Abstract
Supplementation of a chemically defined medium with amino acids or succinate to improve heterologous xylanase production by a prototrophic Saccharomyces cerevisiae transformant was investigated. The corresponding xylanase production during growth on ethanol in batch culture and in glucose-limited chemostat culture were quantified, as the native ADH2 promoter regulating xylanase expression was derepressed under these conditions. The addition of a balanced mixture of the preferred amino acids, Ala, Arg, Asn, Glu, Gln and Gly, improved both biomass and xylanase production, whereas several other individual amino acids inhibited biomass and/or xylanase production. Heterologous protein production by the recombinant yeast was also improved by supplementing the medium with succinate. The production of heterologous xylanase during growth on ethanol or glucose could thus be improved by supplementing metabolic precursors in the carbon- or nitrogen-metabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cultivation of recombinant yeast in defined media for heterologous protein production is advantageous due to easier quantification of growth requirements, simpler purification of the product and the propensity towards rapid scale-up (Greasham and Herber 1997). In the present study, the possible limitation of heterologous protein production in recombinant Saccharomyces cerevisiae by an insufficient availability of amino acids or succinate during growth in defined medium was investigated. A prototrophic recombinant S. cerevisiae strain producing a fungal xylanase under the transcriptional control of the ADH2 promoter was used as a model system. Compared to production levels in complex medium, very low production levels of heterologous xylanases by auxotrophic recombinant S. cerevisiae in defined media have been reported (Donald et al. 1994; Pérez-González et al. 1996; La Grange et al. 1996; Nuyens et al. 2001; our unpublished results). In an auxotrophic recombinant S. cerevisiae strain, heterologous xylanase production improved dramatically by supplementation of the defined medium with an excess of amino acids (Görgens et al. 2004). An apparent limitation in the availability of amino acid building blocks for heterologous xylanase production by auxotrophic yeasts thus existed. In the present study, a prototrophic strain was used to investigate the presence of a similar limitation for amino acids or succinate for heterologous xylanase production. The defined medium was supplemented during growth on ethanol in batch culture or during glucose-limited growth in chemostats, when the native ADH2 promoter, used to regulate xylanase expression, was derepressed.
Supplementation of defined media with exogenous nitrogen sources has previously improved biomass formation by S. cerevisiae, especially during fully respiratory growth on ethanol (Chen et al. 1993; Gu et al. 1991; Thomas and Ingledew 1990, 1992). Nitrogen sources most strongly preferred by S. cerevisiae include glutamine, asparagine and ammonium. These compounds are utilised first from mixtures of nitrogen sources and support higher growth rates than less preferred nitrogen sources (Ter Schure et al. 2000; Dubois and Messenguy 1997; Wiame et al. 1985; Cooper 1982). Exogenous amino acids can be incorporated directly into biomass during consumption (Albers et al. 1996), whilst amino acids have been known to improve the production of heterologous proteins by S. cerevisiae in defined media (Mendoza-Vega et al. 1994; Wittrup and Benig, 1994; Toman et al. 2000; Blechl et al. 1992). The proteolytic degradation of heterologous protein products may be decreased by supplementation of the medium with pure amino acids, such as arginine and lysine (Choi et al. 2000; Kang et al. 2000; Chung and Park 1998), or complex mixtures, such as casamino acids (Coppella and Dhurjati 1989; Boze et al. 2001; Werten et al. 1999; Goodrick et al. 2001; Sreekrishna et al. 1997). Metabolite balancing during recombinant protein production has identified a depletion of amino acids and biosynthetic precursors from the TCA cycle during recombinant protein production (Jin et al. 1997). In the present investigation, medium supplementation was aimed at increasing the availability of amino acid building blocks for biosynthesis or stimulating the TCA cycle by succinate addition, during heterologous xylanase production by a prototrophic S. cerevisiae transformant growing on ethanol or glucose.
Materials and methods
Strains and plasmids
An auxotrophic, recombinant strain of S. cerevisiae Y294 [pDLG5] with genotype [ura3/URA3, fur1::LEU2, trp1, his3], producing heterologous β-1,4-xylanase by expression of a plasmid-based XYN2 gene under the control of the native ADH2-promoter, was constructed previously (La Grange et al. 1996). In the present study, a prototrophic variant of this strain, S. cerevisiae Y294 [ura3/URA3, fur1::LEU2, trp1::TRP1, his3::HIS3, pDLG5], was created by replacement of the trp1, his3 alleles with wild-type TRP1 and HIS3 alleles using Rothstein replacement (Rothstein 1991). This latter prototrophic strain was used throughout the present investigation. Xylanase production was regulated by the native ADH2 promoter, which is derepressed by the diauxic shift to growth on ethanol.
