Abstract
The use of genetically engineered microorganisms is a cost-effective, scalable technology for the production of recombinant human collagen (rhC) and recombinant gelatin (rG). This review will discuss the use of yeast (Pichia pastoris, Saccharomyces cerevisiae, Hansenula polymorpha) and of bacteria (Escherichia coli, Bacillus brevis) genetically engineered for the production of rhC and rG. P. pastoris is the preferred production system for rhC and rG. Recombinant strains of P. pastoris accumulate properly hydroxylated triple helical rhC intracellularly at levels up to 1.5 g/l. Coexpression of recombinant collagen with recombinant prolyl hydroxylase results in the synthesis of hydroxylated collagen with thermal stability similar to native collagens. The purified hydroxylated rhC forms fibrils that are structurally similar to fibrils assembled from native collagen. These qualities make rhC attractive for use in many medical applications. P. pastoris can also be engineered to secrete high levels (3 to 14 g/l ) of collagen fragments with defined length, composition, and physiochemical properties that serve as substitutes for animal-derived gelatins. The replacement of animal-derived collagen and gelatin with rhC and rG will result in products with improved safety, traceability, reproducibility, and quality. In addition, the rhC and rG can be engineered to improve the performance of products containing these biomaterials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The use of recombinant technology to produce animal component-free collagen and gelatin addresses the variability, potential immunogenicity, and risk of infection inherent in the use of collagen and gelatin for medical applications. Recombinant microbial production offers the only known technology for the cost-effective manufacturing of collagen and gelatin with consistent quality that is free of animal components. Recombinant microbial production also offers high productivity and traceable processes that delivers consistent quality products suitable for parenterally delivered products or as components for tissue-engineering applications (Olsen et al. 2003b).
Collagen fibrils and their denatured derivative, gelatin, are the main structural and functional component in many medical products such as implants, hemostats, device coatings, resuscitation fluids, formulation excipients, capsules, and tablets (Olsen et al., unpublished data; Olsen et al. 2003b). The use of animal-derived collagen and gelatin in these medical products results in safety concerns caused by difficulty to trace sources and the inherent risk of transmitting pathogenic vectors, including prions (Dormont 2002; Kaneko 2002). In addition, an increasing and significant portion of the world's population has dietary or religious requirements leading them to avoid the consumption of animal-derived products.
There are many excellent reviews about collagen and gelatin describing their synthesis, structure, physical properties, and use (Bateman et al. 1996; Myllyharju and Kivirikko 2004; Olsen et al. 2003b; Prockop and Kivirikko 1995; Schrieber and Seybold 1993). In humans, there are at least 27 different types of collagens, defined as proteins having repetitive Gly-X-Y triplets where X and Y are frequently proline or hydroxyproline, found in a triple helical structure (Myllyharju and Kivirikko 2004). Most commercial preparations of collagen and gelatin are made from bovine or porcine bone or skin, tissues enriched in type I collagen. These preparations also contain small but variable amounts of type III and other collagens that cannot be cost effectively separated from each other in a commercial process.
During the synthesis and maturation of collagen, several posttranslational modifications take place (Kivirikko KI et al. 1992). The most important of these modifications required to generate stable triple helical collagen is the hydroxylation of specific Y-position proline residues by the enzyme prolyl 4-hydroxylase (P4H; E.C. 1.14.11.2) prior to triple helix assembly (Kivirikko and Pihlajaniemi 1998; Kukkola et al. 2004; Min et al. 2000; Tandon et al. 1998). Other modifications include hydroxylation and glycosylation of specific lysine residues (Yamauchi and Shiiba 2002), N- and C-terminal propeptide removal by specific metalloproteinases after secretion into the extracellular matrix (Kivirikko 1995), and interchain cross-linking occuring at hydroxylysine and lysine residues previously deaminated by lysyl oxidase (Eyre 1987; Yamauchi and Shiiba 2002). The degree of cross-linking of mature collagen is influenced by the age and the physiology of the tissue. These modifications affect both the extractability of collagens from tissues and their biophysical characteristics.
Gelatin derived from denatured collagen is composed of a mixture of collagen chains of different length, structure, and composition. The processing of collagen into gelatin results in fragments of different sizes and isoelectric points yielding products with variable gelling and physical properties (Asghar and Henrickson 1982; Olsen et al. 2005; Saddler and Horsey 1987). The fragment distribution and composition of gelatin is variable depending on the types of collagens available in the tissue to be extracted, extraction method, and the pH and ionic strength of the solution used for processing. This variability in composition and structure presents a significant challenge to those using gelatin in medical applications (Digenis et al. 1994; Olsen et al. 2003).
For these reasons, there is the need to develop cost effective, scalable technology to produce recombinant human collagen (rhC) and recombinant gelatin (rG) for use in medical products with consistent quality and improved safety (Bulleid et al. 2000; Olsen et al. 2003b; Yang et al. 2004). In addition, the use of recombinant production offers the only route to produce engineered rhC and rG for novel products with enhanced performance.
