Abstract
The fatty acid composition of pyruvate-grown Comamonas testosteroni ATCC 17454 was analyzed after growth at 30 and 20°C and after half-maximum growth inhibition caused by different membrane-active chemicals at 30°C. Palmitic acid (16:0), palmitoleic acid (16:1 ω7c) and vaccenic acid (18:1 ω7c) were the dominant fatty acids. At 20°C, the proportion of palmitic acid decreased and those of palmitoleic and vaccenic acid increased. Saturation degree was also lowered when half-maximum growth inhibition was caused by 4-chlorosalicylic acid, 2,4-dichlorophenoxyacetic acid and 2,4-dinitrophenol and, to a lesser extent, in the presence of 2,4-dichlorophenol, phenol and ethanol. It appeared that the dissociated forms of the former group of chemicals were preferentially incorporated near the head group region of the lipid bilayer, thereby somewhat extending the outer region of the membranes, and that the increased amount of bent, unsaturated fatty acids helped to maintain membrane integrity. Irrespective of how the decrease of the saturation degree was triggered, it caused electron transport phosphorylation (adenosine triphosphate synthesis driven by n-hexanol oxidation) to become more sensitive to uncoupling. Apparently, the viscosity and phase stability of the cytoplasmic membrane of C. testosteroni were maintained at the price of a reduced protection against energy toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bacteria adapt to temperature variation and chemical stresses by changing the fatty acid composition of their cell membranes. A few known mechanisms are the variation of saturated-to-unsaturated fatty acids ratio, cis–trans isomerization, chain length alteration and change of branching sites (Marr and Ingram 1962; Ingram 1976, 1984; Kaneda 1977; Okuyama et al. 1991; Suutari and Laakso 1993). For example, an increased membrane fluidity of Escherichia coli at elevated temperatures and in the presence of middle-chain alcohols was counteracted by an increase in the saturation degree of fatty acids (Marr and Ingram 1962; Ingram 1976, 1984). Acinetobacter calcoaceticus 69-V reacted in the same way, and the increased saturation of its fatty acids at high temperatures and after growth on membrane-active substrates was accompanied by an improved resistance of electron transport phosphorylation (ETP) to the uncoupling effect of 2,4-dinitrophenol (2,4-DNP) (Loffhagen et al. 1995a,b). In A. calcoaceticus, a fatty acid desaturase altered the saturation degree of extant fatty acids formed via the so-called aerobic pathway (Härtig et al. 1999).
Several strains of Pseudomonas putida respond to the presence of lipophilic chemicals by increasing the proportion of saturated fatty acids and by converting isomers from cis to trans (Diefenbach et al. 1992; Heipieper et al. 1992, 1996; Weber et al. 1994; Pinkart et al. 1996; Härtig et al. (2004). When heat is used to stimulate the cis–trans fatty acid isomerization of resting cells of P. putida NCTC 10936 without changing the saturation degree, the resistance of the ETP to uncoupling by 2,4-DNP is improved (Loffhagen et al. 2001). This shows that cis–trans isomerization alone influences ETP stability against uncoupling. However, nothing is known about the effects of saturation degree changes on the stability of the energy conservation of this strain, and the isolated investigation of this feature is difficult to achieve because of the presence of the cis–trans isomerase. Recently, we reported a way to investigate the uncoupling effects of chlorophenoxy herbicides on the energy conservation of Comamonas testosteroni ATCC 17454 (Loffhagen et al. 2003). Probably, like P. putida, C. testosteroni uses a carbon- and energy-dependent de novo synthesis of fatty acids via the so-called anaerobic pathway (Wada et al. 1989; Magnuson et al. 1993). However, unlike P. putida, C. testosteroni does not possess a cis–trans isomerase and therefore allows an isolated look on the effects of changes in the saturation degree on ETP stability against uncoupling in an organism using the anaerobic pathway of fatty acid synthesis. Here we report how growth temperature and the presence of membrane-active chemicals influence the fatty acid composition, saturation degree and sensitivity of the ETP of C. testosteroni ATCC 17454 to uncoupling by 2,4-DNP.