Cultivation of recombinant yeast
Medium supplements were first screened individually in baffled shake-flask cultures, prior to quantification in batch culture. The prototrophic transformant was cultivated in a defined minimal medium (Verduyn et al. 1992) containing 20 g l−1 glucose as the carbon source. Batch and continuous fermentation in the defined medium were performed as previously described (Görgens et al. 2001). In batch cultures, amino acid and succinate supplements were added to cultures just before the shift from growth on glucose to growth on ethanol, resulting in maximal availability during the heterologous xylanase production phase. Xylanase production was regulated by the native ADH2 promoter, which is derepressed by the diauxic shift to growth on ethanol. The utilisation of medium supplements for xylanase production, rather than biomass formation, could thus be maximised. The culture pH was maintained at 5.0, unless otherwise specified.
Analytical methods
Cell density was determined by measuring absorbence at 620 nm after diluting samples with 9 g l−1 NaCl into the 0.05–0.2 linear detection range of the spectrophotometer. Absorbency measurements were calibrated to dry weight measurements made in parallel. Glucose, ethanol and succinate concentrations were determined by column liquid chromatography (CLC) in a Gilson CLC system (Middletown, Wis.). Compounds were separated on an HPX87-H column (Bio-Rad, Richmond, Calif.) at 45°C, with 5 mM H2SO4 at 0.6 ml min−1 as mobile phase, and detected with a Shimadzu RID6A refractive index detector (Kyoto, Japan). Samples were assayed for xylanase activity according to Bailey et al. (1992). The substrate [1% birchwood xylan (Sigma, St. Louis, Mo.) suspended in 50 mM citrate buffer, pH 6.0] and enzyme (diluted with 50 mM citrate buffer, pH 6.0) mixtures were incubated for 5 min at 60°C, and the reducing sugar determined (Miller et al. 1960); 1 unit (U) enzyme activity corresponded to 1 μmol reducing sugar released per minute. All enzyme activities were converted to protein amounts (milligrams) by using the specific activity of 1.812 (U μgpure xylanase−1), determined with purified protein (Görgens et al. 2001). For the quantification of intracellular xylanase activity, a sample of the fermentation broth was collected on ice, washed twice with 9 g l−1 NaCl and the cellular protein extracted with Y-PER (Yeast Protein Extraction Reagent; Pierce, Rockford, Ill.). Extracellular free amino acid concentrations were determined by ion exchange chromatography with postcolumn derivatisation (Biochemistry and Nutrition, DTU, Lyngby, Denmark; Barkholt and Jensen 1989). The ammonium ion concentration was determined using the Boehringer Mannheim Ammonia test kit (Cat. Nr 1112732), adapted for use with a Cobas Mira autoanalyser.
Results
The supplementation of defined medium with amino acids or succinate for the improvement of extracellular and intracellular heterologous xylanase production and biomass formation by a prototrophic, recombinant S. cerevisiae strain was investigated. Components were screened in shake-flask cultivation, prior to quantification in batch and continuous culture.
Screening of amino acids in shake-flask cultivation
Individual amino acids were added to shake-flask cultures at a concentration of approximately 4 mM, and at a time point near to glucose depletion, thus maximising their availability during the xylanase production (growth on ethanol) phase of the recombinant strain (Görgens et al. 2001). The total effect of individual amino acids on the extracellular xylanase production was expressed as relative xylanase productivity, which was calculated as the sum of the normalised values of the maximum volumetric xylanase activity (U ml−1), the maximum specific xylanase activity (U gcells−1), and the time-wise integrals of the specific xylanase activity and the cell density (Tables 1, 2). First, the effect of amino acids on proteolytic degradation of extracellular xylanase was determined by cultivating the recombinant yeast in shake-flasks at pH 3.0, where increased extracellular protease activity has been observed (Ogrydziak 1993; Jones 1991). Subsequently, the effect of amino acid supplementation was determined at pH 5.0, where extracellular protease activity was reduced.
