Abstract
Although Bordetella pertussis, the etiologic agent of whooping cough, adheres and grows on the ciliated epithelium of the respiratory tract, it has been extensively studied only in liquid cultures. In this work, the phenotypic expression of B. pertussis in biofilm growth is described as a first approximation of events that may occur in the colonization of the host. The biofilm developed on polypropylene beads was monitored by chemical methods and Fourier transform infrared (FT-IR) spectroscopy. Analysis of cell envelopes revealed minimal differences in outer membrane protein (OMP) pattern and no variation of lipopolysaccharide (LPS) expression in biofilm compared with planktonically grown cells. Sessile cells exhibited a 2.4- to 3.0-fold higher carbohydrate/protein ratio compared with different types of planktonic cells. A 1.8-fold increased polysaccharide content with significantly increased hydrophilic characteristics was observed. FT-IR spectra of the biofilm cells showed higher intensity in the absorption bands assigned to polysaccharides (1,200–900 cm−1 region) and vibrational modes of carboxylate groups (1,627, 1,405, and 1,373 cm−1) compared with the spectra of planktonic cells. In the biofilm matrix, uronic-acid-containing polysaccharides, proteins, and LPS were detected. The production of extracellular carbohydrates during biofilm growth was not associated with changes in the specific growth rate, growth phase, or oxygen limitation. It could represent an additional virulence factor that may help B. pertussis to evade host defenses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The reemergence of the pertussis incidence rates in several countries with high vaccination coverage has led to renewed interest in understanding the colonization and survival strategies of the bacteria in the host (Qiushui et al. 2003; Mooi et al. 2001). Bordetella pertussis expresses several virulence factors, studied in murine models, that play an important role in the interaction between bacteria and host cells (Khelef et al. 1994). The expression of these factors is mainly regulated by the bvg locus in response to environmental signals (Coote 1991; Martínez de Tejeda et al. 1996). Most of the studies of growth, metabolism, adaptability, and physiology of this pathogen have been performed on planktonic cells grown in batch culture (Graeff-Wohlleben et al. 1995; Martínez de Tejeda et al. 1996). Although this traditional way of culturing bacteria has provided extensive information about the basic molecular mechanisms which control the phenotypic expression of B. pertussis, this organism lives, at least during the first steps of infection, adhered to epithelial tissues (Cherry et al. 1988) where it could grow as a biofilm.
Biofilms are defined as sessile communities characterized by cells that are irreversibly attached to a surface or to each other, embedded in a matrix of extracellular polymeric substances (EPS), and that exhibit differential phenotypic characteristics with respect to their planktonic counterparts (Costerton et al. 1987; Donlan and Costerton 2002). Under such growth conditions, exopolysaccharide production is often increased, which, in turn, could form a diffuse exopolymer slime layer or increase the size of bacterial capsule which protect the bacteria against a variety of antimicrobial agents as well as against host attack (Brown and Barker 1999; Brown and Williams 1985). In the case of whooping cough, there is very little information about the physiologic response that B. pertussis displays during the colonization of ciliated epithelial cells and the strategies that the bacterium develop to persist in the host. It was therefore of interest to work in a model of abiotic surface colonization as a first approximation of events that may occur during the growth of this organism adhered to surfaces.
Infrared spectroscopy is a nondestructive technique which allows obtaining information of the overall chemical composition of a sample ( et al. 1985; Naumann et al. 1991; Schmitt and Flemming 1998). This technique has been used over the years in chemical analysis and in the characterization of biological samples. The ability of Fourier transform infrared (FT-IR) spectroscopy to discriminate structural and biochemical changes in the composition of microbial cells accompanying their surface-associated growth has made this technique particularly suitable for monitoring biofilm cultures (Nivens et al. 1995). Previously, we employed FT-IR spectroscopy to describe adherence and growth of B. pertussis on abiotic surfaces (Bosch et al. 2000). In this work, by using this technique, we demonstrated phenotypic changes in B. pertussis cells grown adhered to surfaces with respect to free-floating planktonic cells and cells recovered from solid medium. Complementary and additional information of cells grown under the different growth conditions was obtained by chemical and biochemical methods. A partial characterization of the extracellular matrix recovered from the biofilm was also reported.
Materials and methods
Bacterial strains and culture conditions
B. pertussis Tohama I strain (8132 Collection of Pasteur Institute, CIP) was used throughout this study. This strain is used in vaccine production. Stock cultures were grown on Bordet–Gengou agar (Difco, Detroit, MI, USA) with 1% (wt/vol) bactopeptone (Difco) and 14% (vol/vol) defibrinated sheep blood (Instituto Biológico, La Plata, Argentina; buffered glycerol saline, BGS) at 36°C for 72 h and subcultured for 48 h. Cells recovered from BGS plates were used for FT-IR and chemical analysis.
For batch cultures, colonies from BGS plates were inoculated into 500-ml Erlenmeyer flasks containing 125 ml of chemically defined Stainer–Scholte (SS) medium (Stainer and Scholte 1971), in which glutamate is the growth-limiting nutrient, and incubated at 36°C on a rotatory shaker (160 rpm). Samples were taken at lag, mid-exponential, and stationary phases of growth and tested for optical density at 650 nm (OD650), viable cell counts, and infrared spectroscopy. Subsamples were kept at −70°C for subsequent determinations. Oxygen-limited cultures were carried out in 500-ml Erlenmeyer flasks containing 125 ml of SS medium which were incubated on a rotatory shaker at 25 rpm for 4 days. Samples were taken every 24 h. Continuous cultures were carried out in 4-l bioreactors (LH Inceltech 210 Series, LH Fermentation Ltd., UK) with 700 ml of SS medium, as previously described (Rodríguez et al. 1994). Samples were withdrawn at least 10 generations after the steady state was achieved. Four independent experiments for solid, batch, and continuous cultures were performed.