Materials and methods
Strain and chemicals
Comamonas testosteroni DSM 38/ATCC 17454 was obtained from the German Collection of Microorganisms and Cell Cultures. All chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) and Merck (Darmstadt, Germany). Stock solutions of substituted phenols, phenoxyalkanoate and salicylate were prepared in dimethylsulfoxide (DMSO). The volumes of stock solutions added to the bacteria were so small such that the final DMSO concentrations were no higher than 1%. Control assays demonstrated no toxic effects or influences of DMSO on membrane composition at this concentration.
Culture conditions and growth inhibition
Comamonas testosteroni was grown at 20°C for 66 h and at 30°C for 22 h in 100-ml shake cultures in a medium (pH 7.0) containing 0.76 g/l NH4Cl, 0.76 g/l KH2PO4, 0.87 g/l K2HPO4, trace elements as detailed in Müller and Babel (1986) and 3 g/l sodium pyruvate as a carbon and energy source for growth. To study the effects of membrane-active chemicals, C. testosteroni was grown at 30°C on pyruvate in the presence of different concentrations of either 2,4-dichlorophenol (2,4-DCP), phenol, ethanol or 2,4-DNP during 3 h of exponential growth (addition of the compounds after 6 h). Inhibition of growth caused by different concentrations of the toxicant within 3 h was calculated as the ratio μtox/μcontrol×100%. In a second series of experiments, either 0.3 mM 2,4-DCP, 12 mM phenol, 700 mM ethanol, 1.6 mM 4-chlorosalicylic acid (4-CSA), 2.5 mM 2,4-dichlorophenoxyacetic acid (2,4-D) or 0.3 mM 2,4-DNP was added at the beginning of a growth period of 22 h. Growth was monitored by measuring the light absorbance of culture samples at 700 nm using a CADAS 50S spectrophotometer (Dr. Lange, Germany). At the times indicated, C. testosteroni was harvested by sedimentation at 4,000×g for 20 min. Pellets were washed twice in distilled water, resuspended in 2 mM imidazole buffer (pH 7.0) and stored at −18°C prior to their use for lipid analysis.
Analysis of fatty acid composition
Fatty acids in whole-cell hydrolysates were quantified as methyl esters upon temperature-programmed (170–310°C) gas chromatographic separation on a non-polar HP-Ultra 2 capillary column (25 m, 0.22 mm; Agilent) as described by Härtig et al. (1999). To prepare the esters, harvested cells of approximately 10 mg of dry mass were saponified with sodium hydroxide, methylated with acidic methanol, extracted in a mixture of n-hexane/tert-butyl ether and base-washed according to the procedure described by MIDI (http://www.midi-inc.com) in the technical notes supplied with their microbial identification system. Fatty acid analysis was done using an Agilent 6890 gas chromatograph with a flame ionization detector and hydrogen as the carrier gas. The relative amounts of the fatty acids were measured from the corresponding peak areas using the ChemStation software (Agilent).
Data analysis
The dominant saturated fatty acid hexadecanoic (palmitic) acid (16:0) and the dominant unsaturated 9-cis hexadecenoic (palmitoleic) acid (16:1 ω7c) and 11-cis octadecenoic (vaccenic) acid (18:1 ω7c) were used to calculate the degree of saturation as either the concentration ratio 16:0/[16:0+16:1]×100% (S 16) or 16:0/[16:0+16:1+18:1]×100% (S 16+18).
Determination of respiration
The oxygen concentration of air-saturated buffers was measured by the method of Robinson and Cooper (1970). The respiration of whole-cell suspensions was measured in a reaction chamber (volume 1–5 ml, Cyclobios Oxygraph; A. Paar, Austria), and signals from the polarographic oxygen sensor were digitally stored and analyzed by the Cyclobios program DatGraf v. 2.0. For this purpose, 20 μl of the cell suspensions (40–52 mg/ml dry mass) was added while stirring to 1.0 ml of 20 mM imidazole buffer (pH 7.0) and then incubated at 20, 30 or 40°C for respiration measurements. After 15 min, during which time endogenous respiration was monitored, 10 μl of n-hexanol (final concentration 0.6 mM) was added, and n-hexanol-dependent oxygen consumption was measured. To measure the effect of 2,4-DNP on cell respiration, 10 μl (1:10 dilution of stock solution in DMSO) of 2,4-DNP (final concentration 0.1 mM) was added 60 s before the addition of n-hexanol.