Supplementation of the defined medium with individual amino acids during cultivation at pH 3.0 dramatically improved extracellular xylanase levels (Fig. 1, Table 1). Three typical responses to amino acid supplementation were observed. Firstly, the amino acids Arg, Ala, Asn, Glu, Gln and Gly apparently retarded extracellular proteolysis of the xylanase protein, compared to the control, under the chosen conditions (Jones 1991). The increase in extracellular activity of proteases at low pH (Ogrydziak 1993; Jones 1991), present in the medium due to either secretion or cell lysis, resulted in degradation of the extracellular xylanase (Fig. 1a). In the control experiment, extracellular xylanase activity peaked after 18 h of cultivation, followed by loss of activity up to 30 h when levels were no longer measurable (Fig. 1a). Arg, Ala, Asn, Glu, Gln and Gly retarded the extracellular proteolysis of xylanase, resulting in higher maximum production levels and measurable xylanase activity up to 60 h of cultivation (Fig. 1a). Other responses involved the inhibition of either biomass (Ile, Trp, Met, Cys, Phe, Thr, Leu and Tyr) or xylanase production (Cys, His, Ile, Leu, Lys, Met, Phe, Thr, Trp, Tyr and Val) by the prototrophic strain (Table 1).
Effect of individual amino acids on xylanase production by a prototrophic transformed Saccharomyces cerevisiae strain in shake-flask culture during growth on ethanol at pH 3.0 and 5.0. ○ Control without amino acid addition, ● glutamine, ▲ asparagine, ◆ arginine, ■ alanine, + glycine, ▼ glutamate (pH 3.0) or aspartate (pH 5.0). The pH of shake-flask cultures was controlled by either buffering defined medium to pH 5.0, using 50 mM citrate, or allowing the pH to decrease to approximately 3.0 during growth on glucose in unbuffered defined medium, due to ammonium utilisation (Greasham and Herber 1997)
Amino acid supplementation had a less dramatic effect on extracellular xylanase levels during cultivation at pH 5.0 (Fig. 1b), probably because the extracellular protease activity was reduced (Fig. 1b; Ogrydziak 1993; Jones 1991). The amino acids Ala, Arg, Asn, Asp, Gln, Gly and Lys improved the actual xylanase production by the transformed strain without inhibiting cell growth (Table 2). Biomass formation was inhibited by Cys, His, Ile, Leu, Met, Phe, Ser, Thr, Trp, Tyr and Val during growth at pH 5.0, whereas significantly fewer amino acids (Cys, Ile, Trp, Tyr and Val) inhibited xylanase production (Table 2). The amino acids Ala, Arg, Asn, Glu, Gln and Gly reduced extracellular proteolysis (pH 3.0) and increased actual xylanase production (pH 5.0), and were selected for further testing under fermentation conditions in defined medium.
Amino acid quantification in batch fermentation
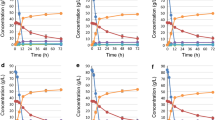
Batch cultures were supplemented with either arginine (40 mM total amino nitrogen), a four amino acid mixture (Arg, Asn, Ala, Gly; 20 mM total amino nitrogen) or a six amino acid mixture (Arg, Asn, Gly, Ala, Gln, Glu; 20 mM total amino nitrogen) during batch growth on ethanol (Fig. 2a–c; Table 3). Biomass formation, extracellular xylanase production (Fig. 2a, b) and ammonium consumption (not shown) increased due to supplementation with the four or six amino acid mixtures. These levels of biomass and xylanase production were comparable to those obtained with complex medium (Fig. 2a, b). The intracellular xylanase levels were improved as a result of amino acid supplementation (Table 4). The growth rate and rate of carbon (ethanol) consumption by the prototrophic transformant increased due to supplementation with the four or six amino acid mixture (Table 3; Fig. 2c).
Defined medium supplemented with either amino acid mixtures (a–c) at different total free amino nitrogen concentrations, or succinate (d–f), during growth on ethanol in batch culture. a, d Biomass; b, e xylanase; c ethanol production. f Succinate consumption during growth on ethanol in batch cultivation. ○ (pH 5.0), ▼ (pH 3.0) Controls without amino acid or succinate addition, + complex (YPD) medium control. Addition of Arg, Asn, Ala, Gly mixture (20 mM; ●), arginine (40 mM; ◆), Arg, Asn, Gly, Ala, Gln, Glu mixture (20 mM; ■), or succinate [9 g l−1 (▲), 11 g l−1 (△)]. