteolytic enzymes released during cell lysis (De Vuyst and Vandamme 1992).
In fed-batch cultures, the maximum nisin titer obtained at the feeding rate of 7 g l−1 h−1 was higher than that at 6 and 10 g l−1 h−1, which was also be found for the model parameter KN (Table 1). Figure 6 shows a comparison of the specific nisin production rate (qN, mg nisin g−1 CDW h−1) during feeding at different feeding rates. A higher feeding rate (8 and 10 g l−1 h−1) resulted in the increase in residual sucrose during feeding (Figs. 4, 5), while the specific nisin production rate was lower than that at the feeding rate of 7 g l−1 h−1 (residual sucrose concentration was maintained at about 3.5 g l−1 during feeding). This result showed that nisin biosynthesis was affected by the sucrose concentration, and that sucrose regulation may be responsible for the difference in the specific nisin production rate. During fed-batch cultures, the cell growth curves were similar for different feeding rates (Figs. 3, 4, 5), while nisin production differed. The model describing growth-associated nisin production (Eq. 8) could fit the experimental data better; it could not explain the difference in nisin biosynthesis in fed-batch cultures and did not reflect the influence of sucrose.
Carbon (especially sucrose) regulation appears to be a major control mechanism for nisin biosynthesis (De Vuyst and Vandamme 1992). There is a genetic link between sucrose fermentation and nisin production (James and Larry 1986) , and the influence of sucrose concentration on nisin production was examined in the batch culture (data not shown). The nisin titer reached its maximum with an initial sucrose concentration of 30 g l−1. When the sucrose concentration exceeded 30 g l−1, although cell growth was not affected, nisin production was inhibited and the nisin titer decreased. So it is necessary to determine the appropriate sucrose concentration to improve nisin production. In this study, nisin production could be enhanced by ensuring a lower, stable sucrose concentration in the fed-batch culture; the maximum nisin titer was 4,815 IU ml−1 at the feeding rate of 7 g l−1 h−1, an increase of 58% relative to that of the batch culture.
References
De Vuyst L, Vandamme EJ (1992) Influence of the carbon source on nisin production in Lactococcus lactis subsp. Lactis batch fermentations. J Gen Microbiol 138:571–578
De Vuyst L, Callewaert R, Crabbe K (1996) Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology 142:817–827
Gross E, Morell JL (1971) The structure of nisin. J Am Chem Soc 93:4634–4639
Hiroshi S, Taiji M, Eiji T, Suteaki S (1999) Nisin production by mixed-culture system consisting of Lactococcus lactis and Kluyveromyces marxianus. Appl Environ Microbiol 65:3134–3141
James LS, Larry LM (1986) Partial characterization of the genetic basis for sucrose metabolism and nisin production in Streptococcus lactis. Appl Environ Microbiol 51:57–64
Luedeking R, Piret EL (1959) A kinetic study of the lactic acid fermentation. Batch process at controlled pH. J Biochem Microbiol Technol Eng 1:393–412
Mallika B, Noppol L, Wallace B, Peter LR (2003) Batch and continuous culture of Lactococcus lactis NZ133: experimental data and model development. Biochem Eng J 14:127–135
Mercier P, Yerushalmi L, Rouleau D, Dochain D (1992) Kinetics of lactic acid fermentation on glucose and corn by Lactobacillus amylophilus. J Chem Technol Biotechnol 55:111–121
Oardini CE, Leloir LF, Ohiriboga J (1955) The biosynthesis of sucrose. J Biol Chem 214:149
Parente E, Ricciardi A (1994) Influence of pH on the production of enterocin 1146 during batch fermentation. Lett Appl Microbiol 19:12–15
Parente E, Ricciardi A, Addario G (1994) Influence of pH on growth bacteriocin production by Lactococcus latis subsp. lactis 140 NWC during batch fermentation. Appl Microbiol Biotechnol 41:388–394
Parente E, Brienza C, Ricciardi A, Addario G (1997) Growth and bacteriocin production by Enterococcus faecium DPC 1146 in batch and continuous culture. J Ind Microbiol Biotechnol 18:62–67
Steinsholt K, Calbert HE (1960) A rapid colorimetric method for the determination of lactic acid in milk and milk products. Milchwissenschaften 15:7–11
Tramer J, Fowler GG (1964) Estimation of nisin in foods. J Sci Food Agric 15:522–528
Van’t Hul JS, Gibbons WR (1997) Neutralization/recovery of lactic acid from Lactococcus lactis: effects on biomass, lactic acid, and nisin production. World J Microbiol Biotechnol 13: 527–532
Yeh PLH, Bajpai RK, Iannotti EL (1991) An improved model for lactic acid fermentation. J Ferment Bioeng 71:75–77
Acknowledgement
The authors would like to acknowledge the financial support of the Tenth National Five-Year Plan of the P.R. China (grant no. 2004BA713B05-02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lv, W., Zhang, X. & Cong, W. Modelling the production of nisin by Lactococcus lactis in fed-batch culture. Appl Microbiol Biotechnol 68, 322–326 (2005). https://doi.org/10.1007/s00253-005-1892-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-1892-7