Abstract
Genetic marker technology designed to detect naturally occurring polymorphisms at the DNA level had become an invaluable and revolutionizing tool for both applied and basic studies of fungi. To eliminate the confusion on the taxonomy of Ganoderma strains, in this study, a collection of 31 accessions representative of morphotypes and some unclassified types was used for analyzing molecular diversity using a novel molecular marker sequence-related amplified polymorphism (SRAP). This collection included commercial cultivars and wild varieties that represented the great diversification of types from different countries and regions. The experimental results showed that 50 out of 95 combinations of primers turned out to be polymorphic, and 85 polymorphism bands were obtained using six combinations. Based on the appearances of markers, the genetic similarity coefficients were calculated, and genetic variations were observed (0∼1) among the 31 different Ganoderma strains. The group of Ganoderma lucidum showed significant differences from the group of Ganoderma sinense. Moreover, G. lucidum in China was also different from G. lucidum in Yugoslavia. At the same time, cluster analysis successfully categorized these 31 Ganoderma strains into five groups. These results revealed the genetic diversity of Ganoderma strains and their correlation with geographic environments. It also suggested SRAP marker could be used in the taxonomic analysis of fungi. To our knowledge, this is the first application of SRAP marker on the systematics of Ganoderma strains within basidiomycetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungi were important sources serving as the raw material in traditional Chinese medicine. There had been many reports that extracts from about 200 species of fungi had several pharmacological activities, such as stimulating immunoactivity (Chang 1996) and inhibiting the growth of different kinds of tumor (Kimura 2005; Pezzuto 1997; Wang et al. 1997). Ganoderma strains belonging to the family Polyporaceae were one of the most important medical fungi of basidiomycetes. The majority of researches were related to the medicinal values of Ganoderma lucidum, or ‘Lingzhi’ in Chinese and ‘Reishi’ in Japanese, such as immunomodulatory action (Gao et al. 2005), antitumor (Jiang et al. 2005), antimicrobial activity (Ofodile et al. 2005) and cardiovascular effects (Lee and Rhee 1990). However, only a few varieties of Ganoderma strains have such pharmacological functions. At present, due to the incorrect naming of newly cultivated strains, the taxonomy of Ganoderma strains was still confusing, which was unfavorable for developing this medicinal fungi source further. So it was very important to investigate the genealogical classification and genetic diversity of Ganoderma strains for the exploration of their medical values penetratingly. With this intention, the molecular marker technology was applied because the genetic marker was an identifiable physical location on a chromosome whose inheritance can be monitored. Moreover, genetic markers were locus specific and polymorphic in the studied populations.
Hitherto, there were various well-established molecular marker techniques that had been widely used in the classification of fungi including isozyme variation (Royse and May 1982), DNA sequences in ITS1 region (Sokol et al. 1999), mitochondrial small-subunit ribosomal RNAs (Gonzalez and Labarere 2000), random amplified polymorphic DNA (RAPD) (Graham et al. 1994), restriction amplified fragment length polymorphism (RFLP) (Toyomasu et al. 1992) and amplified fragment length polymorphism (AFLP) (Flores Berrios et al. 2005). However, limited works were reported about the application of molecular markers on Ganoderma strains, and so far only two articles (Wang and Yao 2005; Qi et al. 2003) discussed the polymorphism of G. lucidum strains using ITS marker with several primer combinations. The methods of isozyme variation and DNA sequences in ITS2 region were complicated because multiple steps were involved. Currently, the abundant information available about genome sequences in fungi made it possible to develop new marker systems. An interesting modified marker technology termed as sequence-related amplified polymorphism (SRAP) (Li and Quiros 2001) was similar to RAPD, but it was a preferential random amplification of coding regions in genome. SRAP had been applied extensively in genetic linkage map construction (Li and Quiros 2001), genetic diversity analysis (Ferriol et al. 2003; Lin et al. 2004), and comparative genetics (Li et al. 2003) of different species. Furthermore, in the genetic diversity analysis, the information derived from SRAP marker was more concordant to the morphological variability and to the evolutionary history of the morphotypes than that of other molecular markers (Ferriol et al. 2003).