Several nonmicrobial systems (mammalian/insect cell culture, transgenics) have been explored to produce rhC and rG. Currently, the productivity, quality, and costs associated with these nonmicrobial rhC and rG production processes are not attractive for commercialization. Mammalian cells transfected with human collagen genes were first used to produce rhC, providing the only known recombinant system that can express properly prolyl hydroxylated full-length rhC that gets secreted (Ala-Kokko et al. 1991; Olsen et al. 1991; Schnieke et al. 1987). Recombinant insect cell cultures transfected with collagen genes accumulated the product intracellularly (Pihlajamaa et al. 1999; Tomita et al. 1995; Tomita et al. 1997; Tomita et al. 1999). However, insect cells did not provide sufficient collagen-specific P4H activity to fully hydroxylate the rhC. To address this deficiency, a multigene expression technology was developed to coexpress rhC with animal or human P4H, allowing the production of fully hydroxylated rhC in insect cells (Annunen et al. 1997; Lamberg et al. 1996; Myllyharju et al. 1997; Veijola et al. 1996). Milk from transgenic animals (John et al. 1999; Toman et al. 1999), secretions from transgenic silkworms (Tomita et al. 2003), transgenic tobacco cell culture (Olsen et al. 2003b; Yang et al. 1999), and transgenic tobacco plants (Merle et al. 2002; Ruggiero et al. 2000) have all been shown to accumulate nonhydroxylated or partially hydroxylated rhC homotrimers by coexpression of P4H. Transgenic mice accumulate underhydroxylated type I human procollagen homotrimers in milk at very high levels (8 g/l) (Toman et al. 1999). Transgenic tobacco cell cultures accumulate recombinant type III collagen coexpressed with human P4H as a triple helical product with 75% of the prolyl hydroxylation level found in collagen obtained from human tissue (Olsen et al. 2003b).
Microbial recombinant collagens
Production of recombinant collagens in Pichia pastoris
P. pastoris had been engineered to coexpress P4H for the production of properly hydroxylated triple-helical type I, II, and III rhC at high levels (1−1.5 g/l) (Myllyharju et al. 2000; Nokelainen et al. 2001; Olsen et al. 2003b). These P. pastoris-derived fibril-forming rhCs have similar structure and hydroxyproline content as human tissue-derived collagens (Kivirikko et al. 1992). The P. pastoris-derived rhCs formed stable triple helices, although P. pastoris probably does not contain HSP47, a folding chaperone thought to be involved in collagen assembly in animals (Nagata and Hosokawa 1996; Vuorela et al. 1997). Cells coexpressing the pro α1 subunit of type I collagen with human P4H accumulated stable homotrimeric rhC. P. pastoris coexpressing both the pro α1 and the pro α2 subunits of type I collagen with P4H accumulated heterotrimeric type I collagen with the expected 2:1 chain ratio (Nokelainen et al. 2001). Over 90% of the helical and nonhelical procollagens accumulated within the ER and does not proceed further in the secretory pathway even when the authentic signal sequence of collagen was replaced with the Saccharomyces cerevisiae α-mating factor prepro-secretion sequence (Keizer-Gunnink et al. 2000).
The recombinant procollagen accumulation levels in P. pastoris fermentations were improved from 15 mg/l to 1.5 g/l by genetic manipulations and optimization of fermentation control parameters (Bodo et al. 2004). Intracellular accumulation of 1.1 g type I collagen/l, 0.7 g type II collagen/l, and 1.5 g type III collagen/l has been reported (Olsen et al. 2003b). The use of synthetic genes optimized for P. pastoris expression resulted in a 25% increase in product accumulation (Bodo et al. 2004). Expression without the N-propeptide yielded a correctly folded and hydroxylated collagen molecule, resulting in a 20% increase in type III rhC accumulation compared to the expression of the same molecule with the N-propeptide. This result indicates that the N-propeptide does not play an essential role in collagen assembly. Substitution of the C-propeptide with a short bacterial polypeptide, foldon, involved in the assembly of phage T4 fibritin, resulted in the efficient formation of triple-helical rhC (Pakkanen et al. 2003). Production of stable tetrameric P4H is enhanced by the expression of collagen polypeptides as the substrate increases the half-life of P4H (Vuorela et al. 1999). No active P4H is formed in P. pastoris when the P4H α subunit is expressed alone. Expression of the animal P4H β subunit is essential for P4H activity in P. pastoris, suggesting that the endogenous P. pastoris protein disulfide isomerase does not efficiently form tetramers with recombinant human P4H α subunits. Cultures containing a single copy of the P4H gene accumulated more rhC compared to cultures containing multiple copies of the P4H genes (Bodo et al. 2004).
The P. pastoris fermentation process was conducted in a fed-batch mode at pH 6.0. Unlike most Pichia fermentation processes, the fermentation of strains expressing rhC was performed at 32°C. Using this temperature, it was found that rhC yields were slightly lower, but the hydroxylation of Y-position prolines was more efficient. The fermentation process achieved high cell densities of >300 g/l wet cell weight using stirred tanks sparged with oxygen (Bodo et al. 2004). Oxygen enrichment is critical to achieve full rhC hydroxylation as the human P4H needs high concentrations of molecular oxygen (Kivirikko and Pihlajaniemi 1998). A chemically defined minimal media is used to achieve process consistency and low cost, to avoid the use of complex components susceptible to contamination, and to produce an animal component-free product. To achieve high biomass and product accumulation, a glycerol feed is used to reach a wet cell weight of 300 g/l. The use of hexametaphosphate and of 10 g/l ammonium sulfate was determined to enhance growth (Bodo et al. 2004). The fed-batch phase is followed by a methanol feed to induce procollagen and P4H gene expression. The expression of these products is under the control of the alcohol oxidase 1 promoter. Fig. 1 illustrates the accumulation of recombinant type III procollagen in a P. pastoris fermentation.
