Abstract
Rhodococcus opacus 1CP, a potent degrader of (chloro-) aromatic compounds was found to utilise C10–C16 n-alkanes as sole carbon sources. Highest conversion rates were observed with n-tetradecane and n-hexadecane, whereas the utilisation of n-dodecane and n-decane was considerably slower. Thin-layer chromatography of organic extracts of n-alkane-grown 1CP cultures indicated the growth-associated formation of a glycolipid which was characterised as a trehalose dimycolate by 1H-NMR spectroscopy and mass spectrometry. Total chain lengths between 48 and 54 carbons classify the fatty acid residues as nocardiomycolic acids. The presence of two double bonds in each mycolic acid is another feature that distinguishes the corresponding trehalose dinocardiomycolates from trehalose dicorynomycolates reported for Rhodococcus erythropolis DSM43215 and Rhodococcus ruber IEGM231. R. opacus 1CP was not found, even under nitrogen limitation, to produce anionic trehalose tetraesters which have previously been reported for R. erythropolis DSM43215.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Numerous microorganisms of aquatic and terrestrial origin are able to utilise aliphatic and aromatic hydrocarbons of extremely low water solubility as carbon and energy sources. Although the actual uptake of such compounds by bacteria has been reported to be a passive process (Ratledge 1978), the bottleneck of low bioavailability has been overcome by several adaptive mechanisms of which the formation of biosurfactants probably is the most important one (for extensive reviews see Banat et al. (2000), Lang (2002), and Ron and Rosenberg (2001)). Accelerated dissolution of the substrate, the formation of fine substrate droplets (pseudosolubilisation) and the facilitation of cell/substrate contact are discussed to be the three principal modes of action of (bio-) surfactants (Schippers et al. 2000).
The formation of biosurfactants by rhodococci and related nocardioform actinobacteria has been subject of many studies, and several trehalose-containing glycolipids which often occur as a complex mixture during growth of rhodococci on alkanes were structurally elucidated. Trehalose monomycolates (Kretschmer and Wagner 1983), trehalose dimycolates (Philp et al. 2002; Rapp et al. 1979), trehalose trimycolates (Tomiyasu et al. 1986), as well as mono-, di-, tetra-, hexa- and octa-acylated derivatives of trehalose (Kretschmer and Wagner 1983; Philp et al. 2002; Singer et al. 1990) represent the non-ionic trehalose lipid type. Trehalose tetraesters (Rapp and Gabriel-Jürgens 2003; Ristau and Wagner 1983) and succinoyl trehalose lipids (Uchida et al. 1989), which additionally harbour one or two succinoyl residues, represent the anionic trehalose lipid type. Because the structural diversity of those glycolipids originates from a very limited number of investigated strains (mainly Rhodococcus erythropolis and Rhodococcus ruber) the existence of additional biosurfactant types with interesting surface-active properties is likely.
Due to their high-performance emulsifying and emulsion-stabilising properties and their low toxicity, trehalose lipids are favoured in several environmental applications such as microbial-enhanced oil recovery (MEOR) and oil-spill treatment (Kretschmer et al. 1982). In addition, certain trehalose lipids from rhodococci might have the potential for medical application because they have recently been shown to possess immune-stimulating and cell-differentiating properties (Kitamoto et al. 2002; Ryll et al. 2001).
To supplement the structural knowledge on trehalose lipids for the species Rhodococcus opacus and as a step towards elucidation of the genetic and biochemical basis of trehalose lipid formation, the genetically well investigated and metabolically extraordinary versatile model organism R. opacus 1CP was characterised for its ability to produce glycolipid-type biosurfactants. Strain 1CP was originally isolated due to its ability to mineralise 2,4-dichlorophenol (Gorlatov et al. 1989) and was shown to degrade a broad spectrum of (chloro-) aromatic compounds (Moiseeva et al. 1999). The protocatechuate-, the catechol-, and two different chlorocatechol-metabolic pathways have been extensively studied on biochemical and molecular basis, and the latter ones were found to be located on a huge linear megaplasmid p1CP (König et al. 2004). We report here on the growth of R. opacus 1CP with long-chain n-alkanes and on the detection, isolation and structural characterisation of novel trehalose dinocardiomycolates which were shown to be overproduced during growth on this type of substrates.