However, up to now, the SRAP molecular marker had not been used in the genealogical classification of medicinal fungi. So, in this work, polymerase chin reaction (PCR)-based SRAP marker was adopted to study the genetic variability present in 31 Ganoderma strains and to set up a system for identification between strains. This paper showed the results of genetic diversity analysis of 31 Ganoderma strains using the SRAP marker for the first time. This research will be of great importance to the genetics study of basidiomycetes. Meanwhile, this will lay the foundations of studying the genetic systematics of other medicinal mushrooms at the molecular level.
Materials and methods
Ganoderma strains and culture conditions
The 31 Ganoderma strains used to investigate genetic diversity in this study were listed in Table 1. Among the total strains, 15 strains were purchased from Institute of Microbiology, Chinese Academy of Sciences (Beijing, China) were collected from many regions in China covering a wide range of ecological conditions from north to south and from mountains to flat irrigated lands, two strains (No. 10, 11) were derived from Korea, and one strain (No. 20) came from Yugoslavia. Thirteen strains were obtained from Green Valley Institutes of Bio-pharmaceutical Technology (Fuzhou, China). Stock cultures of the different strains were prepared on potato dextrose agar (PDA) slants (potato 200 g/l, glucose 20 g/l, and agar 20 g/l, pH 7.0). After being incubated at 25°C for 5 days, these strains were stored at 4°C for future use. Liquid cultures of vegetative mycelia were grown in liquid PDA medium at 120 rpm in an orbital shaker and maintained at 25°C for 10 days. The mycelium was harvested by filtration and washed for three times with sterilized double-distilled water.
DNA extraction
Total DNA was purified from 1 g of wet mycelia following the procedure described by Zeng and Zhang (1996) and analyzed by electrophoresis at 5 V/cm, on a 1.0% agarose gel in 0.5×TBE buffer (45 mM Tris–borate and 1 mM EDTA at pH 8.0) stained with 0.5 μg/ml ethidium bromide. Gel images were captured on a Gel Doc 2000 imaging system (Bio-Rad, Hercules, CA). The concentration of DNA was determined by comparison with the molecular weight marker using the Quantity One software (Bio-Rad).
Protocol for the SRAP marker system
The SRAP technique was a PCR-based marker system employing a combination of two primers, a forward primer of 17 bases and a reverse primer of 18 bases, which consisted of preferential amplification of open reading frames (ORFs). The primers were composed of a core sequence of 13 to 14 bases, where the first 10 or 11 bases starting at the 5' end were ‘filler’ sequences of no specific constitution, followed by the sequence CCGG in the forward primer and AATT in the reverse primers. Three selective bases were added to the 3' ends of the primers. A variation in these three selective nucleotides generated a set of primers sharing the same core sequence. The forward primer preferentially amplified exonic regions. The reverse primer preferentially amplified intronic regions. The observed polymorphism fundamentally originated in the variation of the length of these introns, promoters, and spacers, both among individuals and among species (Li and Quiros 2001). In this assay, six different combinations were employed using four forward primers and five reverse primers (Table 2).
DNA amplification
Polymerase chain reactions with chromosomal DNAs as templates were performed in a final volume of 25 μl containing the following reagents: 1.0 μg of chromosomal DNA, 0.25 μM of each primer, 200 μM dNTPs, 2.5 U Taq polymerase (TaKaRa Biotech, Japan), 2.5 μl PCR buffer, and sterile double-distilled water. A thermal cycle was implemented with the following program: the first five cycles were run at 94°C (5 min), 94°C (1 min), 35°C (1 min), and 72°C (1 min) for predenaturing, denaturing, annealing, and extension, respectively. Then, the annealing temperature was raised to 50°C for another 35 cycles. After the completion of the 35 cycles, the reaction mixture was incubated for 10 min at 72°C. The amplification products were analyzed by electrophoresis in 1.6% (w/v) agarose gels in 0.5×TBE running buffer and detected by autoradiography after being stained with ethidium bromide. DL2000 plus DNA (TaKaRa Biotech, Japan) was used as size markers.
Analysis of the data
Photographs of agarose gels containing the different types of markers were digitized and analyzed using the Fragment Analysis Program (Amersham, Buckinghamshire, UK) to determine marker sizes. Data was scored as the presence (1) and absence (0) for amplification and non-amplification fragments, respectively. The Jaccard’s coefficients were generated based on the definition of Jaccard (1908). Similarity for each pair of species was calculated according to the formula:
Sij was the Jaccard’s coefficients of similarity between samples i and j, xij was the number of shared bands between both samples, and xi and xj were the numbers of bands unique to each sample, respectively.