SDS-PAGE analysis of PC-type III collagen (type III procollagen with the N-propeptide sequence deleted) expression. P. pastoris strain ΔNsCIIIz1-10 was fermented at the 20-l scale. Aliquots were harvested at the indicated times, and lysates were prepared in 0.1 M Tris–HCl pH 8.0, 0.2 M NaCl by glass bead lysis. The lysates were reduced with β mercaptoethanol and fractionated on a 4−12% Tris−glycine gel and stained with Gelcode Blue
The purification process for rhC from P. pastoris was developed to avoid the use of chromatographic methods and to deliver rhC with low endotoxin, host DNA, and host cell impurity levels (Bodo et al. 2004). As P. pastoris accumulates procollagen intracellularly, the cells are first washed free of media salts by tangential flow filtration (500 kDa cut-off) and lysed by physical disruption. No significant procollagen proteolysis was detected during cell disruption at acidic pH using procedures resulting in cell breakage efficiency of 85 to 95% (Bodo et al. 2004). The procollagen is converted to collagen by pepsin treatment at 4−8°C to remove propeptides and to degrade host cell proteins. P. pastoris-derived recombinant human pepsin was used for procollagen processing to avoid the use of animal-derived pepsin, resulting in a rhC production process completely free of animal-derived contaminants. The soluble, processed rhC is separated from cellular debris by sedimentation or filtration. Three salt precipitations are then performed using different pHs resulting in highly purified rhC preparation. The final removal of endotoxin and trace amounts of host cell-derived impurities was done by raising the pH of the preparation to 9.2 and adding calcium salts to precipitate the contaminants. The residual bioburden is removed by 0.2 μ membrane filtration. The final bulk product is a solution of highly purified, sterile filtered rhC in 0.01 M HCl ready for use in the products discussed in Sect. 4 of this review.
Characterization of P. pastoris-derived rhC indicates that the prolyl hydroxylation, measured as the ratio of hydroxyproline to proline plus hydroxyproline, is similar to the levels obtained in tissue-derived collagen. The completeness of the prolyl hydroxylation is also reflected in the similarity between the melting temperature (T m) as measured by circular dichroism of tissue-derived collagens with the P. pastoris-derived rhC (Olsen et al. 2003b). The T m of P. pastoris-derived types I and III rhC and bovine type I collagen was 40.5, 40.3, and 42.6°C, respectively. Comparison of purified type I, II, and III rhC with bovine type I collagen by sodium dodecyl sulfate−polyacrylamide gel electrophoresis (SDS-PAGE) indicated that over half of the bovine collagen is covalently cross-linked as dimers, trimers, and higher order molecular aggregates. The rhC consists predominately of monomers with only 5−10% of the material appearing as covalently cross-linked dimers (Olsen et al. 2003b). The rhC is a more homogeneous preparation than collagen obtained from tissues. Transmission electron microscopy analysis of rhC fibrils formed at neutral pH in phosphate buffer indicates that the fibrils have the characteristic banded pattern typical of native collagen. Fig. 2 illustrates the structure of fibrils formed from rhC III.
Transmission electron micrograph of glutaraldehyde cross-linked rhCIII. rhCIII expressed in P. pastoris was purified, and fibrils were formed in 20 mM phosphate buffer pH 7.4, 0.15 M NaCl and cross-linked with 0.0035% glutaraldehyde. The fibrils were collected by centrifugation, embedded in EPON resin, sectioned, and negatively stained with uranyl acetate. Fibrils were analyzed using a Phillips 12 CM transmission electron microscope. The magnification of the photograph is 10,000x
The rhC products derived from Pichia were shown to have excellent biocompatibility as determined in animal studies (Yang et al. 2004). The rhC was also tested for a wide array of potential contaminants including host cell protein, residual pepsin, host cell DNA, residual carbohydrate, bioburden, endotoxin, and heavy metals. Based on the low levels of these impurities, the collagen was suitable for intradermal injection in humans.
Production of recombinant collagens in S. cerevisiae
Stable heterotrimeric type I rhC has been produced by coexpressing the α1(I) and α2(I) procollagen genes in S. cerevisiae with chicken P4H (Toman et al. 2000). The S. cerevisiae-derived rhC heterotrimer was resistant to proteases at temperatures as high as 40°C. The hydroxyproline levels were 82% of values for tissue-derived collagen. Supplementation with glutamate was required to obtain high expression levels of type I procollagen. Another study involving type I rhC coexpression with chicken P4H indicated that triple helical conformation could be obtained even if both N- and C- propeptides are removed (Olsen et al. 2001). This result indicated that propeptides are not required for triple-helical folding and that their presence may limit rhC expression in S. cerevisiae. However, the ratio of pepsin-resistant α1(I) and α2(I) chains was 5:1, not 2:1 as in native type I collagen. The interpretation of this result is that both homotrimeric and heterotrimeric type I collagen species are produced in the same amount. Collagen fibrils with the characteristic-banding pattern seen in native collagen were prepared using the S. cerevisiae-derived rhC expressed without either propeptide.
A fragment of the human type III collagen helical domain was expressed in S. cerevisiae in a triple helical form (Vaughn et al. 1998). Expression was achieved by coexpressing the 255 amino acid collagen fragment of the helical domain with the complete C-telopeptide and C-propeptide domains with human P4H. The hydroxylated proline/nonhydroxylated ratio was 0.37 compared with the expected 0.81 in the corresponding human collagen fragment.
Microbial recombinant gelatins
The current process for manufacturing gelatin involves the extraction of collagen from tissue and its conversion to gelatin. This process yields a heterogeneous mixture of polypeptides that differ in both size and charge. The use of microbial systems to make rG has led to an alternative strategy. This strategy, illustrated in the examples below, consists of directly generating rG by expressing collagen gene fragments of specified length and composition. Studies using these collagen fragments indicated that they can be used as replacement for animal-derived gelatin for many of the current medical applications of gelatin (Olsen et al., unpublished data; Olsen et al. 2005).
Production of recombinant gelatins in P. pastoris
P. pastoris strains were engineered to secrete rG as collagen fragments with defined molecular weights and pI. These rGs exhibit lot-to-lot reproducibility, and the expression systems allow tailoring of the rG by genetic engineering to match specific applications. In addition, the use of high productivity P. pastoris-based rG processes results in low cost and the possibility to deliver the large amounts of material required for several gelatin applications. Approximately 50,000 metric tons of gelatins are used annually for medical use. Over 80% of this material is used in capsule production, with the rest used for injectable formulations and medical devices.