Biofilms were formed on polypropylene cylindrical beads (150 g, ∅=4.2 mm, h=2 mm, with an average density of 0.901 g cm−3; Petroken, Argentina) in glass column reactors (∅=5 cm, h=55 cm) (Bosch et al. 2000). A planktonic culture of B. pertussis (125 ml, OD650=1.0) grown in SS medium for 15 h at 160 rpm was added as inoculum to each column and incubated for 5 h at 36°C to allow cell attachment to the beads. Then, the suspension was drained to remove unattached cells, and 125 ml of fresh SS medium was added to each column. The bioreactors were incubated aerobically (0.1 l min−1) for different periods of time (24, 48, 72, 96, and 120 h) at 36°C. At 24-h intervals, the spent medium was replaced by a fresh one. The bioreactors incubated for different periods of time were independent and repeated four times each. In order to investigate the biofilm formation by FT-IR spectroscopy in a relatively undisturbed state, optical plates of IR transparent material (ZnSe windows of 13×2 mm, Korth Kristalle GmbH) were used as support of growth. This technique adapted from Nivens et al. (2001) was performed to evaluate the biofilms in situ. Sterile windows were placed inside Petri dishes with 8 ml of SS medium, inoculated with a 15-h culture of B. pertussis planktonic cells (2 ml) and incubated at 37°C with slight agitation. Half of the culture medium was replaced everyday by an equal volume of fresh medium. After 96 h, the culture medium was removed, and the windows were gently washed with distilled water to eliminate unattached cells and dried in vacuum (3–6 kPa) for 1 h.
Recovery of attached cells and EPS
Biofilm cultures were harvested after 24, 48, 72, 96, and 120 h of incubation. Beads from each column were washed three times with sterile distilled water to remove unattached cells. Sessile cells were disaggregated from the biofilm matrix by slight agitation in PBS buffer with 270 mM KCl (Mallinckrodt, St. Louis, MO, USA) for 15 min at room temperature (Figueroa and Silverstein 1989). The suspended cells were removed from the beads, pelleted by centrifugation at 3,000×g for 15 min at 4°C, and then suspended in sterile distilled water. Subsamples of cells were analyzed by FT-IR spectroscopy and viable cell counts. The rest of the cells were frozen at −70°C for subsequent analyses. The supernatant thus obtained (containing exopolysaccharide and other components of the biofilm matrix) was filtered (0.22 μm). Three volumes of cold 95% ethanol were added to this supernatant and incubated overnight at −20°C for EPS precipitation. The pellet was collected by centrifugation (10,000×g, 15 min) and suspended in sterile distilled water. After repeating this procedure twice, the final pellet was dialyzed and lyophilized.
Characterization of bacterial cells
In order to examine the biofilm development, scanning electron microscopy (SEM) was used. Samples of beads with attached bacteria withdrawn from different times of biofilm growth were fixed with 2.5% glutaraldehyde (Merck KGaA, Darmstadt, Germany) in PBS (pH 7.2) for 3 h at 4°C. The samples were dehydrated in a graded ethanol series (30, 50, 70, and 100% two times for 15 min each) and then dried by critical point using liquid carbon dioxide. Finally, the beads were coated with gold (fine coat sputter JFC-1100, JEOL) and examined using a scanning electron microscope (JEOL JSM-T100, Museo de La Plata, Argentina).
For the extraction and analyses of lipopolysaccharide (LPS), bacterial cells were washed and suspended in distilled water, adjusting all the samples to the same OD650. After extracting the LPS from the different types of cells by EDTA/triethylamine/polymyxin B (ETP) method, as described by Valverde et al. (1997), protein digestion was performed (60°C for 1 h) with proteinase K (Merck) at a final concentration of 50 μg ml−1. Then, β-mercaptoethanol (BioRad, Hercules, CA, USA) was added, and the samples were heated at 100°C for 10 min and finally sonicated for 15 min. LPS profiles were characterized by tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis (tricine-SDS-PAGE) as indicated by Lesse et al. (1990). After running the gels for 5 h at 20 mA, bands were visualized performing a silver stain (BioRad), according to Hitchcock and Brown’s (1983) technique. Alternatively, when indicated, gels were first treated with Alcian blue (Sigma, St. Louis, MO, USA), a specific dye used for capsule carbohydrate staining (Pelkonen et al. 1988; Qadri et al. 1994), and then stained with silver.
The outer membrane proteins (OMPs) of the different types of cells were obtained by lysozyme–shock thermal treatment (Maagd and Lugtenberg 1986) with differential solubilization of cell envelopes with Triton X-100. The protein concentration of each OMP preparation was determined and adjusted prior to SDS-PAGE analysis (15%, wt/vol; Laemmli 1970). After electrophoresis, the gels were stained with Coomassie brilliant blue R250 (BioRad). Molecular weights were estimated by means of a calibration kit from Pharmacia Biotech.
FT-IR spectra of cells were recorded on a Spectrum One FT-IR spectrometer (Perkin-Elmer, USA) and/or a Bruker 113V FT-IR spectrometer (Bruker Optics, Germany), equipped with a mercury–cadmium–telluride (MCT) detector cooled with liquid N2. The cells were washed and suspended in sterile distilled water to OD650 of approximately 10. One hundred microliters of each bacterial suspension was transferred to a ZnSe optical plate and dried in vacuum (3–6 kPa) to obtain transparent films (Naumann 2000). Absorbance spectra of all samples were recorded between 4,000 and 650 cm−1. To avoid spectral water vapor interferences, each spectrum was measured under a continuous purge of dried air, co-adding 128 scans at 6 cm−1 resolution. Prior to the analysis, baseline offset correction and vector normalization in the full spectral range were performed (Naumann 2000). In order to determine spectral fine structure of overlapped bands, second derivatives of the IR spectra were calculated. The procedures mentioned above and band area calculation were carried out by means of Grams/32 software (Galactic Industries Corporation, USA), OPUS 4.1 (Bruker Optics, USA), and Perkin-Elmer software (Spectrum 3.0).