The result using the between-groups linkage method of hierarchical cluster analysis (Su et al. 2002) was depicted as dendrograms, in which the closest strains were those that show the highest similarity between them. All statistical analysis was performed using the SPSS 11.5 Program (SSPS, Chicago, IL, USA).
Results
Polymorphism within Ganoderma strains detected by SRAP
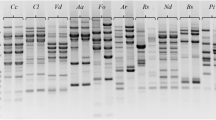
A total of 95 different combinations of primers were employed using five forward primers and 17 reverse primers. Fifty combinations of primers revealed some polymorphisms between the Ganoderma strains, among which six combinations turned out to be monomorphic (Table 1). The results of SRAP profile of DNAs from 31 strains of Ganoderma strains with six combinations showed none of all strains shared the same bands (Figs. 1 and 2). Only 20 more strains among 31 strains displayed homology for possessing the same bands. Eighty-five polymorphic bands were obtained by the six combinations (Table 1). The number of fragments amplified using each primer combination was ranging from 11 to 20, with an average of 14 polymorphic bands per combination of primers. The combination of primers showing most polymorphism was the combination of me-2 and em-12, which produced 20 polymorphic bands as shown in Fig. 1.
SRAP profiles of DNA from 31 strains of Ganoderma using combination primers me-2/em-7, me-2/em-12, and me-5/em-13. A high-stringency temperature profile applying 50°C as annealing temperature was used. Lane M represented a DL2000 plus DNA ladder (TaKaRa Biotech, Japan). Lanes 1–31 corresponded to the strains of Ganoderma listed in Table 1
SRAP profiles of DNA from 31 strains of Ganoderma using combination primers me-3/em-3, me-4/em-15, and me-2/em-13. A high-stringency temperature profile applying 50°C as annealing temperature was used. Lane M represented a DL2000 plus DNA ladder (TaKaRa Biotech, Japan). Lanes 1–31 corresponded to the strains of Ganoderma listed in Table 1
Similarity coefficients among 31 Ganoderma strains
The difference among strains was based on the existence of fragments unique to one certain strain. A similarity matrix of the 31 Ganoderma strains used in SRAP analysis showed the range of similarity varying between 0 and 1 (data not shown). The result showed that genetic relationships of 31 Ganoderma strains were different. The similarity of strain No. 23 is the lowest with other Ganoderma strains with an average similarity coefficient of 0.05, which indicated the genetic relationships of strain No. 23 (Chang Bai Shan) was most far from other Ganoderma strains. The similarity was 1 between strains No. 15 (Ganoderma capense) and No. 27 (G. capense), so they were possible duplicates in origin collection.
Analysis of phylogenetic dendrogram based on SRAP
The phylogenetic dendrogram (Fig. 3) based on SRAP constructed using the hierarchical cluster analysis clearly discriminated the difference among species used. We selected 21.5 as standard measure value for classification. Thirty-one strains of Ganoderma were divided into five clusters: A, B, C, D, and E (Fig. 3). The 22 strains from different geographical locations were classified into cluster B, which belonged to G. lucidum group. Strain No. 20 from Yugoslavia in cluster C was different from G. lucidum in China. Strain No. 23 from the northeast area in China, which had been previously classified into G. lucidum based on traditional morphological traits and classified index, was classified into cluster E. According to the result of phylogenetic dendrogram analysis, there were three strains (Nos. 15, 17, and 27) in cluster A, which belonged to G. capense. Four strains (Nos. 3, 14, 18, and 28) in cluster D, whose fruit bodies were purple, nearly black in color, were systematized into Ganoderma sinense. This showed that there was a marked difference between G. lucidum group and G. sinense group.