Material and methods
Microorganism
R. opacus 1CP has originally been isolated from soil by enrichment on 2,4-dichlorophenol and is able to utilise various (chloro-) aromatic compounds as sole sources of carbon and energy (Gorlatov et al. 1989).
Media and culture conditions
Batch cultivation of R. opacus 1CP on n-alkanes was performed in mineral medium (Dorn et al. 1974) with doubled phosphate buffer concentration and 0.5 or 1% (w/v) of the respective hydrocarbon (n-decane, n-dodecane, n-tetradecane, n-hexadecane). In case of nitrogen-limiting conditions, the above mineral medium did not contain ammonium sulphate and thus had a high carbon surplus. Cultures were grown in baffled Erlenmeyer flasks with shaking (130 rpm) at 30°C. For storage and purity control R. opacus 1CP was kept on nutrient broth solid media (Oxoid) or on mineral solid media containing 1 mM glucose.
Quantification and characterisation of bacterial growth
Alkane consumption was determined by respirometric control with an OxiTopC system (WTW, Weilheim, Germany). Continuously stirred 100-ml cultures, containing mineral medium and 0.1% (w/v) of the respective carbon source (n-decane, n-dodecane, n-tetradecane, n-hexadecane), were inoculated with 0.5 ml of a glucose-grown pre-culture of strain 1CP and incubated at 20°C (±0.1°C) for 10 days. Basal respiration was determined by running a control without n-alkane. The experiment was performed in duplicate. Biomass formation was measured indirectly via quantification of total protein. Thus, whole 20-ml n-tetradecane-grown cultures were extracted three times each with 10 ml chloroform. Rapid phase separation was achieved by centrifugation at 5,000×g for 5 min. Finally, the aqueous layer was carefully removed, and the cell-containing chloroform extracts were combined. After separating the cells from the solvent by another centrifugation step, the organic phase was subjected to alkane and glycolipid quantification, whereas the pelleted biomass was resuspended in the aqueous phase. After concentrating this mixture to dryness under reduced pressure, total protein was determined of the residue by the Biuret protocol (Bast 1999). The whole procedure was done in duplicate.
To document the characteristic growth behaviour of R. opacus 1CP in the presence of n-alkanes, 20-ml samples from a 300-ml (1% w/v) n-tetradecane culture were transferred into petri dishes and were photographed in reflected light using a macro lens.
Quantification of n-alkanes
Residual n-tetradecane in chloroform extracts was quantified by gas chromatography using a Hewlett Packard 437A instrument equipped with a flame ionisation detector (FID) and a glass column (length 1 m, i.d. 2 mm, 10% SE30 on Chromosorb WHP, 80-100 mesh, Alltech). The system was connected with an interface (Dionex), and signals were analysed with the Chromeleon software V6.11 (Dionex). The column was operated isothermally at 150°C at an N2 flow rate of 25 ml min−1. Calibration was performed with an external tetradecane standard.
Detection, quantification and purification of glycolipids
Cultures (100 ml) of R. opacus 1CP grown exponentially with 0.5% (w/v) n-alkanes (n-decane, n-dodecane, n-tetradecane, n-hexadecane) were acidified to pH 3–4 by addition of HCl. Solid NaCl was added to saturation, and the whole broth was extracted twice each with 300 ml of ethyl acetate or, at a later point, with the same volume of chloroform. Rapid phase separation was achieved by centrifugation of the slurry at 17,700×g for 20 min. The organic phase was separated, pooled, and after drying with anhydrous Na2SO4 and filtration through Whatman paper, was freed from solvent under reduced pressure. The residual viscous oil was re-dissolved in a defined volume of chloroform, and 5-μl samples were subjected to thin-layer chromatography (TLC) analysis.