Hydrophobicity was studied to evaluate the bacterial adhesion to hydrocarbons (BATH). It was estimated by the removal coefficient (K) obtained by the kinetic approach described by Doyle and Rosenberg (1995) using hexadecane as organic phase.
Ruthenium red (RR; Sigma) adsorption onto cells was used to determine semiquantitatively acid polysaccharides on the outer cell surface using the method described by Figueroa and Silverstein (1989).
Protein quantification was performed by the Lowry et al. (1951) method using bovine serum albumin as standard (Sigma). Results were expressed as micrograms of protein per milligram of biomass. Total neutral carbohydrates were estimated by the phenol–sulfuric acid method (Dubois et al. 1956) using d-glucose (glc) as standard. Results were expressed as micrograms of glc per milligram of biomass.
EPS characterization
Total neutral carbohydrate and protein contents in EPS samples recovered from 120-h biofilm cultures were determined as indicated for cells. Uronic acid content was measured by photometry with m-hydroxydiphenyl (MHDP) at 520 nm using glucuronic acid as standard (Sigma). The value expressed the total content of uronic carboxylic groups as galacturonic units (Blumenkrantz and Asboe-Hansen 1973). For FT-IR spectroscopy analysis, a solution of the lyophilized EPS (10 mg ml−1) was prepared, and 100 μl was transferred to a ZnSe window and dried in vacuum. FT-IR absorbance spectra of the EPS films were recorded using the parameters described in the previous section.
Statistical analysis
Four independent experiments of the three culture types described above (solid, liquid, and biofilm) were performed. For each growth condition, averages and standard deviations of the experimental data obtained were reported. The differences in the mean values of chemical determinations and FT-IR data among the diverse types of cultures were analyzed by one-way analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test at the 5% level.
Results
SEM images of B. pertussis attached to polypropylene beads are shown in Fig. 1. Images of different magnifications revealed at 48 h the formation of clusters of bacteria in microcolonies and a confluent biofilm at longer-term colonization (120 h). Viable cell counts, determined every 24 h over 6 days of growth, showed that sessile cell populations increased throughout the 120 h of incubation. At that time, the viable sessile cell count recovered from the beads was 2.8×108 colony-forming units (CFU) cm−2. The specific growth rate (μ) determined for sessile population was 0.03 h−1.
Influence of the attachment on the physicochemical characteristics of cell envelope
The OMP pattern of biofilms cells and cells recovered from liquid and solid media is shown in Fig. 2. Although major bands appeared similar in all preparations, differences in minor bands (15–16 and 25–28 kDa) and in a few band intensities (from 30 to 14 kDa) between sessile and planktonic cells were observed. Similarly, when the LPS expression was compared among the different types of cells studied, no main differences on their profiles were found. In all cases, the expression of a rough-type LPS (Fig. 3A), which consists of two bands, a strongly stained LPS A band and a faster migrating minor LPS B band, was found. These bands have been described for B. pertussis in the literature (Peppler 1984). Nevertheless, when the same samples were analyzed by tricine-SDS-PAGE but first stained with Alcian blue and then with silver, the sessile cells showed an additional band which was assigned to a polysaccharide. This band was not observed in LPS samples of cells recovered from liquid and solid media treated in the same way (Fig. 3B). The presence of polysaccharides could be the consequence of the biofilm growth. Taking into account that this component could affect the physicochemical surface properties of sessile cells, we studied the hydrophobicity (one of the most characteristic physicochemical properties of cell surface; van Loosdrecht et al. 1990) of adhered cells in a comparative manner with planktonic cells and with cells from solid medium. Polysaccharide expression may be associated to the oxygen limitation and to a reduction of the μ value, conditions normally present in the biofilm growth. Thus, we included in this study the analysis of planktonic cells grown at μ value of 0.03 h−1 (similar μ value determined for biofilm population) and under oxygen-limited conditions. The hydrophobicity was determined in the different types of B. pertussis cells by means of the removal coefficient (K). Table 1 shows significant differences (p<0.05) among the hydrophobicity values of planktonic cells from different phases of growth in batch culture and also among the values obtained for cells recovered from solid and biofilm cultures. Besides, it is important to note that among all the cells studied, bacteria recovered from biofilm were the most hydrophilic ones, showing a K value more than 10-fold lower than the different types of planktonic cells and almost 6-fold lower than cells obtained in solid medium. To asses whether the higher hydrophilic characteristics of sessile cells could be due to an increased quantity of cell-bound exopolymer, a semiquantitative assay using RR stain was used to detect acid polysaccharides on the outer surface of cells (Figueroa and Silverstein 1989; Waller et al. 2004). As shown in Table 1, the uptake of RR dye with 25±3% was significantly higher for biofilm cells compared with the cells recovered from the other assayed cultures types (p<0.05).
Fifteen percent sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of outer membrane proteins of B. pertussis cells recovered from solid medium (lane 1), mid-exponential phase of batch culture (lane 2), and biofilm of 48, 72, 96, and 120 h of growth (lanes 3, 4, 5, and 6, respectively)
Tricine-SDS-PAGE of lipopolysaccharide (LPS) from B. pertussis cells. A Silver-stained and B first treated with Alcian blue and then silver-stained. The lanes correspond to the LPS obtained from cells 1 grown 48 h in solid medium, 2 cells recovered after 15 h of growth in batch culture, and 4 cells obtained after 120 h of biofilm growth. Lane 3 contains loading buffer
FT-IR spectroscopy of whole cells
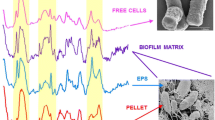
The biochemical composition of the cells collected from biofilm, liquid, and solid cultures were also compared using FT-IR spectroscopy. Fig. 4 shows the infrared spectra of sessile cells recovered from the polypropylene beads, planktonic cells grown at similar growth rate in continuous culture, exponential phase cells from batch cultures, and cells grown under oxygen-limited conditions. Spectrum (E) in this figure represents the difference spectrum between sessile cells and cells from continuous culture in the range from 1,800 to 700 cm−1. Table 2 gives a tentative assignment of the bands corresponding to the functional groups in biomolecules mentioned in this work and their respective references. The region 1,800–700 cm−1 includes the absorption of two main distinct cellular components: proteins (1,700–1,480 cm−1) and carbohydrates (1,200–900 cm−1). The difference spectrum (E) showed some important spectral modifications in bands referred to as biofilm growth markers (Helm et al. 1991; Nichols et al 1985): a band at 1,458 cm−1, three bands in the window dominated by carbohydrates vibrations (1,123, 1,079, and 1,020 cm−1), and two other bands in the region 900–800 cm−1 (860 and 800 cm−1) which is sensitive to anomeric configuration of carbohydrates (Table 2). Other differences involved the increase in the relative intensity of the bands corresponding to antisymmetric and symmetric stretching of carboxylate ion (1,627 and 1,405 cm−1, respectively), bands assigned to vibrational modes of esters (1,733 and 1,257 cm−1), and bands corresponding to polysaccharide C–O–C ring vibrations, suggesting an increase in the production of a uronic-acid-containing polysaccharide by biofilm cells (Table 2). Similar differences with slight shifts were observed in the resultant spectrum of the subtraction between biofilm cell spectrum and the spectrum of the other types of planktonic cells mentioned in Fig. 4 (data not shown). A further biofilm characterization was performed to analyze the spectra obtained from cells grown directly on the ZnSe windows (Fig. 5). Thus, the removal of the biofilm from its support was avoided, and the measurement of the spectra was performed in a nondestructive way (Nivens et al. 2001). These spectra obtained in situ of bacterial biofilms produced a shoulder in the amide I band at 1,629 cm−1 (asymmetric carboxylate ion stretching), bands at 1,372 and near 1,400 cm−1 (symmetric carboxylate ion stretching), a shoulder in amide II band at 1,516 cm−1, and a broad band at 1,200–900 cm−1 (polysaccharide region). It is important to remark that the bands at 1,733 and 1,260 cm−1 (Fig. 5) might indicate the presence of O-acetyl groups in B. pertussis sessile cells. These bands were reported in the spectra of P. aeuroginosa as an important component in the biofilm architecture (Nivens et al. 2001).
Infrared spectra of B. pertussis cells harvested from A 120 h of biofilm culture, B exponential growth phase of batch culture, C continuous culture (D=0.03 h−1), and D oxygen-limiting conditions in batch cultures. Spectra were normalized to amide II band. The difference spectrum A minus C is shown by curve E
Effect of attachment on the biomass macromolecular composition
As the macromolecular composition of biomass is an integrated indicator of the physiological state of organisms, we analyzed whether the biofilm mode of growth affects the cells of B. pertussis, in relation to free-floating grown cells, using FT-IR spectroscopy (Grube et al. 1999) and chemical analyses. Statistically significant differences were detected among the areas of the main biomolecules’ absorption bands in the spectra of biofilm and planktonic cells. The areas of the bands assigned to fatty acids, carbohydrates, and proteins were calculated in full-range vector-normalized spectra for each type of cells (Table 3; Naumann 2000). The value of the carbohydrate/protein ratio of biofilm cells was from 2.4- to 3.0-fold higher compared with the values obtained for the different types of planktonic cells and 2.1-fold higher than cells recovered from solid medium. When carbohydrate and protein contents were analyzed by chemical methods, the corresponding ratio for biofilm cells was from 2.1- to 2.7-fold and approximately 1.7-fold higher compared with the different types of planktonic cells and cells from solid medium, respectively. Sessile cell spectra showed 1.5-fold higher values for band area of the band assigned to fatty acids as obtained for the other types of cells. Furthermore, it is noteworthy that the ratio of carbohydrate/fatty acid band areas for sessile cells was from 1.5- to 2.5-fold higher compared with the values obtained for cells grown both in liquid and solid media.
EPS analyses
While the methodology used to recover the EPS allowed its isolation from biofilm cultures, the same procedure did not give satisfactory results when applied to cells grown in liquid or solid cultures. Chemical analysis of EPS showed that sessile cells produced 45.6±7.2 μg of neutral carbohydrates expressed as glucose per milligram of biomass. This EPS contained 2% (wt/wt) uronic acid and 7.6% (wt/wt) protein. SDS-PAGE analysis indicated that these proteins had the same pattern as those present in culture supernatants (data not shown). The presence of LPS in EPS was analyzed by tricine-SDS-PAGE. The gel first stained with Alcian blue and then with silver showed that the EPS contained the two LPS bands expressed by sessile cells (LPS A and LPS B) and a band of polysaccharide similar to the one found in biofilm cells, which was observed only when the gel was treated with Alcian blue (data not shown). Taken together, these results show that B. pertussis EPS contain polysaccharides, uronic acids, LPS, and proteins.
IR spectra of the EPS (three independent replicates) and the corresponding second derivatives are shown in Fig. 6. The typical bands assigned to uronic acids were observed at 1,628 cm−1, 1,408 cm−1, and 1,373 cm−1; an important band at 1,260 cm−1 was associated with O-acetyl groups with a complementary band at 1,731 cm−1 for >C=O carbonyl stretch and intensive bands in the region 1,200 and 900 cm−1 assigned to carbohydrate C–O–C ring absorptions. In addition, the two bands in the region 900–800 cm−1 were associated to the glycosidic linkage types in polysaccharides (Table 2). The LPS content in EPS was confirmed by IR spectroscopy as characteristic bands of fatty acids present in the spectral region between 3,000 and 2,800 cm−1 and by the distinct band at 720 cm−1 resulting from the >CH2 rocking vibration modes of the fatty acid chains (data not shown).