A series of rGs produced as fragments of the human pro α1(I) chain ranging in size from 56 to 1,014 amino acids are secreted by P. pastoris in high density fermentations (Olsen et al. 2000; Olsen et al. 2003b). The S. cerevisiae α-mating factor was used as the signal peptide to direct these fragments to the yeast secretory pathway. N-terminal sequencing demonstrated that accurate yeast secretion signal peptide processing takes place. The purification process to produce rG suitable for use as a human injectable product consists of cell separation, precipitation, solvent extraction, filtration, and ion exchange chromatography. Characterization of the resulting product indicated greater than 95% purity by SDS-PAGE densitometry analysis with no single impurity over 0.5%. As described above for rhC from P. pastoris, very low levels of potential contaminants were detected using a battery of analytical assays (Olsen et al. 2003).
A low molecular weight P. pastoris-derived 8.5 kDa rG with a pI of 9.4 was developed for use as a stabilizer for various injectable biologics (Olsen et al. 2003a; Olsen et al. 2005b). Characterization of the secreted 8.5 kDa rG by cation exchange chromatography indicated the presence of eight charge isoforms; 75% of the expressed gelatin was either phosphorylated or truncated at the C-terminus. Alanine-scanning mutagenesis was used to identify the modified amino acids responsible for the charged variants, and an engineered 8.5 kDa rG product free of these modifications was produced (Olsen et al. 2005b). This product has been successfully evaluated by human safety studies.
The high level expression and secretion of rat type III and mouse type I collagen fragments ranging in size from 21 to 53 kDa has been reported in P. pastoris. One of these fragments, a 21 kDa rG derived from the helical domain of rat type III collagen, was secreted at one of the highest accumulation levels for a secreted heterologous protein ever obtained from recombinant yeast: 14.8 g/l (Werten et al. 1999). Proteolytic degradation was minimized by conducting the fermentation at pH 3, the addition of extracts containing amino acids, and protein engineering to alter the amino acid sequence. The accumulation of 14.8 g/l was obtained using a strain containing 15 copies of the coding sequence per cell. Single-copy transformants accumulated rG in the range of 3 to 6 g/l (Werten et al. 2001). These secreted rGs could be purified to near homogeneity by differential ammonium sulfate precipitation.
Production of recombinant gelatins in S. cerevisiae and Hansenula polymorpha
Secretion of rG has been reported in H. polymorpha but at lower accumulation levels compared to P. pastoris (de Bruin et al. 2000). Secretion of an endogenous collagen-like protein and a heterologously expressed collagen fragment hydroxylated in the Y position of Gly-X-Y triplets was reported using H. polymorpha without coexpression of P4H (de Bruin et al. 2002). Hydroxylated rG accumulation occurred in both constitutive, glucose-fed fermentations, and methanol-induced fermentations, but only when a complex nutrient source (peptone) was added to the media. No hydroxylation was obtained when the fermentation was conducted with a medium containing only salts and methanol. In contrast, when the same fragment was expressed in P. pastoris in the presence of peptone, no hydroxylation occured, indicating the presence of an endogenous P4H activity specific to H. polymorpha. This accumulation of hydroxylated rG represents the first report of an endogenous yeast prolyl hydroxylase activity and of an endogenous yeast-derived collagen-like protein. The smut fungus Microbotryum violaceum also produces an endogenous collagen-like protein (Celerin et al. 1996). Cell fractionation data suggest that in both systems, these fungal collagen-like proteins are cell-surface attached.
Production of recombinant gelatin in bacterial systems
The production of gelatin using Escherichia coli has seen limited success. Bovine collagen α2 (I) chain fragments ranging in size from 93 to 245 amino acids were expressed in E. coli using the T7 promoter as a fusion protein containing a 6-histidine tag and a portion of the phage T7 gene 10 leader (Hori et al. 2002). No information was provided about the accumulation levels or purification yields, but enough material was produced to test the reactivity of rGs with antigelatin antibodies. A totally synthetic gelatin made of 32 repeats of the sequence Gly-Pro-Pro fused to bacteriophage protein CII was expressed in E. coli as a 22 kDa fusion protein under the control of the thermoinducible lambda pL promoter (Goldberg et al. 1989). This fusion protein containing the synthetic gelatin was shown to accumulate in inclusion bodies. Genetic inhibition of the heat shock response of E. coli significantly stabilized the expressed synthetic gelatin product. The expression of other synthetic gelatin-like proteins in E. coli had been reported in low yield because of the apparent instability of these highly repetitive genes in E. coli (Cappello et al. 1990; Cappello 1990).
An E. coli strain was engineered for the cotranslational incorporation of hydroxyproline to accumulate fragments as well as full-length type I rhC (Buechter et al. 2003). This was achieved by growing an E. coli culture engineered for increased prolyl aminoacyl-tRNA synthase accumulation in a hyperosmotic media supplemented with hydroxyproline. The resulting α1(I) collagen fragment was different from tissue-derived collagen in that hydroxyproline was present at both X and Y positions of the Gly-X-Y triplets. The expressed collagen fragment was assembled into a triple helix as determined by circular dichroism analysis. By supplementing the cultures with a mixture of hydroxyproline and proline and using genetic approaches, the authors speculate that it may be possible to control the level and specificity of hydroxyproline incorporation. Accumulation of the collagen chain fragment polypeptide did not occur using a hyperosmotic media containing only proline or rich media.