Determination of adenosine triphosphate synthesis
In parallel to respiration measurements, the adenosine triphosphate (ATP) content of the cells was determined in air-sparged 10-ml tubes that were incubated in a thermostat at 20, 30 and 40°C. One hundred microlitres of cell suspension was injected into 0.4 ml of 20 mM imidazole buffer (pH 7.0). After 15 min, 5 μl of n-hexanol (final concentration 0.6 mM) was added. After a further 20 s, the reactions were stopped by injecting 0.25 ml of ice-cold 1.3 M perchloric acid, which contained 23 mM ethylenediaminetetraacetic acid (EDTA) and was stored at 4°C. To quantify uncoupling, after 15 min, 5 μl of 2,4-DNP in DMSO (final 2,4-DNP concentration 0.1 mM) was injected. Twenty seconds later, the reactions were either stopped or 5 μl of n-hexanol (final concentration 0.6 mM) was added and the reactions were stopped after a further 20 s. Controls without n-hexanol and 2,4-DNP were stopped after 15 min and 20 s.
After 30 min, the reactions were centrifuged at 4°C for 15 min at 16,000×g. Five hundred microlitres of each supernatant was neutralized to pH 7.7 with 0.72 M KOH containing 0.16 M KHCO3, centrifuged once again and used to determine ATP concentration through a luciferin–luciferase bioluminescence assay using a Wallac Multilabel Counter 1420 (Turku, Finland). For this, a microplate well containing a mixture of 20 μl of diluted supernatant and 180 μl of 20 mM Tris–H2SO4 buffer (pH 7.75; with 2 mM EDTA and 10 mM MgSO4) was placed in the luminometer, and 50 μl of a dissolved luciferin–luciferase mixture (Thermo Life Sciences Labsystems, Egelsbach, Germany) was injected with a dispenser. After mixing for 10 s, the light intensity was recorded for 10 s, and the ATP concentration was calculated, taking into account errors due to the inhibition of bioluminescence using the constant addition technique (Lundin and Thore 1975).
Results
Influence of growth temperature and membrane-active chemicals on fatty acid composition
The fatty acid profiles of C. testosteroni were similar under all conditions, with saturated, unsaturated and hydroxy fatty acids of 12- to 18-carbon atoms. They were dominated (>5%) by palmitic acid (16:0), palmitoleic acid (16:1 ω7c) and vaccenic acid (18:1 ω7c). Table 1 shows the proportions of these three fatty acids after growth on pyruvate at 20 and 30°C. The growth temperature strongly influences the saturation degrees of the fatty acids. S 16 and S 16+18 are significantly higher after growth at 30°C than at 20°C (Table 1).
The presence of 2,4-DCP, phenol and ethanol during 3 h of exponential growth at 30°C decreased the saturation degree. 2,4-DCP had already caused this effect at concentrations leading to only less than a half-maximum inhibition of growth, whereas phenol and ethanol decreased the saturation up to concentrations exhibiting more than a half-maximum inhibition of growth (Fig. 1a–c). The most significant decrease of the saturation at low concentrations and upon return to the unchanged levels at concentrations resulting in half-maximum to full growth inhibition was observed in the presence 2,4-DNP (Fig. 1d). In all cases, a decreased saturation hints at sublethal effects that allow the cells to adapt to the toxicants. In order to provoke more pronounced changes of the saturation degree, the concentrations of the toxicants in ranges causing half-maximum growth inhibition were added at the beginning of a 22-h growth period (Table 1). Non-poisoned exponential cells sampled after 9 h of growth (Fig. 1) had a somewhat lower saturation degree S 16+18 than stationary cells sampled after 22 h (Table 1)—a difference that can be attributed to different physiological states. A close inspection of the concentrations required to cause a half-maximum growth inhibition during permanent exposure (cf. “Materials and methods”) as opposed to a short-term exposure of exponential cells (Fig. 1) suggests that there is also a differential susceptibility of stationary and exponential cells to toxicants. Long-time incubation in the presence of toxicants reduced saturation degrees to similarly slight extents for 2,4-DCP, phenol and ethanol, whereas with the remaining compounds, more drastic decreases were observed on the order of 4-CSA<2,4-D<2,4-DNP, with the latter lowering the saturation degree as strongly as growth at 20°C. The differences between the concentration ranges at which the individual toxicants inhibited growth and induced membrane modifications can be obtained from “Materials and methods” (Fig. 1 and Table 1).