Effect of detachment on exopolymer expression
IR spectroscopy was used in order to exclude the possibility that the higher amounts of exopolysaccharide produced by sessile cells may result from the presence of a subpopulation of sticky mucoid variants. To test for this possibility, sessile cells from 120 h of growth were recovered from the beads by shaking in an Erlenmeyer flask by hand, washed, and then used as inoculum in an SS liquid medium culture. A typical IR spectrum of the resultant planktonic cells was recorded after 20 h of growth (exponential phase) and is shown in Fig. 7, spectrum B. Fig. 7 also shows the spectrum of these cells compared with the corresponding spectrum of cells recovered from liquid medium (20 h of growth, spectrum C) not previously attached and to the IR spectrum of cells from 120 h of biofilm growth (spectrum A). The area of the bands related to carbohydrates (1,146–980 cm−1) and fatty acids (2,960–2,800 cm−1) in the sessile cell spectrum decreased significantly after 20 h of growth in liquid medium and was not significantly different from that for cells that had not been previously attached.
FT-IR absorption spectra in the 3,700–750 cm−1 region of B. pertussis cells: a sessile cells recovered after 120 h of biofilm growth, b sessile cells recovered after 120 h of biofilm growth and cultivated for 20 h in liquid medium, and c cells cultivated in liquid medium for 20 h. Spectra were full-range vector-normalized
Discussion
Although the incidence of whooping cough has decreased dramatically with the introduction of vaccines based on B. pertussis planktonic cells, the disease was not eradicated yet (van Rie and Hethcote 2004). We speculate about the efficacy of current vaccines produced in bioreactors with free-floating cells in controlling a disease which is caused by cells that grow, at least during the first stages of the infection, adhered to ciliated epithelium of the upper respiratory tract (Cherry et al. 1988; Costerton et al. 2003). Based on the finding that B. pertussis may grow as biofilm (Bosch et al. 2000), we studied the phenotype expression of a vaccinal strain growing on abiotic surfaces as a first model of environmental conditions that it would have in the host.
In our experimental conditions, the μ value determined for biofilm cells was more than two times less than the maximum μ (0.07 h−1) obtained for planktonic cells in batch culture with the same culture medium (Rodríguez et al. 1994). However, as biofilms are not homogeneous structures and gradients of nutrients produce different environments where cells grow at different rates (Costerton et al. 1995), this μ value must be considered as an average of the population growth rate.
It was reported that nutrient availability and growth rate result in variations in phenotypes such as expression of wall components or production of extracellular polysaccharides (Weiser et al. 2001; Uhlinger and White 1983). Thus, in this paper, we tried to explain if the phenotypic expression of B. pertussis sessile cells is a result of a phenotypic adaptation to the biofilm lifestyle or due to changes in availability of nutrients (O2) or reduction in the specific growth rate. In the previous results of continuous cultures, we demonstrated that OMP profiles of B. pertussis were not affected by growth rate (Rodríguez et al. 1994). Consequently, the different OMP patterns expressed in biofilm cells compared with their counterpart planktonic cells and cells from solid medium (Fig. 2) could not be associated with the growth rate. These results are also consistent with the differences observed in the OMP pattern of biofilm cells in Escherichia coli (Prigent-Combaret et al. 1999). The similar LPS profiles found among the different types of cells studied (Fig. 3) are in agreement with the limited variation in LPS expression by B. pertussis, both at different growth rates (Rodríguez et al. 1994) and in modulation conditions (Valverde et al. 1997). These data are also comparable to the ones reported for P. aeruginosa cells grown as biofilm, which showed no modification in the LPS expression (De Kievit et al. 2001).
The presence of an extracellular matrix surrounding B. pertussis cells (Tohama I strain) observed in biofilms on polypropylene beads by SEM (Fig. 1), the significantly lower hydrophobicity found for attached cells as indicated by BATH assays, and the higher amount of polysaccharides on the envelope of sessile cells detected by RR dye adsorption (Table 1) are in excellent correlation with the results obtained from FT-IR spectroscopy. Our comparative FT-IR spectroscopy analysis showed, as has been demonstrated for other bacteria growing attached to surfaces (Sutherland 1997; Davies et al. 1993), that the major difference among the spectra of the different types of cells can be assigned to an increase in the amount of a uronic-acid-containing polysaccharide produced by sessile cells (Fig. 4). Furthermore, the same uronic acid bands and spectroscopic biofilm growth markers (mostly associated to the carbohydrate absorption region) were also found when a nondestructive FT-IR characterization of the B. pertussis biofilm on ZnSe surface was performed (Fig. 5).
The activation mechanism of exopolymer synthesis in B. pertussis attached cells is not known yet, even though our results (Fig. 4; Table 3) from batch and continuous cultures suggest that this activation does not depend on growth phase or the growth rate. The higher level of exopolysaccharide production found for the attached populations in relation to their counterpart planktonic cells growing at a similar rate is consistent with those reported by Evans et al. (1994) and Vandevivere and Kirchman (1993) for soil subsurface bacterial isolates. Exopolymer production may also change as a function of the type of limiting nutrient (Sutherland 2001) and as a response to toxic substances like toluene as found in environmental biofilm investigations by FT-IR spectroscopy (Schmitt et al. 1995). Weiser et al. (2001) reported that, depending on the oxygen availability, the expression of capsular polysaccharide changes in the case of phenotypic variants of Streptoccoccus pneumoniae. However, FT-IR spectroscopy analyses showed that B. pertussis growing in SS medium under oxygen-limited conditions did not present a higher production of exopolysaccharides (Fig. 4). The higher amount of exopolysaccharide found for B. pertussis sessile cells due to the preferential attachment of a subpopulation of variants differing in the exopolymer synthesis was also considered. Here, the inoculation of sessile cells into a liquid medium resulted in a reduction of the exopolysaccharide content to similar levels than those found in planktonic cells (Fig. 7). Therefore, the behavior of B. pertussis cells (Tohama I strain) adhered to a surface represents a physiological adaptation and disregards the possibility that attached cells constitute a hyperproductive subpopulation. The reversible production of EPS is consistent with the fact that the EPS synthesis is an energy-intensive process, which can be down-regulated when the EPS production is not required, as for the growth of planktonic cells (Costerton 1985).