Analytical TLC was performed on silica gel 60F254 plates (Merck) using the mobile phase chloroform/methanol/water (65:10:0.9 v/v/v). Developed plates were dried, sprayed with sugar-specific 4-methoxybenzaldehyde/acetic acid/sulphuric acid reagent (1:10:2 v/v/v) (Kretschmer et al. 1982) and heated to 150°C for 2–4 min. Glycolipids gave green- to blue-coloured spots. However, the reagent also reacts with other compounds such as fatty acids, triglycerides and alkanes. A preliminary assignment of detected glycolipids was achieved by comparison of their retention with those of authentic reference compounds for trehalose monocorynomycolate, trehalose dicorynomycolate and anionic trehalose tetraester (Kretschmer et al. 1982).
The purification of milligram amounts of glycolipids for structural analysis was achieved by low pressure liquid chromatography on a Lobar column (Chroprep Si60, size B, Merck) using the mobile phase already applied in TLC. If necessary, crude organic extracts were pre-purified and freed from excess n-alkane by flash-chromatography. Silica gel 60 (63–200 μm, Fluka) was used as the stationary phase, and elution was achieved with solvent systems of increasing polarity in the following order: cyclohexane, cyclohexane/chloroform (1:1), chloroform/methanol (9:1), chloroform/methanol/water (65:10:0.9). The purity of glycolipids was controlled by TLC as described above.
Trehalose lipids were quantified after alkaline hydrolysis due to their carbohydrate content with the anthrone method (Pan et al. 1996). Reducing and non-reducing sugars react with the anthrone reagent in the presence of strongly oxidising sulphuric acid to yield a blue to green colour. From the chloroform extracts of n-alkane-grown 1CP cultures, 1-ml samples were taken and freed from solvent. The residue was hydrolysed for 1 h with 250 μl 1 M NaOH in a boiling water bath under frequent shaking. After cooling on ice, an equimolar amount of 1 M HCl was added, and the mixture was centrifuged for 15 min at 16,100×g to separate residual n-alkane. One hundred and thirty microliters of the aqueous phase was mixed with the 3-fold volume of freshly prepared anthrone reagent (0.2 g anthrone in 96% sulphuric acid), and the mixture was heated for 15 min in a boiling water bath. After cooling, the absorption of the solution was measured at 620 nm and compared with a calibration curve prepared with trehalose in a concentration range from 0 to 0.3 mM. An average molar mass of 1,824 g mol−1 as later determined from structural analysis was used for the calculation of weight amounts of the trehalose dinocardiomycolate.
Structural analysis of the glycolipid
Detailed structural elucidation of the glycolipid isolated and purified from exponentially n-decane- and n-tetradecane-grown R. opacus 1 CP was achieved using a combination of 1H-NMR-spectroscopic and electrospray ionisation (ESI) mass-spectrometric data. 1D (1H) and 2D (COSY-45) NMR spectra were recorded on a Bruker DPX-300 NMR spectrometer locked to the major deuterium resonance of the solvent CDCl3/CD3OD (70:30). Chemical shifts are given in parts per million relative to TMS, and coupling constants in hertz. ESI mass spectra were recorded on a Micromass QTOF2 mass spectrometer.
Results and discussion
Utilisation of long-chain n-alkanes by R. opacus 1CP
Growth on aliphatic hydrocarbons has frequently been reported to induce glycolipid formation in rhodococci. Accordingly, strain 1CP was tested for its ability to utilise several long-chain n-alkanes (n-decane, n-dodecane, n-tetradecane and n-hexadecane) as sole carbon sources. Mineralisation of the hydrocarbons (1% w/v in mineral medium) led to a characteristic growth behaviour (Fig. 1). One day after inoculation, the formation of fine filamentous particles presumably consisting of alkane and biomass occurred. During the following 2 days, the filaments grew in length and subsequently aggregated into pellets of increasing size. Similar observations were made during the cultivation of R. erythropolis DSM 43215 with n-alkanes (Rapp et al. 1979), and a limitation of nutrients as well as of oxygen during this growth phase was discussed. These cell/alkane particles impede growth determination by classical turbidity measurement and, because of their attachment to glass surfaces, make representative sampling unreliable.
Appearance of an R. opacus 1CP culture growing on n-tetradecane. A batch culture of strain 1CP, inoculated with glucose-grown cells, was incubated on mineral medium containing 1% (w/v) n-tetradecane as sole source of carbon. Samples (20 ml), taken after 1, 2, 4 and 6 days of incubation (30°C, 130 rpm), were transferred into a petri dish and photographed in reflected light
The respirometric determination of oxygen consumption was chosen as an alternative technique for growth control. Taking into account the basal respiration rate of the inoculum on the mineral medium in the absence of hydrocarbon, all tested n-alkanes were shown to give rise to a more or less fast increase of oxygen demand in strain 1CP (Fig. 2). From the obtained curves, it is evident that n-tetradecane and n-hexadecane are converted with highest rates (175 and 138 mg O2 l−1 day−1, respectively), whereas the utilisation of n-dodecane and n-decane was considerably slower (13 and 3 mg O2 l−1 day−1, respectively). The slow conversion of n-decane probably indicates some kind of toxic action which, for this type of compound, may result from the dissolution of phospholipid cell membrane constituents (Sikkema et al. 1995). The respiration curves of the n-hexadecane and n-tetradecane culture show a two-phase course and can be divided into an exponential and a linear part that probably reflects the typical growth behaviour described above. Whereas the exponential part represents non-limited cell growth within thin cell/alkane filaments, the linear kinetic might be attributed to the above-mentioned limitation occurring in the centre of growing particles.
Oxygen consumption of R. opacus 1CP during growth with different n-alkanes. Bottles (500 ml) each containing 100 ml of mineral medium and 30 mg of the n-alkane were inoculated with identical amounts of glucose-grown 1CP cells. After installation of pressure sensors (OxiTopC), cultures were continuously stirred and incubated at 20±0.1°C
Formation of a trehalose lipid during growth on n-alkanes
Cultures of strain 1CP growing on all four n-alkanes were extracted with an organic solvent to isolate glycolipids because it was shown in former studies that, due to their low hydrophilic–lipophilic balance (HLB), up to 90% of trehalose lipids may remain cell-associated (Rapp et al. 1979). Analytical TLC of the extracts indicated, after sugar-specific derivatization, for each of the alkane substrates the formation of a glycolipid with similar retention to a trehalose dicorynomycolate reference from R. erythropolis DSM 43215 (Rapp et al. 1979) (Fig. 3a). Neither trehalose monocorynomycolate, which was originally isolated from the same organism and postulated to be an intermediate of trehalose dicorynomycolate biosynthesis (Kretschmer and Wagner 1983), nor anionic trehalose tetraester were detected in the extracts. Concomitantly, spots pointing to the presence of free triglycerides and fatty acids appeared. R f values of the substances were similar to data given in the literature (Kretschmer et al. 1981). No trehalose dicorynomycolate was detected from extracts of glucose-grown R. opacus 1CP (data not shown). Because trehalose dimycolates represent regular extractable components of the rhodococcal cell envelope (Sutcliffe 1998), they can be isolated in traces even during growth on non-hydrocarbon substrates (Datta and Takayama 1993; Ueda et al. 2001). Thus, in the above experiment, their concentration must have been below the detection limit.
Identification of glycolipids of R. opacus 1CP produced during growth on different n-alkanes under balanced C/N ratio (a) and under nitrogen limitation (b). Strain 1CP was incubated in batch cultures (20 ml), containing mineral medium (including 7.6 mM ammonium sulphate) and 1% (w/v) of n-decane, n-dodecane, n-tetradecane or n-hexadecane. Three days after inoculation, whole-cell-containing broth was extracted with chloroform, and samples of the dried organic phase were subjected to TLC. Trehalose monocorynomycolate (THL1), trehalose dicorynomycolate (THL2) and anionic trehalose tetraester (THL4) from R. erythropolis DSM43215 served as trehalose lipid references, and major spots belonging to these standards are marked by arrows. To investigate the influence of nitrogen limitation on the glycolipid formation of strain 1CP, cultures were grown with n-tetradecane in usual mineral medium (N limitation, −) as well as in a modified mineral medium in which ammonium sulphate was omitted (N limitation, +). In the latter culture, the residual nitrogen concentration was 0.075 mM nitrate. The increased retention of THL2 in b resulted from slight changes in the composition of the mobile phase
Nitrogen limitation was frequently shown to specifically favour the formation of anionic trehalose tetraesters during growth of rhodococci on hydrocarbons (Kim et al. 1990; Ristau and Wagner 1983). To test R. opacus 1CP under these conditions, cultures grown on 1% (w/v) n-tetradecane in the presence of only 0.075 mM nitrate (C/N ratio approximately 1:300) were analysed for their glycolipid content. In contrast to R. erythropolis B7g, which under these conditions produced large amounts of anionic trehalose tetraester (Zimmermann 2003), no such trehalose lipid was detected from strain 1CP, and again, THL2 was the only glycolipid detected (Fig. 3b).
To estimate the yields of trehalose lipid produced by strain 1CP with each of the four n-alkanes, cultures grown in parallel on 0.5% (w/v) of each hydrocarbon were sacrificed at particular times and analysed for their maximum glycolipid content by the anthrone method. Total amounts were calculated on the basis of a molar mass of 1,824 g mol−1 and were found to range from 247 mg l−1 trehalose dinocardiomycolate for n-decane up to 420 mg l−1 for n-dodecane. Growth on C14 and C16 gave yields between 333 and 315 mg l−1. The ratio between these yields obviously differs from the one, which can be assessed from the spots of initial TLC detection (Fig. 3a). This reflects the fact that for the experiment pictured in Fig. 3a, all n-alkane-grown cultures were harvested at the same time point at which the progress of conversion might have been quite different. In contrast, for the determination of yields, the time points that gave the maximum values were chosen.
Isolation and structural characterisation of a novel trehalose dinocardiomycolate
For preparative separation of the trehalose lipid from residual n-alkane and other extracted contaminants (fatty acids, lipids, carotinoids), ethyl acetate extracts from an 8-day-old n-decane- and n-tetradecane-grown culture of strain 1CP were purified by low pressure liquid chromatography.
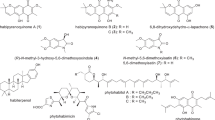
The purified trehalose lipids obtained from growth of R. opacus 1CP on n-decane and n-tetradecane were subjected to 1H-NMR spectroscopy. However, only data of the glycolipid from the C10 cultivation were reliably interpretable (Fig. 4), due to the presence of residual contamination in the C14 preparation. For closer identification of the sugar moiety, a 2D homonuclear 1H,1H-correlated spectrum (COSY) was recorded. The signals of the sugar moiety that appeared in the shift range of 5.1 to 3.0 were shown to correspond to the disaccharide trehalose whose substitution, due to the detection of only one H-1 signal of the α-pyranosyl system, has to be symmetrical. The low-field signals of the methylene group of this residue are characteristic of acylation at the C-6 hydroxyl group. The presence of single H-2 and H-3 protons of the lipid moiety is typical for 2-alkyl-3-hydroxy-fatty acids and from their integral together with the symmetry of the disaccharide is indicative of the presence of a trehalose-6,6′-dimycolate. An important feature of the 1H spectrum was the presence of olefinic systems that from the COSY spectrum are located more than six carbon atoms away from the ester grouping. Integration of 8 olefinic protons and 16 adjacent methylene protons indicated that in total four double bonds occur per trehalose lipid molecule. Taking into account the apparent symmetrical substitution, each mycolic acid molecule should contain two double bonds. Integration of the methylene protons including the ones adjacent to the double bonds gave values of 40–48 CH2 units per mycolic acid residue and allowed only an approximate estimation of the total chain length.
Structure of the trehalose dinocardiomycolate of R. opacus 1CP and assignment of obtained 1H-NMR data. a The spin system H-5/H-6′AB is a second order system, hence the respective couplings have not been evaluated. b The positive ion ESI-MS data indicate the mycolic acid component consists of a range components with a carbon content from C-48 to C-54 (n+x+y+z from 37 to 43). For further details see text
More precise data were afforded by positive ion ESI mass spectrometry. This spectrum showed a broad envelope corresponding to a cluster of overlapping signals for the protonated, [M+H]+, and sodiated, [M+Na]+, molecular ions centred at m/z 1824.3 and 1846.3, respectively, in a ratio of approximately 2:1. These can be attributed to a molecule with a molecular formula C114H214O15. Taking into account the presence of two methyl groups, two olefin systems and the branched C-3 unit at the carboxyl side, this formula corresponds to an average total number of 42 methylene groups per mycolic acid residue. With an average total C number of 51 carbon atoms, the 2-alkyl-3-hydroxy-fatty acid can be categorised as a typical nocardiomycolic acid which per definition cover chain lengths between 44 and 60 (Hommel and Ratledge 1993). The presence of an envelope of signals showing a Gaussian distribution with molecular masses in integers of ±14 relative to the main signal was evident of a mixture of mycolic acids, the main components of which range from C-48 to C-54. Presumably, the apparently odd-numbered carbon-containing mycolic acids are, considering their normal mode of biosynthesis, in fact mixtures of two even-numbered acids (one more and one less) on the trehalose unit. Such arrangements would have no visible effects on the appearance of the 1H-NMR spectra.
Although the exact lengths of the alkyl side chains and the position and nature of the double bonds cannot be determined with the available techniques, data of other mycolic acids from the cell envelope of rhodococci suggest that both double bonds will be located in the long meromycolate backbone. In contrast, the second alkyl branch of mycolic acids from this genus typically is 10–14 carbons in length and is fully saturated (Sutcliffe 1998).
The novel trehalose dinocardiomycolates of strain 1CP differ in both, chain length and the occurrence of double bonds, from trehalose dicorynomycolates reported for R. erythropolis DSM 43215 (Rapp et al. 1979) and R. ruber IEGM231 (Philp et al. 2002). With a total C number of 32 to 36 and 34 to 46 (centred at 40), respectively, the mycolic acids in these trehalose lipids are considerably shorter, and both are virtually devoid of any double bonds.
Mycolic acids, most of which are covalently linked to cell wall components, of species of the genus Rhodococcus cover an overall length of 30 to 54 carbons (Sutcliffe 1998). The longest representatives of these fatty acids containing 48 to 54 carbons as well as up to three double bonds were found in R. opacus (Klatte et al. 1994). Taking into account the fact that mycolic acids present in the free lipid fraction are apparently of similar size ranges as those in the bound lipid fraction (Sutcliffe 1998), the novel trehalose dinocardiomycolate reflects this species very well and should represent the most hydrophobic example for this type of glycolipid biosurfactant from all Rhodococcus species identified so far. It is likely that differences in hydrophobicity of certain trehalose lipids have an impact on the availability of hydrophobic compounds to the organism and thus might influence its substrate spectrum. Varying levels of hydrophobicity might also result in changes of the sensitivity against alkanes of shorter chain length, which to a certain extent can act as membrane-disintegrating agents (Sikkema et al. 1995). For the related genus Mycobacterium, it was recently shown that the presence of polyaromatic hydrocarbons induces a significant increase in cell wall hydrophobicity which promotes enhanced cell-substrate contact and which relies on a modulation of the mycolic acid pattern (Wick et al. 2002). In respect thereof, it would be interesting to find out differences in the utilisation of hydrophobic compounds between R. erythropolis and R. opacus.
Kinetic of trehalose dinocardiomycolate formation
To investigate whether the formation of trehalose lipids by R. opacus 1CP is growth- or non-growth-associated, several n-tetradecane-growing cultures which had been started in parallel were analysed for both, total protein and trehalose lipid content. As shown in Fig. 5, trehalose lipid formation correlated with biomass formation and n-alkane consumption and was obviously growth-associated. A similar growth-associated formation kinetic was reported for a trehalose dicorynomycolate from R. erythropolis DSM43215 (Rapp et al. 1979), whereas the formation of anionic trehalose ester by this organism seems, at least to a large extent, to be uncoupled from growth and to occur in the stationary phase or by resting cells (Kim et al. 1990).
Correlation of growth (biomass formation), alkane consumption and glycolipid formation of R. opacus 1CP. Batch cultures (20 ml) each containing mineral medium and 1% (w/v) n-tetradecane were inoculated with identical amounts of glucose-grown R. opacus 1CP and incubated at 30°C with constant shaking (130 rpm). The cultivation of single flasks was stopped at different times by extracting the whole content with chloroform. The organic phase was analysed for residual n-alkane (GC) and trehalose lipid (anthrone method), and after combining aqueous phase and cell pellet, total protein was determined with the Biuret method
After the n-alkane was exhausted by R. opacus 1CP, the trehalose lipid concentration decreased significantly (Fig. 5), indicating that in the absence of substrate, the biosurfactant might serve as a carbon source. Investigations concerning this utilisation, the specificity of growth-stimulation as well as the identification of biosynthetically relevant genes are under progress.
References
Banat IM, Makkar RS, Cameotra SS (2000) Potential commercial applications of microbial surfactants. Appl Microbiol Biotechnol 53:495–508
Bast E (1999) Mikrobiologische Methoden Spektrum Akad. Verl. Heidelberg, Berlin
Datta AK, Takayama K (1993) Isolation and purification of trehalose 6-mono- and 6,6′-dicorynomycolates from Corynebacterium matruchotii. Structural characterization by 1H NMR. Carbohydr Res 245:151–158
Dorn E, Hellwig M, Reineke W, Knackmuss HJ (1974) Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch Microbiol 99:61–70
Gorlatov SN, Maltseva OV, Shevchenko VI, Golovleva LA (1989) Degradation of chlorophenols by Rhodococcus erythropolis. Microbiology (English translation of Mikrobiologiya) 58:647–651
Hommel RK, Ratledge C (1993) Biosynthetic mechanisms of low-molecular-weight surfactants and their precursor molecules. In: Kosaric N (ed) Biosurfactants (Surfactant Science Series Vol 48). Marcel Dekker, pp 3–63
Kim JS, Powalla M, Lang S, Wagner F, Lünsdorf H, Wray V (1990) Microbial glycolipid production under nitrogen limitation and resting cell conditions. J Biotechnol 13:257–266
Kitamoto D, Isoda H, Nakahara T (2002) Functions and potential applications of glycolipid biosurfactants—from energy-saving materials to gene delivery carriers. J Biosci Bioeng 94:187–201
Klatte S, Kroppenstedt RM, Rainey FA (1994) Rhodococcus opacus sp. nov., an unusual nutritionally versatile Rhodococcus species. Syst Appl Microbiol 17:355–360
König C, Eulberg D, Gröning J, Lakner S, Seibert V, Kaschabek SR, Schlömann M (2004) A linear megaplasmid, p1CP, carrying the genes for chlorocatechol catabolism of Rhodococcus opacus 1CP. Microbiology 150:3075–3087
Kretschmer A, Wagner F (1983) Characterization of biosynthetic intermediates of trehalose dicorynomycolates from Rhodococcus erythropolis grown on n-alkanes. Biochim Biophys Acta 753:306–313
Kretschmer A, Lang S, Marwede G, Ristau E, Wagner F (1981) Formation of surface active glycolipids by n-alkane utilizing microorganisms. In: Vezina C, Singh K (eds) Advances in Biotechnology. Pergamon, Canada, pp 475–479
Kretschmer A, Bock H, Wagner F (1982) Chemical and physical characterization of interfacial-active lipids from Rhodococcus erythropolis grown on n-alkanes. Appl Environ Microbiol 44:864–870
Lang S (2002) Biological amphiphiles (microbial biosurfactants). Curr Opin Colloid Interface Sci 7:12–20
Moiseeva OV, Lin'ko EV, Baskunov BP, Golovleva LA (1999) Degradation of 2-chlorophenol and 3-chlorobenzoate by Rhodococcus opacus 1CP. Microbiology (English translation of Mikrobiologiya) 68:400–405