Recently, an active human P4H tetramer was expressed in E. coli (Neubauer et al. 2005). Production of active enzyme required a strain of E. coli with a relatively oxidizing cytosol (Kersteen et al. 2004). This result indicates that it may be possible to generate prolyl hydroxylated collagen chain fragments in E. coli by P4H coexpression.
Artificial gelatins comprising tandemly repeated 30-amino acid peptide units derived from type I collagen sequences were successfully expressed using B. brevis (Kajino et al. 2000). The artificial gelatins containing the eight-unit and six-unit repeats were secreted at levels as high as 0.5 g/l. Product was recovered by ammonium sulfate fractionation and anion-exchange chromatography. The purified artificial gelatins had the predicted N-terminal sequences and amino acid compositions.
Commercial use of yeast-derived recombinant collagens and gelatins
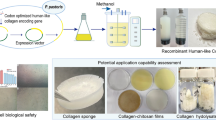
rhC and rG are being evaluated for use in many medical devices and pharmaceutical products. P. pastoris-derived rhC can self-assemble into fibrils that can be used directly or can be fabricated into other structural forms (e.g., porous matrices, films, gels, or sponges) (Yang et al. 2004). In a 3-D sponge matrix, P. pastoris-derived rhC was shown to have superior mechanical integrity, greater surface area, and increased hemostatic activity in animal models compared with the same structure made from bovine type I collagen. In addition, subcutaneous implantation studies in rats indicated that the animal-derived collagen sponges induced an inflammatory response, while the rhC sponges were more biocompatible (Yang et al. 2001; Yang et al. 2004). Sponges made from P. pastoris-derived type I rhC are less porous and more resistant to bacterial collagenase compared to animal-derived collagen sponges (Yang et al. 2004). These properties allow P. pastoris-derived rhC to provide a suitable matrix for tissue-engineering application such as repair or replacement of failing or aging body parts and treatment of periodontal disease. Sponges containing recombinant P. pastoris-derived type II collagen may be a natural choice as a carrier for chondrogenic growth factors for cartilage repair (Yang et al. 2004). P. pastoris-derived rhC was also used to prepare collagen membranes that could be used for dural closures, wound dressings, reinforcement and support of weak tissues, and guided tissue regeneration. A P. pastoris-derived rhC I matrix was shown to be effective as a dermal layer (Yang et al. 2004).
Studies indicated that knitted DACRON vascular grafts coated with rhC III provide a suitable substrate for cell attachment and spreading (Olsen et al. 2003b). The use of collagen-coated stents may provide supplementary functions such as local drug delivery, gene transfer, reduction of operative blood loss, and facilitation of endothelial cell in-growth. Drugs may be incorporated into or added to the collagen matrix for drug delivery applications. The use of rhC offers the opportunity to develop products enabling process standardization and consistent drug delivery output.
Studies had shown that rhC and rG can be used as substrates for attachment of various cell types (Yang et al. 2004). Vero cells are routinely grown on gelatin-coated microcarriers to prepare high-titer viral stocks for vaccines. The growth of Vero cells on rG-coated and animal gelatin-coated macrocarriers was shown to be similar. Microscopic evaluation showed cells on the surface of both the original beads and smaller diameter beads added to the culture after the cultures were confluent, demonstrating the effective expansion of the culture in a bioreactor without using an animal-derived protease, such as trypsin to passage cells (Olsen et al. 2003b).
A low molecular weight 8.5 kDa P. pastoris-derived rG was shown to be equivalent to animal-derived gelatin as a stabilizer of an influenza virus (Olsen 2004). The immunogenicity profile of this rG was tested using serum from children with confirmed gelatin allergies. Antibodies recognizing bovine and human collagens did not recognize P. pastoris-derived 8.5 kDa rG, indicating its low allergenic potential. Animal studies indicated the potential use of P. pastoris-derived rG as a safer resuscitation fluid, providing an alternative to animal-derived gelatin and human serum albumin (Olsen et al, unpublished data). P. pastoris-derived rhG could also be used to replace the gel-forming gelatins, such as those used in foods, capsules, and other applications (Olsen et al, unpublished data). Replacement of animal-derived gelatin with a safer, higher quality product has been a difficult challenge for the capsule industry. Gelatin has certain attributes, such as viscosity and gel strength, making it a unique material for producing capsules that dissolve at physiologically relevant temperatures and pH.
Conclusion
This review discussed the use of recombinant microbial expression systems as a cost-effective, scalable production technology suitable for manufacturing two novel biomaterials with consistent quality and improved safety: rhC and rG. Recombinant microbial technology has been proposed for the production of other important protein-based biopolymers such as elastin and dragline spider silk. Some of these biopolymers can only be commercialized using recombinant technology (silk, gelatin as defined collagen fragments); in other cases, the use of recombinant technology provides attractive substitutes for animal and human tissue-derived biomaterials, especially for use in pharmaceutical applications. The technology also enables the engineering of biopolymers to improve the performance of products containing these biomaterials.
P. pastoris was demonstrated to be an effective production system for the manufacture of rhC and rG. Four important aspects of this production system that are critical for the commercialization of recombinant protein-based biopolymers were discussed: first, the accumulation of high levels of recombinant protein, 1 to 1.5 g/l for rhC and 3 to 14 g/l for rG; second, the proper intracellular assembly of triple helical rhC and the secretion of intact rG fragments; third, the posttranslational modification of collagen by coexpression of P4H to obtain the required structure and stability; and finally, the recovery of material suitable for diverse medical applications such as tissue engineering, resuscitation fluids, capsule manufacturing, stabilization of pharmaceutical formulation, and hemostats could be achieved with these systems. In addition to P. pastoris, rG can be produced as collagen fragments with defined molecular weight, composition, and physiochemical properties using S. cerevisiae, H. polymorpha, E. coli, and B. brevis.