Bacterial growth in biofilm cultures may reach high densities. In other microbial aggregates, sensing systems play a key role in cell-to-cell communications. This fact may be responsible for the biofilm specific physiological state (Donlan 2002). Although this mechanism has not been reported as being for B. pertussis yet, the existence of quorum-sensing signaling systems in this high-density culture (2.8×108 CFU cm−2) could explain the phenotypic expression of B. pertussis sessile cells in a population-dependent manner.
Chemical analysis of EPS recovered from B. pertussis biofilm cultures showed that it is composed of polysaccharides (with uronic acids), LPS, and proteins. FT-IR spectrum analysis of EPS confirmed the presence of these components and spectroscopic biofilm markers. The general feature of these spectra has also been previously found in exopolymer spectra produced by other microorganisms (Beech et al. 1999; Nivens et al. 2001, Nebenzahl et al. 2004).
LPS plays an important role in the bacterial pathogenesis of B. pertussis, and it is known to be highly immunogenic owing to the expected properties of an endotoxin (Amano et al. 1990). Taking into account previous results (Hozbor et al. 1993), we consider that the presence of LPS and proteins in the extracellular slime layer of B. pertussis cells could be the consequence of a trapping of these components which are released by the cells during growth rather than their overproduction as typical for polysaccharides. The presence of LPS in the extracellular slime layer has also been reported for Shigella dysentereae type 1 (Qadri et al. 1994). The resultant structure of B. pertussis biofilm could constitute, as it is generally accepted (Mayer et al. 1999; Sutherland 2001), a network of polysaccharides and other molecules, including cell products or virulence factors that give rise to a gel-like matrix, which may behave as other virulence attribute. In B. pertussis as in other species like E. coli (Yasuda et al. 1994), Pasteurella multocida (Chung et al 2001), S. dysenteriae (Qadri et al. 1994), Staphylococcus epidermidis (Shiau and Wu 1998), and Pseudomonas aeruginosa (Meluleni et al. 1995), the biofilm matrix could contribute to the pathogenesis and might play a role in overcoming host defense immune mechanisms (Brown and Williams 1985). Likewise, reports in other biofilm-forming bacteria suggest that the presence of uronic acids in EPS may play an important role for virulence. These acid sugars may promote the stabilization of glycosidic linkages by means of carboxylic acid moiety, conferring sessile bacteria a higher resistance to acid hydrolysis (Flemming et al. 2000; Fett et al. 1995). As a consequence, the chemical properties of EPS might favor the survival of B. pertussis sessile cells at acid pHs similar to the condition they find in phagosomes during the host attack, representing an important mechanism of immune evasion. It is also important to remark that the production of a uronic-acid-containing extracellular capsule or exopolymer by bacteria that colonize both the respiratory and enteric tracts may help them to aggregate and adhere to cells (Pace et al. 1997).
The results of our model could be associated with that reported by Belcher et al. (2000), which showed that B. pertussis induces mucin gene transcription by epithelial cells. It is interesting to speculate whether the exopolymer and mucus are acting in unison in the pertussis infection and in the persistence of the disease, by creating a favorable microenvironment for the bacterial survival in terms of nutrient acquisition, localization of extracellular products, and protection against phagocytes. These insights might also assist in the development of novel strategies for controlling this bacterial infection.
References
Amano KL, Fukushi K, Watanabe M (1990) Biochemical and immunological comparison of lipopolysaccharides from Bordetella species. J Gen Microbiol 136:481–487
Batsoulis AN, Nacos MK, Pappas CS, Tarantilis PA, Mavromoustakos T, Polissiou MG (2004) Determination of uronic acids in isolated hemicelluloses from kenaf using diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) and the curve-fitting deconvolution method. Appl Spectrosc 58:199–202
Beech I, Hanjagsit L, Kalaji M, Neal AL, Zinkevich V (1999) Chemical and structural characterization of exopolymers produced by Pseudomonas sp. NCIMB 2021 in continuous culture. Microbiology 145:1491–1497
Belcher CE, Drenkow J, Kehoe B, Gingeras TR, McNamara N, Lemjabbar H, Basbaum C, Relman DA (2000) The transcriptional responses of respiratory epithelial cells to Bordetella pertussis reveal host defensive and pathogen counter-defensive strategies. Proc Natl Acad Sci U S A 97:13847–13852
Blumenkrantz N, Asboe-Hansen G (1973) New method for quantitative determination of uronic acids. Anal Biochem 54:484–489
Bosch A, Massa NE, Donolo AS, Yantorno OM (2000) Molecular characterisation by infrared spectroscopy of Bordetella pertussis growing in biofilm. Phys Status Solidi 220:635–640
Brown M, Barker J (1999) Unexplored reservoirs of pathogenic bacteria: protozoa and biofilms. Trends Microbiol 7:46–50
Brown MR, Williams P (1985) The influence of environment on envelope properties affecting survival of bacteria in infections. Annu Rev Microbiol 39:527–556
Cherry JD, Brunell PA, Golden GS, Karzon DT (1988) Report of the task force on pertussis and pertussis immunization. Pediatrics 81:939–984