Phylogenetic dendrogram showing relationships among 31 accessions of Ganoderma using SRAP markers based on the between-groups linkage method of hierarchical cluster analysis. Thirty-one strains of Ganoderma strains were divided into five clusters: A, B, C, D, and E by standard measure value (21.5). Group A including three strains (Nos. 15, 17, and 27) belonged to G. capense. Group B belonging to G. lucidum included 22 strains. Four strains (Nos. 3, 14, 18, and 28) in group D were systematized into G. sinense. Strain No. 20 and strain No. 23 were involved in group C and E, respectively
Genetic variation of the 31 Ganoderma strains
The intragroup and intergroup average similarity coefficients were summarized in Table 3. From the table, the average similarity coefficient was lowest (0.08) between group C (strain No. 20) and E (strain No. 23), while it was highest (1.00) within group C and E. The average similarity coefficients within group B (strain Nos. 2, 4, 5, 6, 7, 8, 9, 10, 11, 13, 16, 19, 21, 22, 24, 25, 26, 29, 30, and 31) and group D were 0.42 and 0.35, respectively. According to the theory of Nei and Li (1979) that genetic variation in population could be well reflected by the average similarity coefficients, there were great variations within group B and D. The genetic variation within group A was less with an average similarity coefficient of 0.83. The small average similarity coefficients between or within groups indicated that there was a marked difference in heredity, which may be also explained by the diversity of their region distribution. The studied strains were from different countries and regions; they grow in various ecological and climatic conditions. The genetic variation may be caused by the different evolution routes they follow over the long period.
Discussion
The polymorphism of Ganoderma strains was very rich that may relate with their complex growing environments and genetic background. This result also proved the following viewpoint of Lin (2001): G. lucidum should be many species which were close to one another in fruit body color. Among 22 strains that belonged to cluster B, the 11 strains (bold in Table 1), which were cultivars or parental varieties in the breeding process with undetermined scientific names, were included to determine the usefulness of SRAP analysis in identifying possible duplicates in the original collection or the same species with different names. Accordingly, G. lucidum with the same scientific name were possibly different species. Therefore, Ryvarden (1994) emphasized that the results of identification of G. lucidum only based on traditional systematics deserved doubt occasionally.
In this study, the novel PCR-based SRAP marker had been successfully used. Ganoderma strains were classified into five clusters using this method, which supplied molecular evidences for the exploit of this rich source in China. PCR-based SRAP marker may have various applications for genetic studies and practical breeding programs in fungi, which had been applied to the polymorphism study of other mushrooms, such as Agricus bisporus, Lentinla edodes, Volvaria volvacea, and Flammulina velutipes. Among these basidiomycetes, we obtained good amplification and easily found polymorphism. So, SRAP marker was suitable for the molecular characterization and the investigation of phylogenic relationships in mushroom. Furthermore, these researches proved that the use of SRAP approach was more efficient to examine the genetic diversity in basidiomycetes. This provided an alternative method to identify the phylogenetic relationships within Ganoderma collections. Currently, research is in progress by the amplified fragments sequencing to shed light on the genetic linkage relationship between genotype and phenotype. In addition, it would be necessary to enlarge the number of varieties either to deepen the insight of the genetic diversity within Ganoderma or to detect molecular markers that allowed discrimination between genotypes.
In conclusion, the SRAP marker system was a simple and efficient marker system that had several advantages over other systems: simple, with a reasonable throughput rate, able to disclose numerous co-dominant markers, allows easy isolation of bands for sequencing and, most importantly, it targets ORFs, which could be adapted for a variety of purposes in different fungi including SCAR marker, map construction, and gene chip. As the dominant SRAP markers could provide more accurate information on population genetic diversity than the traditional methods, the SRAP molecular genetic distance based on monokaryons derived from different dikaryotic parents should also be useful for predicting hybrid performance in outbreeding of mushrooms.