Influence of growth temperature on the stability of ETP against uncoupling
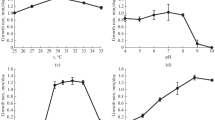
For energizing C. testosteroni, its capacity to oxidize middle-chain alcohols, probably mainly via a pyrroloquinoline-quinone-dependent periplasmic alcohol dehydrogenase, was used. This served as the basis for investigating the influence of membrane lipid fatty acid composition on the stability of ETP. Long-time-exposed cells were used for this purpose. The provision of low n-hexanol concentrations caused respiration and ATP synthesis via ETP at rates sufficient for decoupling experiments. After growth at 20°C and a 15-min preincubation at either 20, 30 or 40°C, the endogenous and n-hexanol-dependent respiration rates increased with increasing incubation temperature. Although 2,4-DNP stimulated endogenous respiration, its presence in addition to n-hexanol reduced the respiration (Fig. 2a). Respiration rates correlated well with the corresponding ATP pools of the cells, except for the generally negative influence of 2,4-DNP on ATP pools (Fig. 2b). At any incubation temperature, the ATP level in the cells was increased by n-hexanol oxidation and reduced by 2,4-DNP. When the cells were grown at 30°C, a comparison of respiration rates and ATP pools indicated a lower sensitivity to the effects of 2,4-DNP than of cells grown at 20°C (Fig. 2c and d). There were clear trends that endogenous respiration was much less stimulated by 2,4-DNP and that the n-hexanol oxidation-driven ATP synthesis was unaffected by 2,4-DNP at all incubation temperatures.
Effects of 2,4-DNP on respiration (a, c) and ATP pools (b, d) of C. testosteroni grown at 20°C (a, b) and 30°C (c, d) in the absence and presence of n-hexanol measured at different temperatures. At least two independent experiments were performed. The standard error of the mean (SEM) did not exceed 15% for respiration and 8% for ATP measurements
Influence of growth inhibition on the stability of ETP against uncoupling
Figure 3 presents respiration rates and ATP pools obtained with cells of C. testosteroni that were grown in the presence of different membrane-active chemicals. Growth and measurements occurred at 30°C. Normalized results were presented because of differing endogenous respiration rates and ATP pools. Respiration rates and ATP concentrations measured in the presence of n-hexanol were set to 100%.
Effects of 2,4-DNP on respiration (normalized on n-hexanol oxidation rates; a) and ATP pools (normalized on ATP contents in the presence of n-hexanol; b) of C. testosteroni after half-maximum growth inhibition at 30°C in the absence and presence of n-hexanol measured at 30°C. At least two independent experiments were performed. The SEM did not exceed 12% for respiration and 9% for ATP measurements
The addition of 2,4-DNP generally enhanced endogenous respiration (Fig. 3a), although to levels lower than that of n-hexanol. The oxidation of n-hexanol in the presence of 2,4-DNP was stimulated (values >100%), except for cells grown in the presence of 2,4-DCP (Fig. 3a). However, ATP pools formed by endogenous respiration and n-hexanol oxidation in the presence of 2,4-DNP through cells grown in the presence of 2,4-DCP, phenol or ethanol were only slightly changed but were clearly reduced in cells grown in the presence of 4-CSA, 2,4-D and 2,4-DNP (Fig. 3b).
Discussion
When the growth temperature of C. testosteroni is decreased from 30 to 20°C, the main change in the fatty acid composition is an increase in the proportion of vaccenic acid (18:1 ω7c) at the expense of palmitic acid (16:0). The same phenomenon has been described for E. coli by Marr and Ingram (1962). As these organisms use the anaerobic pathway of fatty acid synthesis, this does not reflect the conversion of palmitic acid into vaccenic acid, but a de novo fatty acid synthesis with an increased conversion of trans-decenoyl-ACP into cis-decenoyl-ACP driven by an increased activity of β-hydroxydecanoyl-ACP-dehydrase and/or β-ketoacyl-ACP-synthase I. Chain elongation then leads to vaccenic acid (18:1 ω7c) (Magnuson et al. 1993). A. calcoaceticus 69-V, in contrast, converts palmitic acid to oleic acid (18:1 ω9c) via chain elongation and desaturation (aerobic pathway; Härtig et al. 1999).