Chung JY, Wilkie I, Boyce JD, Townsend KM, Frost AJ, Ghoddusi M, Adler B (2001) Role of capsule in the pathogenesis of fowl cholera caused by Pasteurella multocida serogroup A. Infect Immun 69:2487–2492
Coote JG (1991) Antigenic switching and pathogenicity: environmental effects on virulence gene expression in Bordetella pertussis. J Gen Microbiol 137:2493–2503
Costerton JW (1985) The role of bacterial exopolysaccharides in nature and disease. Dev Ind Microbiol 26:249–261
Costerton JW, Cheng KJ, Geesey GG, Ladd TI, Nickel JC, Dasgupta M, Marie TJ (1987) Bacterial biofilms in nature and disease. Annu Rev Microbiol 41:435–464
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995) Microbial biofilms. Annu Rev Microbiol 711–745
Costerton JW, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G (2003) The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest 112:1466–1477
Davies DG, Chakrabarty AM, Geesey GG (1993) Exopolysaccharide production in biofilms: substratum activation of alginate gene expression by Pseudomonas aeruginosa. Appl Environ Microbiol 59:1181–1186
De Kievit TR, Gillis R, Marx S, Brown C, Iglewski BH (2001) Quorum-sensing genes in Pseudomonas aeruginosa biofilms: their role and expression patterns. Appl Environ Microbiol 67:1865–1873
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890
Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193
Doyle RJ, Rosenberg M (1995) Adhesion of microbial pathogens. In: Doyle RJ, Ofek I (eds) Methods in enzymology, vol 253. Academic, San Diego, pp 542–550
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Evans E, Brown MRW,Gilbert P (1994) Iron chelator, exopolysaccharide and protease production in Staphylococcus epidermidis: a comparative study of the effects of specific growth rate in biofilm and planktonic culture. Microbiology 140:153–157
Fett WF, Wells JM, Cescutti P, Wijey C (1995) Identification of exopolysaccharides produced by fluorescent pseudomonads associated with commercial mushroom (Agaricus bisporus) production. Appl Environ Microbiol 61:513–517
Figueroa LA, Silverstein JA (1989) Ruthenium red adsorption method for measurement of extracellular polysaccharides in sludge flocs. Biotechnol Bioeng 33:941–947
Flemming H-C, Wingender J, Griegbe T, Mayer C (2000) Physico-chemical properties of biofilms. In: Evans LV (ed) Biofilm: recent advances in their study and control. Harwood, Amsterdam, pp 19–34
Graeff-Wohlleben H, Deppisch H, Gross R (1995) Global regulatory mechanisms affect virulence gene expression in Bordetella pertussis. Mol Gen Genet 247:86–94
Grube M, Zagreba E, Gromozova E, Fomina M (1999) Comparative investigation of the macromolecular composition of mycelia forms Thielavia terrestris by infrared spectroscopy. Vibr Spectrosc 19:301–306
Helm D, Naumann D (1995) Identification of some bacteria cell components by FT-IR spectroscopy. FEMS Microbiol Lett 126:75–80
Helm D, Labischinsky H, Schallehn G, Naumann D (1991) Classification and identification of bacteria by Fourier-transform infrared spectroscopy. J Gen Microbiol 137:69–79
Hitchcock P, Brown T (1983) Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol 154:269–277
Hozbor D, Rodríguez ME, Samo A, Lagares A, Yantorno OM (1993) Release of lipopolysaccharide during Bordetella pertussis growth. Res Microbiol 144:201–209
Kacuráková M, Capek P, Sasinková V, Wellner N, Ebringerová A (2000) FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohydr Polym 43:195–203
Khelef N, Bachelet C-M, Vargaftig BB, Guiso N (1994) Characterization of murine lung inflammation after infection with parental Bordetella pertussis and mutants deficient in adhesins or toxins. Infect Immun 62:2893–2900
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 117:680–685
Lesse AJ, Campagnari AA, Bittner WE, Apicella MA (1990) Increased resolution of lipopolysaccharides and lipoligosaccharides utilising tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Immunol Methods 126:109–117
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Maagd R, Lugtenberg B (1986) Fractionation of Rhizobium leguminosarum cells into outer membrane, cytoplasmic membrane, periplasmic, and cytoplasmic components. J Bacteriol 167:1083–1085
Martínez de Tejeda G, Miller JF, Cotter PA (1996) Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica. Mol Microbiol 22:895–908
Mayer C, Moritz R, Kirschner C, Borchard W, Maibaum R, Wingender J, Flemming H-C (1999) The role of intermolecular interactions: studies on model systems for bacterial biofilms. Int J Biol Macromol 26:3–16
Meluleni G, Grout JM, Evans DJ, Pier GB (1995) Mucoid Pseudomonas aeruginosa growing in a biofilm in vitro are killed by opsonic antibodies to the mucoid exopolysaccharide capsule but not by antibodies produced during chronic lung infection in cystic fibrosis patients. J Immunol 155:2029–2038
Mooi FR, van Loo IHM, King AJ (2001) Adaptation of Bordetella pertussis to vaccination: a cause of its reemergence? Emerg Infect Dis 7:526–528
Moreno J, Vargas MA, Madiedo JM, Muños J, Rivas J, Guerrero MG (2000) Chemical and rheological properties of an extracellular polysaccharide produced by the cyanobacterium Anabaena sp. ATCC 33047. Biotechnol Bioeng 67:283–290
Naumann D (2000) Infrared spectroscopy in microbiology. Wiley, Chichester
Naumann D, Helm D, Labischinski H (1991) Microbiological characterizations by FT-IR spectroscopy. Nature 351:81–82
Navarini L, Stredansky M, Matulova M, Bertocchi C (1997) Production and characterization of an exopolysaccharide from Rhizobium hedysari HCNT1. Biotechnol Lett 19:231–1234