References
Ala-Kokko L, Hyland J, Smith C, Kivirikko KI, Jimenez SA, Prockop DJ (1991) Expression of a human cartilage procollagen gene (COL2A1) in mouse 3T3 cells. J Biol Chem 266:14175−14178
Annunen P, Helaakoski T, Myllyharju J, Veijola J, Pihlajaniemi T, Kivirikko KI (1997) Cloning of the human prolyl 4-hydroxylase alpha subunit isoform alpha(II) and characterization of the type II enzyme tetramer. The alpha(I) and alpha(II) subunits do not form a mixed alpha(I)alpha(II)beta2 tetramer. J Biol Chem 272:17342−17348
Asghar A, Henrickson RL (1982) Chemical, biochemical, functional, and nutritional characteristics of collagen in food systems. Adv Food Res 28:231−372
Bateman JF, Lamande SR, Ramshaw JAM (1996) Collagen superfamily. In: Comper WD (ed) Extracellular matrix, 2nd edn. Harwood Academic, Amsterdam pp 22−67
Bodo B, Chang R, Hamalainen E, Leigh S, McMullin H, Olsen D, Revak T, Yang C, Polarek J (2004) Production of triple-helical recombinant human collagen in P. pastoris. Annual meeting industrial microbiology and biotechnology, Anaheim, CA, USA
Buechter DD, Paolella DN, Leslie BS, Brown MS, Mehos KA, Gruskin EA (2003) Co-translational incorporation of trans-4-hydroxyproline into recombinant proteins in bacteria. J Biol Chem 278:645−650
Bulleid NJ, John DC, Kadler KE (2000) Recombinant expression systems for the production of collagen. Biochem Soc Trans 28:350−353
Cappello J (1990) The biological production of protein polymers and their use. Trends Biotechnol 8:309−311
Cappello J, Crissman J, Dorman M, Mikolajczak M, Textor G, Marquet M, Ferrari F (1990) Genetic engineering of structural protein polymers. Biotechnol Prog 6:198−202
Celerin M, Ray J, Schisler N, Day A, Stetler-Stevenson W, Laudenbach D (1996) Fungal fimbriae are composed of collagen. EMBO J 15:4445−4453
de Bruin EC, de Wolf FA, Laane NC (2000) Expression and secretion of human alpha1(I) procollagen fragment by Hansenula polymorpha as compared to Pichia pastoris. Enzyme Microb Technol 26:640−644
de Bruin EC, Werten MW, Laane C, de Wolf FA (2002) Endogenous prolyl 4-hydroxylation in Hansenula polymorpha and its use for the production of hydroxylated recombinant gelatin. FEMS Yeast Res 1:291−298
Digenis GA, Gold TB, Shah VP (1994) Cross-linking of gelatin capsules and its relevance to their in vitro-in vivo performance. J Pharm Sci 83:915−921
Dormont D (2002) Prions, BSE and food. Int J Food Microbiol 78:181−189
Eyre D (1987) Collagen cross-linking amino acids. Methods Enzymol 144:115−139
Goldberg I, Salerno AJ, Patterson T, Williams JI (1989) Cloning and expression of a collagen-analog-encoding synthetic gene in Escherichia coli. Gene 80:305−314
Hori H, Hattori S, Inouye S, Kimura A, Irie S, Miyazawa H, Sakaguchi M (2002) Analysis of the major epitope of the alpha2 chain of bovine type I collagen in children with bovine gelatin allergy. J Allergy Clin Immunol 110:652−657
John DC, Watson R, Kind AJ, Scott AR, Kadler KE, Bulleid NJ (1999) Expression of an engineered form of recombinant procollagen in mouse milk. Nat Biotechnol 17:385−389
Kajino T, Takahashi H, Hirai M, Yamada Y (2000) Efficient production of artificially designed gelatins with a Bacillus brevis system. Appl Environ Microbiol 66:304−309
Kaneko K (2002) Potential risk of bovine spongiform encephalopathy (BSE) to human beings and therapeutic approaches to prion disease. Shokuhin Eiseigaku Zasshi 43:J221−J227
Keizer-Gunnink I, Vuorela A, Myllyharju J, Pihlajaniemi T, Kivirikko KI, Veenhuis M (2000) Accumulation of properly folded human type III procollagen molecules in specific intracellular membranous compartments in the yeast Pichia pastoris. Matrix Biol 19:29−36
Kersteen EA, Higgin JJ, Raines RT (2004) Production of human prolyl 4-hydroxylase in Escherichia coli. Protein Expr Purif 38:279−291
Kivirikko KI (1995) Principles of medical biology. In: Bittar EE, Bittar N (eds) Posttranslational processing of collagens, 3rd edn. JAI Press, Greenwich pp 233−254
Kivirikko KI, Pihlajaniemi T (1998) Collagen hydroxylases and the protein disulfide isomerase subunit of prolyl 4-hydroxylases. Adv Enzymol Relat Areas Mol Biol 72:325−398
Kivirikko KI, Myllyla R, Pihlajaniemi T (1992) Hydroxylation of proline and lysine residues in collagens and other animal and plant proteins. In: Harding JJ, Crabbe MJC (eds) Post-translational modifications of proteins. CRC Press, Inc., Boca Raton pp 1−51
Kukkola L, Koivunen P, Pakkanen O, Page AP, Myllyharju J (2004) Collagen prolyl 4-hydroxylase tetramers and dimers show identical decreases in Km values for peptide substrates with increasing chain length: mutation of one of the two catalytic sites in the tetramer inactivates the enzyme by more than half. J Biol Chem 279:18656−18661
Lamberg A, Helaakoski T, Myllyharju J, Peltonen S, Notbohm H, Pihlajaniemi T, Kivirikko KI (1996) Characterization of human type III collagen expressed in a baculovirus system. Production of a protein with a stable triple helix requires coexpression with the two types of recombinant prolyl 4-hydroxylase subunit. J Biol Chem 271:11988−11995