In C. testosteroni, the observed decrease of the saturation degree after growth at lower temperatures went along with the lowered resistance of n-hexanol oxidation to inhibition, and of ETP (ATP synthesis driven by endogenous respiration) to uncoupling by 2,4-DNP. This appeared to be a common behaviour of different organisms, since the same effect of a decreased saturation on ETP stability was found for A. calcoaceticus 69-V, an organism using the aerobic pathway of fatty acid synthesis (Loffhagen et al. 1995b; Härtig et al. 1999). For P. putida grown at lower temperatures and thus spossessing a lowered saturation degree, a stronger growth inhibition was found in the presence of phenolic compounds, which exhibited toxicity via polar narcosis (Margesin et al. 2004).
Accordingly, an increase in the saturation degree of Bacillus subtilis caused by a mutation of a gene coding for the fatty acid desaturase used by this organism was accompanied by a gain of resistance to uncoupling by carbonylcyanide m-chlorophenylhydrazone (CCCP) (Guffanti et al. 1987; Dunkley et al. 1991). A more solid state of the membrane could have fostered a complex formation between components of the respiratory chain and the ATP synthase, thus leading to an improved and localized coupling (Krulwich et al. 1990; Lewis et al. 1994). It thus appears that a high saturation degree protects the ETP against the effects of uncoupling substances. As a consequence, bacteria should synthesize more saturated fatty acids while being growth-inhibited by this kind of chemicals, as they do after increasing the growth temperature.
Surprisingly, the growth of E. coli in the presence of the uncoupler CCCP led to the synthesis of more unsaturated fatty acids and thus decreased the saturation degree (Herring et al. 1985). A similar effect was observed when E. coli was grown on ethanol or in the presence of short-chain alcohols (Ingram 1976; Dombek and Ingram 1984), or when the growth of Oenococcus oeni was inhibited by ethanol (Graca da Silveira et al. 2003). In A. calcoaceticus, saturation decreased more when the shorter compounds of the homologous series from ethanol to n-hexanol were used to inhibit growth (Kabelitz et al. 2003). Unfortunately, the impacts of decreasing saturation on the stability of energy conservation were not reported by these authors. A separate study with A. calcoaceticus 69-V grown on the same series of alcohols from n-hexanol to ethanol confirmed that, with decreasing chain lengths, the saturation degree decreased and that this was accompanied by decreased stabilities of the ETP against uncoupling by 2,4-DNP (Loffhagen et al. 1995b). In the present study, the growth of C. testosteroni in the presence of inhibiting concentrations of ethanol, 2,4-DCP, phenol, 4-CSA, 2,4-D and 2,4-DNP also led to decreased saturation and, with the latter three chemicals, to an increased sensitivity of the ETP to uncoupling. Because of its physicochemical properties, ethanol is preferentially incorporated near the outer region of the lipid bilayer, thereby causing a swelling effect on the hydrophilic head group region of the membrane lipids (Weber and de Bont 1996). In contrast, 2,4-DCP is highly lipophilic and should rather accumulate between the hydrophobic tails of the fatty acid residues (Sikkema et al. 1995). The observed increase of the saturation degree at concentrations around 1 mM 2,4-DCP (Fig. 1a) could be interpreted as a cellular response to this. A fraction of this compound (pK a=7.9) is dissociated at pH 7 (pH of the culture medium). The phenol/phenoxide mixture could spontaneously form heterodimers that lead to surface layers at the water/lipid interface, as was shown to occur with 2,4,5-trichlorophenol at model lipid layers (Siam et al. 2004). The electrostatic interaction exerted by adsorbed ionic species of 2,4-DCP and, obviously, of 4-CSA, 2,4-D and 2,4-DNP might cause conformation changes in the region of the fatty acid ester groups. It may be that this effect is counteracted by the synthesis of an increased proportion of bent, unsaturated fatty acids that stabilize membrane integrity by maintaining hydrophobic interactions in a lipophilic region of reduced overall density.