Nebenzahl YM, Porat N, Lifshitz S, Novick S, Levi A, Ling E, Liron O, Mordechai S, Sahu RK, Dagan R (2004) Virulence of Streptoccoccus pneumoniae may be determined independently of capsular polysaccharide. FEMS Microbiol Lett 233:147–152
Nichols P, Henson J, Guckert J, Nivens DE, White DC (1985) Fourier transform-infrared spectroscopic methods for microbial ecology: analysis of bacteria–polymer mixtures and biofilms. J Microbiol Methods 4:79–94
Nivens DE, Palmer JR Jr, White DC (1995) Continuous nondestructive monitoring of microbial biofilms: a review of analytical techniques. J Ind Microbiol 15:263–276
Nivens DE, Ohman DE, Williams J, Franklin M (2001) Role of alginate and its O acetylation in formation of Pseudomonas aeurginosa microcolonies and biofilms. J Bacteriol 183:1047–1057
Pace JL, Chai T, Rossi HA, Jiang X (1997) Effect of bile on Vibrio parahaemolyticus. Appl Environ Microbiol 63:2372–2377
Pelkonen S, Hayrinen J, Finne J (1988) Polyacrylamide gel electrophoresis of the capsular polysaccharide of Escherichia coli K1 and other bacteria. J Bacteriol 170:2646–2653
Peppler MS (1984) Two physically and serologically distinct lipopolysaccharide profiles in strains of Bordetella pertussis and their phenotype variants. Infect Immun 43:224–232
Prigent-Combaret C, Vidal O, Dorel C, Lejeune P (1999) Abiotic surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli. J Bacteriol 181:5993–6002
Qadri F, Haque MdA, Hossain A, Albert MJ (1994) Production of slime polysaccharides by Shigella dysenteriae type 1. Microbiol Immunol 38:11–18
Qiushui H, Makinen J, Berbers G, Mooi FR, Viljanesn MK, Arvilommi H, Mertsola J (2003) Bordetella pertussis protein pertactin induces type-specific antibodies: one possible explanation for the emergence of antigenic variants? J Infect Dis 187:1200–1205
Rodríguez ME, Hozbor DF, Samo AL, Ertola R, Yantorno OM (1994) Effect of dilution rate on the release of pertussis toxin and lipopolysaccharide of Bordetella pertussis. J Ind Microbiol 13:273–278
Schmitt J, Flemming H-C (1998) FTIR-spectroscopy in microbial and material analysis. Int Biodeterior Biodegrad 41:1–11
Schmitt J, Nivens D, White DC, Flemming H-C (1995) Changes of biofilm properties in response to sorbed substances—an FT-IR/ATR study. Water Sci Technol 32:S149–S155
Shiau AL, Wu CL (1998) The inhibitory effect of Staphylococcus epidermidis slime on the phagocytosis of murine peritoneal macrophages is interferon-independent. Microbiol Immunol 42:33–40
Stainer DW, Scholte MJ (1971) A simple chemically defined medium for production of phase I Bordetella pertussis. J Gen Microbiol 63:211–220
Sutherland IW (1997) Bacterial exopolysaccharides—their nature and production. In: Sutherland IW (ed) Surface carbohydrates of the prokaryotic cell. Academic, London, pp 27–96
Sutherland IW (2001) Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147:3–9
Synytsya A, Copíková J, Matejka P, Machovic V (2003) Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr Polym 54:97–106
Uhlinger DJ, White DC (1983) Relationship between physiological status and formation of extracellular polysaccharide glycocalyx, in Pseudomonas atlantica. Appl Environ Microbiol 45:64–70
Valverde C, Hozbor DF, Lagares A (1997) Rapid preparation of affinity-purified lipopolysaccharide samples for electrophoretic analysis. BioTechniques 22:230–236
Vandevivere P, Kirchman DL (1993) Attachment stimulates exopolysaccharide synthesis by a bacterium. Appl Environ Microbiol 59:3280–3286
van der Mei H, Noordmans J, Busscher HJ (1989) Physicochemical surface properties of nonencapsulated and encapsulated coagulase-negative Staphylococci. Appl Environ Microbiol 55:2806–2814
van Loosdrecht MCM, Lyklema J, Norde W, Schraa G, Zehnder AJB (1990) The role of bacterial cell wall hydrophobicity in adhesion. Appl Environ Microbiol. 53:1893–1897
van Rie A, Hethcote HW (2004) Adolescent and adult pertussis vaccination: computer simulations of five new strategies. Vaccine 22:3154–3165
Waller LN, Fox N, Fox KF, Fox A, Price RL (2004) Ruthenium red staining for ultrastructural visualization of a glycoprotein layer surrounding the spore of Bacillus anthracis and Bacillus subtilis. J Microbiol Methods 58:23–30
Weiser J, Bae ND, Epino H, Gordon SB, Kapoor M, Zenewcz LA, Shchepetov M (2001) Changes in availability of oxygen accentuate differences in capsular polysaccharide expression by phenotypic variants and clinical isolates of Streptococcus pneumoniae. Infect Immun 69:5430–5439
Yasuda H, Ajiki Y, Aoyama J, Yokota T (1994) Interaction between human polymorphonuclear leucocytes and bacteria released from in-vitro bacterial biofilm models. J Med Microbiol 41:359–367
Zeroual W, Choisy C, Doglia SM, Bobichon H, Angiboust J-F, Manfait M (1994) Monitoring of bacterial growth and structural analysis as probed by FT-IR spectroscopy. Biochim Biophys Acta 1222:171–178
Acknowledgements
This work was supported by a grant from FONCYT, PICT 98-06-03824. A. Bosch is a member of the CIC Provincia de Buenos Aires, D. Serra is a recipient of a fellowship from CONICET, and C. Prieto is a recipient from FOMEC. We are grateful to M.E. Rodríguez for providing us B. pertussis cells grown in continuous culture. We thank J. Figari for his excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bosch, A., Serra, D., Prieto, C. et al. Characterization of Bordetella pertussis growing as biofilm by chemical analysis and FT-IR spectroscopy. Appl Microbiol Biotechnol 71, 736–747 (2006). https://doi.org/10.1007/s00253-005-0202-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0202-8