References
Chang R (1996) Functional properties of edible mushrooms. Nutr Rev 54:91–93
Ferriol M, Picó B, Nuez F (2003) Genetic diversity of a germplasm collection of Cucurbita pepo using SRAP and AFLP marker. Theor Appl Genet 107:271–282
Flores Berrios EP, Alba Gonzalez JF, Arrizon Gavino JP, Romano P, Capece A, Gschaedler Mathis A (2005) The uses of AFLP for detecting DNA polymorphism, genotype identification and genetic diversity between yeasts isolated from Mexican agave-distilled beverages and from grape musts. Lett Appl Microbiol 41:147–152
Gao Y, Tang W, Dai X, Gao H, Chen G, Ye J, Chan E, Koh HL, Li X, Zhou S (2005) Effects of water-soluble Ganoderma lucidum polysaccharides on the immune functions of patients with advanced lung cancer. J Med Food 8:159–168
Gonzalez P, Labarere J (2000) Phylogenetic relationships of Pleurotus species according to the sequence and secondary structure of the mitochondrial small-subunit RNA. Microbiology 146:209–221
Graham GC, Mayers P, Henry RJ (1994) A simplified method for the preparation of fungal genomic DNA for PCR and RAPD analysis. Biotechniques 16:49–50
Jaccard P (1908) Nouvelles recherches surla distribution florale. Bull Soc Vaudoise Sci Nat 44:223–270
Jiang Y, Wang H, Lu L, Tian GY (2005) Chemistry of polysaccharide Lzps-1 from Ganoderma lucidum spore and anti-tumor activity of its total polysaccharides. Yao Xue Xue Bao 40:347–350
Kimura Y (2005) New anticancer agents: in vitro and in vivo evaluation of the antitumor and antimetastatic actions of various compounds isolated from medicinal plants. In Vivo 19:37–60
Lee SY, Rhee HM (1990) Cardiovascular effects of mycelium extract of Ganoderma lucidum: inhibition of sympathetic of sympathetic outflow as a mechanism of its hypotensive action. Chem Pharm Bull 38:1359–1364
Li G, Quiros CF (2001) Sequence-related amplified polymorphism (SRAP), a new marker system based on a simple PCR reaction: its application to mapping and gene tagging in Brassica. Theor Appl Genet 103:455–461
Li G, Gao M, Yang B, Quiros CF (2003) Gene for gene alignment between the Brassica and Arabidopsis genomes by direct transcriptome mapping. Theor Appl Genet 107:168–180
Lin ZB (2001) Modern study on Ganoderma lucidum. Peking Medical Science University, Beijing
Lin ZX, Zhang XL, Nie YC (2004) Evaluation of application of a new molecular marker SRAP on analysis of F2 segregation population and genetic diversity in cotton. Acta Genetica Sinica 31:622–626
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonuclease. Proc Natl Acad Sci U S A 76:5269–5273
Ofodile LN, Uma NU, Kokubun T, Grayer RJ, Ogundipe OT, Simmonds MS (2005) Antimicrobial activity of some Ganoderma species from Nigeria. Phytother Res 19:310–313
Pezzuto JM (1997) Plant-derived anticancer agents. Biochem Pharmacol 53:121–133
Qi JJ, Ma RC, Chen XD, Lan J (2003) Analysis of genetic variation in Ganoderma lucidum after space flight. Adv Space Res 31:1617–1622
Royse DJ, May B (1982) Use of isozyme variation to identify genotypic classes of Agaricus brunnescens. Mycologia 74:93–102
Ryvarden L (1994) Can we trust morphology in Ganoderma. In: Buchanan PK, Hseu RS, Moncalvo JM (eds) Ganoderma: systematics, phytopathology and pharmacology. Proceedings of contributed symposium 59 A, B. Fifth International Mycological Congress, Vancouver, August 14-21, 1994, pp 19–24
Sokol S, Kaldorf M, Bothe H (1999) Molecular characterization and taxonomic affinities of species of the white rot fungus Ganoderma. Z Naturforsch 54:314–318
Su JM, Fu RH, Zhou JB, Zhang LH (2002) Applying practice of SPSS serial of statistics software: cluster analysis. Publishing House of Electronics Industry, Beijing
Toyomasu T, Takazawa H, Zennyoji A (1992) Restriction fragment length polymorphisms of mitochondrial DNAs from the basidiomycetes Pleurotus species. Biosci Biotechnol Biochem 56:359–361
Wang DM, Yao YJ (2005) Intrastrain internal transcribed spacer heterogeneity in Ganoderma species. Can J Microbiol 51:113–121
Wang SY, Hsu ML, Hsu HC, Tzeng CH, Lee SS, Shiao MS, Ho CK (1997) The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int J Cancer 70:699–705
Zeng FY, Zhang YZ (1996) Preparation of edible fungus DNA from polysaccharide-rich sample. Acta Edulis Fungi 3:13–17
Acknowledgements
This work was financially supported by a grant from Science & Technology Department of Fujian Province, People’s Republic of China (2003EAT20021). We would like to sincerely thank Dr. Hu Zhu and Tianwen Wang of East China University of Science and Technology for their critical reading of the manuscript and useful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, SJ., Gao, W., Lin, SQ. et al. Analysis of genetic diversity in Ganoderma population with a novel molecular marker SRAP. Appl Microbiol Biotechnol 72, 537–543 (2006). https://doi.org/10.1007/s00253-005-0299-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0299-9