It may be asked why the biological response to such membrane-active chemicals sensitizes the ETP to the effects of subsequent doses of similar compounds instead of triggering a defence mechanism. It appears that the induced reduction in density and the increased disorder of the lipophilic region facilitate uncoupling by 2,4-DNP. This illogical behaviour is most evident for cells grown in the presence of 2,4-DNP, which exhibit decreased resistance to uncoupling by the same compound. It seems that uncoupling by 2,4-DNP is simply inversely correlated with the degree of saturation, regardless of the environmental trigger of the saturation change, and that mechanisms counteracting a higher sensitivity to uncoupling do not exist. This view is corroborated by the observed destabilization of the ETP via temperature-induced desaturation. Further support comes from the observations that both cis–trans isomerization and increased saturation as a response to lipophilic chemicals stabilize the ETP against uncoupling (Loffhagen et al. 1995a,b, 2003). Apparently, homeoviscous adaptation (Cossins and Sinensky 1986) at lowered growth temperatures, as well as maintenance of phase stability (Denich et al. 2003) of the cytoplasmic membrane in the presence of compounds interacting with the hydrophilic head groups, is accomplished at the price of reduced protection against energy toxicity.
References
Cossins AR, Sinensky M (1986) Adaptation of membranes to temperature, pressure, and exogenous lipids. In: Shinitzky M (ed) Physiology of membrane fluidity. RC Press, Boca Raton, pp 1–20
Denich TJ, Beaudette LA, Lee H, Trevors JT (2003) Effect of selected environmental physico-chemical factors on bacterial cytoplasmic membranes. J Microbiol Methods 52:149–182
Diefenbach R, Heipieper HJ, Keweloh H (1992) Conversion of cis into trans unsaturated fatty acids in Pseudomonas putida P8: evidence for a role in the regulation of membrane fluidity. Appl Microbiol Biotechnol 38:382–387
Dombek KM, Ingram LO (1984) Effects of ethanol on the Escherichia coli plasma membrane. J Bacteriol 157:233–239
Dunkley EA, Clejan S, Krulwich TA (1991) Mutants of Bacillus species isolated on the basis of protonophore resistance are deficient in fatty acid desaturase activity. J Bacteriol 173:7750–7755
Graca da Silveira M, Golovina EA, Hoekstra FA, Rombouts FM, Abee T (2003) Membrane fluidity adjustments in ethanol-stressed Oenococcus oeni cells. Appl Environ Microbiol 69:5826–5832
Guffanti AA, Clejan S, Falk H, Hicks DB, Krulmich TA (1987) Isolation and characterization of uncoupler-resistant mutants of Bacillus subtilis. J Bacteriol 169:4469–4478
Härtig C, Loffhagen N, Babel W (1999) Glucose stimulates a decrease of the fatty acid saturation degree in Acinetobacter calcoaceticus. Arch Microbiol 171:166–172
Härtig C, Loffhagen N, Harms H (2004) The formation of trans fatty acids is not involved in the growth-linked membrane adaptation of Pseudomonas putida. Appl Environ Microbiol 71:1915–1922
Heipieper HJ, Diefenbach R, Keweloh H (1992) Conversion of cis unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading from substrate toxicity. Appl Environ Microbiol 58:1847–1852
Heipieper HJ, Meulenbeld G, van Oirschot Q, de Bont JAM (1996) Effect of environmental factors on the trans/cis ratio of unsaturated fatty acids in Pseudomonas putida S12. Appl Environ Microbiol 62:2773–2777
Herring FG, Krisman A, Sedgwick EG, Bragg PD (1985) Electron spin resonance studies of lipid fluidity changes in membranes of an uncoupler-resistant mutant of Escherichia coli. Biochim Biophys Acta 819:231–240
Ingram LO (1976) Adaptation of membrane lipids to alcohols. J Bacteriol 125:670–678
Ingram LO (1984) Effect of alcohols on microorganisms. Adv Microb Physiol 25:253–300
Kabelitz N, Santos PM, Heipieper HJ (2003) Effect of aliphatic alcohols on growth and degree of saturation of membrane lipids in Acinetobacter calcoaceticus. FEMS Microbiol Lett 220:223–227