Pan YT, Drake RR, Elbein AD (1996) Trehalose-P synthase of mycobacteria: its substrate specificity is affected by polyanions. Glycobiology 6:453–461
Philp JC, Kuyukina MS, Ivshina IB, Dunbar SA, Christofi N, Lang S, Wray V (2002) Alkanotrophic Rhodococcus ruber as a biosurfactant producer. Appl Microbiol Biotechnol 59:318–324
Rapp P, Gabriel-Jürgens LHE (2003) Degradation of alkanes and highly chlorinated benzenes, and production of biosurfactants, by a psychrophilic Rhodococcus sp. and genetic characterization of its chlorobenzene dioxygenase. Microbiology 149:2879–2890
Rapp P, Bock H, Wray V, Wagner F (1979) Formation, isolation, and characterization of trehalose dimycolates from Rhodococcus erythropolis grown on n-alkanes. J Gen Microbiol 115:491–503
Ratledge C (1978) Degradation of aliphatic hydrocarbons. Dev Biodegrad Hydrocarb 1:1–46
Ristau E, Wagner F (1983) Formation of novel anionic trehalose tetraesters from Rhodococcus erythropolis under growth limiting conditions. Biotechnol Lett 5:95–100
Ron EZ, Rosenberg E (2001) Natural roles of biosurfactants. Environ Microbiol 3:229–236
Ryll R, Kumazawa Y, Yano I (2001) Immunological properties of trehalose dimycolate (cord factor) and other mycolic acid-containing glycolipids—a review. Microbiol Immunol 45:801–811
Schippers C, Gessner K, Müller T, Scheper T (2000) Microbial degradation of phenanthrene by addition of a sophorolipid mixture. J Biotechnol 83:189–198
Sikkema J, de Bont JA, Poolman B (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59:201–222
Singer MEV, Finnerty WR, Tunelid A (1990) Physical and chemical properties of a biosurfactant synthesized by Rhodococcus species H13-A. Can J Microbiol 36:746–750
Sutcliffe IC (1998) Cell envelope composition and organisation in the genus Rhodococcus. Antonie van Leeuwenhoek 74:49–58
Tomiyasu I, Yoshinaga J, Kurano F, Kato Y, Kaneda K, Imaizumi S, Yano I (1986) Occurrence of a novel glycolipid, ‘trehalose 2,3,6′-trimycolate’ in a psychrophilic, acid-fast bacterium, Rhodococcus aurantiacus (Gordona aurantiaca). FEBS Lett 203:239–242
Uchida Y, Tsuchiya R, Chino M, Hirano J, Tabuchi T (1989) Extracellular accumulation of mono- and di-succinoyl trehalose lipids by a strain of Rhodococcus erythropolis grown on n-alkanes. Agric Biol Chem 53:757–763
Ueda S, Fujiwara N, Naka T, Sakaguchi I, Ozeki Y, Yano I, Kasama T, Kobayashi K (2001) Structure–activity relationship of mycoloyl glycolipids derived from Rhodococcus sp. 4306. Microb Pathog 30:91–99
Wick LY, Wattiau P, Harms H (2002) Influence of the growth substrate on the mycolic acid profiles of mycobacteria. Environ Microbiol 4:612–616
Zimmermann K (2003) Biotenside aus Actinobakterien: Herstellung und Charakterisierung anionischer Trehalosetetraester aus verschiedenen Stämmen der Gattung Rhodococcus. Diploma thesis. TU Bergakademie Freiberg
Acknowledgements
We thank Rolf Heckmann for his technical assistance during the glycolipid purification and Manfred Nimtz for MS data. This work was kindly supported by the Deutsche Bundesstiftung Umwelt and by a grant from the European community ICA2-CT-2000-10006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niescher, S., Wray, V., Lang, S. et al. Identification and structural characterisation of novel trehalose dinocardiomycolates from n-alkane-grown Rhodococcus opacus 1CP. Appl Microbiol Biotechnol 70, 605–611 (2006). https://doi.org/10.1007/s00253-005-0113-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0113-8