Merle C, Perret S, Lacour T, Jonval V, Hudaverdian S, Garrone R, Ruggiero F, Theisen M (2002) Hydroxylated human homotrimeric collagen I in Agrobacterium tumefaciens-mediated transient expression and in transgenic tobacco plant. FEBS Lett 515:114−118
Min W, Begley TP, Myllyharju J, Kivirikko KI (2000) Mechanistic studies on prolyl-4-hydroxylase: demonstration that the ferryl intermediate does not exchange with water. Bioorganic Chem 28:261−265
Myllyharju J, Kivirikko KI (2004) Collagens, modifying enzymes and their mutations in humans, flies and worms. Trends Genet 20:33−43
Myllyharju J, Lamberg A, Notbohm H, Fietzek PP, Pihlajaniemi T, Kivirikko KI (1997) Expression of wild-type and modified proalpha chains of human type I procollagen in insect cells leads to the formation of stable [alpha1(I)2alpha2(I) collagen heterotrimers and [alpha1(I)3 homotrimers but not [alpha2(I)3 homotrimers. J Biol Chem 272:21824−21830
Myllyharju J, Nokelainen M, Vuorela A, Kivirikko KI (2000) Expression of recombinant human type I−III collagens in the yeast Pichia pastoris. Biochem Soc Trans 28:353−357
Nagata K, Hosokawa N (1996) Regulation and function of collagen-specific molecular chaperone, HSP47. Cell Struct Funct 21:425−430
Neubauer A, Neubauer P, Myllyharju J (2005) High-level production of human collagen prolyl 4-hydroxylase in Escherichia coli. Matrix Biol 24:59−68
Nokelainen M, Tu H, Vuorela A, Notbohm H, Kivirikko KI, Myllyharju J (2001) High-level production of human type I collagen in the yeast Pichia pastoris. Yeast 18:797−806
Olsen D (2004) Recombinant human gelatin vaccine stabilizer: A substitute for animal-derived gelatin with superior features. The seventh annual conference on vaccine research. Arlington, VA, USA (abstract)
Olsen AS, Geddis AE, Prockop DJ (1991) High levels of expression of a minigene version of the human pro alpha 1 (I) collagen gene in stably transfected mouse fibroblasts. Effects of deleting putative regulatory sequences in the first intron. J Biol Chem 266:1117−1121
Olsen D, Chang R, Jiang J, Pirskanen A, Myllyharju J, Yang C, Bodo M, Jarvinen M, Nevalainen T, Hamalainen E-R, Perala-Heape M, Nokelainen M, Kivirikko KI, Polarek J (2000) Development of Recombinant Human Gelatins and Specific Molecular Type Human Gelatins. Cambridge Healthtech Institute's 2nd annual international transmissible spongiform encephalopathies (TSE Issues). Alexandria, VA (abstract)
Olsen DR, Leigh SD, Chang R, McMullin H, Ong W, Tai E, Chisholm G, Birk DE, Berg RA, Hitzeman RA, Toman PD (2001) Production of human type I collagen in yeast reveals unexpected new insights into the molecular assembly of collagen trimers. J Biol Chem 276:24038−24043
Olsen D, Chang R, Sakaguchi M, Leigh S, Lundgard R, Buschman F, Lonergan M, McMullin H, Luehrs C, Beardsley A, Revak T, Polarek J (2003a) Formulation strategies for biopharmaceuticals. Development of recombinant human gelatin for use as a stabilizer in biopharmaceuticals. Philadelphia, PA (abstract)
Olsen D, Yang C, Bodo M, Chang R, Leigh S, Baez J, Carmichael D, Perala M, Hamalainen ER, Jarvinen M, Polarek J (2003b) Recombinant collagen and gelatin for drug delivery. Adv Drug Deliv Rev 55:1547−1567
Olsen D, Chiang R, Williams KE, and Polarek JW, (2005a) The development of novel recombinant human gelatins as replacements for animal-derived gelatin in pharmacuetical applications In: Pasupuleti VK, and Sai International (eds) Protein Hydrolysates in Nutrition and Biotechnology, Kluwer Academic; Dordrecht, The Netherlands. Unpublished
Olsen D, Jiang J, Chang R, Duffy R, Sakaguchi M, Leigh S, Lundgard R, Ju J, Buschman F, Truong-Le V, Pham B, Polarek JW (2005b) Expression and characterization of a low molecular weight recombinant human gelatin: development of a substitute for animal-derived gelatin with superior features. Protein Expr Purif 40:346−357
Pakkanen O, Hamalainen ER, Kivirikko KI, Myllyharju J (2003) Assembly of stable human type I and III collagen molecules from hydroxylated recombinant chains in the yeast Pichia pastoris. Effect of an engineered C-terminal oligomerization domain foldon. J Biol Chem 278:32478−32483
Pihlajamaa T, Perala M, Vuoristo MM, Nokelainen M, Bodo M, Schulthess T, Vuorio E, Timpl R, Engel J, Ala-Kokko L (1999) Characterization of recombinant human type IX collagen. Association of alpha chains into homotrimeric and heterotrimeric molecules. J Biol Chem 274:22464−22468
Prockop DJ, Kivirikko KI (1995) Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem 64:403−434
Ruggiero F, Exposito JY, Bournat P, Gruber V, Perret S, Comte J, Olagnier B, Garrone R, Theisen M (2000) Triple helix assembly and processing of human collagen produced in transgenic tobacco plants. FEBS Lett 469:132−136