Kaneda T (1977) Fatty acids of the genus Bacillus: an example of branched-chain preference. Bacteriol Rev 41:391–418
Krulwich TA, Quirk PG, Guffanti AA (1990) Uncoupler-resistant mutants of bacteria. Microbiol Rev 54:52–65
Lewis K, Naroditskaya V, Ferrante A, Fokina I (1994) Bacterial resistance to uncouplers. J Bioenerg Biomembranes 20:639–646
Loffhagen N, Härtig C, Babel W (1995a) The glucose dehydrogenase-mediated energization of Acinetobacter calcoaceticus as a tool for evaluating its susceptibility to, and defence against, hazardous chemicals. Appl Microbiol Biotechnol 42:738–743
Loffhagen N, Härtig C, Babel W (1995b) Fatty acid patterns of Acinetobacter calcoaceticus 69-V indicate sensitivity against xenobiotics. Appl Microbiol Biotechnol 44:26–531
Loffhagen N, Härtig C, Babel W (2001) Suitability of the trans/cis ratio of unsaturated fatty acids in Pseudomonas putida NCTC 10936 as an indicator of the acute toxicity of chemicals. Ecotoxicol Environ Saf 50:65–71
Loffhagen N, Härtig C, Babel W (2003) Energization of Comamonas testosteroni ATCC 17454 for indicating toxic effects of chlorophenoxy herbicides. Arch Environ Contam Toxicol 45:317–323
Lundin A, Thore A (1975) Analytical information obtainable by evaluation of the time course of firefly bioluminescence in the assay of ATP. Anal Biochem 66:47–63
Magnuson K, Jackowski S, Rock CO, Cronan JE (1993) Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev 57:522–542
Margesin R, Bergauer P, Gander S (2004) Degradation of phenol and toxicity of phenolic compounds: a comparison of cold-tolerant Arthrobacter sp. and a mesophilic Pseudomonas putida. Extremophiles 8:201–207
Marr AG, Ingram JL (1962) Effect of temperature on the composition of fatty acids in Escherichia coli. J Bacteriol 84:1260–1267
Müller RH, Babel W (1986) Glucose as an energy donor in acetate growing Acinetobacter calcoaceticus. Arch Microbiol 144:62–66
Okuyama H, Okajima N, Sasaki S, Higashi S, Murata N (1991) The cis/trans isomerization of the double bond of a fatty acid as a strategy for adaptation to changes in ambient temperature in the psychrophilic bacterium, Vibrio sp. strain ABE-1. Biochim Biophys Acta 1084:13–20
Pinkart HC, Wolfram JW, Rogers R, White DC (1996) Cell envelope changes in solvent-tolerant and solvent-sensitive Pseudomonas putida strains following exposure to o-xylene. Appl Environ Microbiol 62:1129–1132
Robinson J, Cooper JM (1970) Method of determining oxygen concentrations in biological media, suitable for calibration of the oxygen electrode. Anal Biochem 33:95–102
Siam M, Reiter G, Hunziker R, Escher B, Karpfen A, Simperler A, Baurecht D, Fringeli UP (2004) Evidence for heterodimers of 2,4,5-trichlorophenol on planar lipid layers. A FTIR-ATR investigation. Biochim Biophys Acta 1664:88–99
Sikkema J, de Bont JAM, Poolman B (1995) Membrane toxicity of cyclic hydrocarbons. Microbiol Rev 59:201–222
Suutari M, Laakso S (1993) Effect of growth temperature on the fatty acid composition of Mycobacterium phlei. Arch Mikrobiol 159:119–123
Wada M, Fukunaga N, Sasaki S (1989) Mechanism of biosynthesis of unsaturated fatty acids in Pseudomonas sp. strain E-3, a psychrotrophic bacterium. J Bacteriol 171:4267–4271
Weber FJ, de Bont JAM (1996) Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochem Biophys Acta 1286:225–245
Weber FJ, Isken S, de Bont JAM (1994) Cis/trans isomerization of fatty acids as a defence mechanism of Pseudomonas putida strains to toxic concentrations of toluene. Microbiology 140:2013–2017
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loffhagen, N., Härtig, C. & Harms, H. Impact of membrane fatty acid composition on the uncoupling sensitivity of the energy conservation of Comamonas testosteroni ATCC 17454. Appl Microbiol Biotechnol 70, 618–624 (2006). https://doi.org/10.1007/s00253-005-0104-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0104-9