Saddler JM, Horsey PJ (1987) The new generation gelatins. A review of their history, manufacture and properties. Anaesthesia 42:998−1004
Schnieke A, Dziadek M, Bateman J, Mascara T, Harbers K, Gelinas R, Jaenisch R (1987) Introduction of the human pro alpha 1(I) collagen gene into pro alpha 1(I)-deficient Mov-13 mouse cells leads to formation of functional mouse-human hybrid type I collagen. Proc Natl Acad Sci U S A 84:764−768
Schrieber R, Seybold U (1993) Gelatine production, the six steps to maximum safety. Dev Biol Stand 80:195−198
Tandon M, Wu M, Begley TP, Myllyharju J, Pirskanen A, Kivirikko K (1998) Substrate specificity of human prolyl-4-hydroxylase. Bioorg Med Chem Lett 8:1139−1144
Toman PD, Pieper F, Sakai N, Karatzas C, Platenburg E, de Wit I, Samuel C, Dekker A, Daniels GA, Berg RA, Platenburg GJ (1999) Production of recombinant human type I procollagen homotrimer in the mammary gland of transgenic mice. Transgenic Res 8:415−427
Toman PD, Chisholm G, McMullin H, Giere LM, Olsen DR, Kovach RJ, Leigh SD, Fong BE, Chang R, Daniels GA, Berg RA, Hitzeman RA (2000) Production of recombinant human type I procollagen trimers using a four-gene expression system in the yeast Saccharomyces cerevisiae. J Biol Chem 275:23303−23309
Tomita M, Ohkura N, Ito M, Kato T, Royce PM, Kitajima T (1995) Biosynthesis of recombinant human pro-alpha 1(III) chains in a baculovirus expression system: production of disulphide-bonded and non-disulphide-bonded species containing full-length triple helices. Biochem J 312(Pt 3):847−853
Tomita M, Kitajima T, Yoshizato K (1997) Formation of recombinant human procollagen I heterotrimers in a baculovirus expression system. J Biochem (Tokyo) 121:1061−1069
Tomita M, Yoshizato K, Nagata K, Kitajima T (1999) Enhancement of secretion of human procollagen I in mouse HSP47-expressing insect cells. J Biochem (Tokyo) 126:1118−1126
Tomita M, Munetsuna H, Sato T, Adachi T, Hino R, Hayashi M, Shimizu K, Nakamura N, Tamura T, Yoshizato K (2003) Transgenic silkworms produce recombinant human type III procollagen in cocoons. Nat Biotechnol 21:52−56
Vaughn PR, Galanis M, Richards KM, Tebb TA, Ramshaw JA, Werkmeister JA (1998) Production of recombinant hydroxylated human type III collagen fragment in Saccharomyces cerevisiae. DNA Cell Biol 17:511−518
Veijola J, Pihlajaniemi T, Kivirikko KI (1996) Co-expression of the alpha subunit of human prolyl 4-hydroxylase with BiP polypeptide in insect cells leads to the formation of soluble and insoluble complexes. Soluble alpha-subunit-BiP complexes have no prolyl 4-hydroxylase activity. Biochem J 315(Pt 2):613−618
Vuorela A, Myllyharju J, Nissi R, Pihlajaniemi T, Kivirikko KI (1997) Assembly of human prolyl 4-hydroxylase and type III collagen in the yeast Pichia pastoris: formation of a stable enzyme tetramer requires coexpression with collagen and assembly of a stable collagen requires coexpression with prolyl 4-hydroxylase. EMBO J 16:6702−6712
Vuorela A, Myllyharju J, Pihlajaniemi T, Kivirikko KI (1999) Coexpression with collagen markedly increases the half-life of the recombinant human prolyl 4-hydroxylase tetramer in the yeast Pichia pastoris. Matrix Biol 18:519−522
Werten MW, van den BTJ, Wind RD, Mooibroek H, de Wolf F (1999) High-yield secretion of recombinant gelatins by Pichia pastoris. Yeast 15(15):1087−1096
Werten MW, Wisselink WH, Jansen-van den Bosch TJ, de Bruin EC, de Wolf FA (2001) Secreted production of a custom-designed, highly hydrophilic gelatin in Pichia pastoris. Protein Eng 14:447−454
Yamauchi M, Shiiba M (2002) Lysine hydroxylation and crosslinking of collagen. Methods Mol Biol 194:290
Yang C, Bodo M, Chang R, Perala-Heape M, Hamalainen E, Russell D, Polarek J (1999) Development of recombinant gelatin by expression of recombinant collagen in yeast and plants. AAPS annual meeting. New Orleans, LA (abstract)
Yang C, Balan J, Tang J, Lee S, Bodo M, Ho F, Duffy R, Chin E, Perala-Heape M, Hamalainen, E-R, Polarek, J (2001) Biocompatibility of recombinant human collagen I produced with multigene expression system for biomaterial application. Society for biomaterials 27th annual meeting. Saint Paul, MN, USA (Abstract)
Yang C, Hillas PJ, Baez JA, Nokelainen M, Balan J, Tang J, Spiro R, Polarek JW (2004) The application of recombinant human collagen in tissue engineering. BioDrugs 18:103−119
Acknowledgement
The authors would like to recognize the technical contribution to this work provided by Michael Bodo, Frank Buschman, Robert Chang, Robert Duffy, Jenny Jiang, Julia Ju, Scott Leigh, Robert Lundgard, Hugh McMullin, Timothy Revak, Kim Williams, and Chunlin Yang. The authors would also like to thank Elaine Lee for the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Báez, J., Olsen, D. & Polarek, J.W. Recombinant microbial systems for the production of human collagen and gelatin. Appl Microbiol Biotechnol 69, 245–252 (2005). https://doi.org/10.1007/s00253-005-0180-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0180-x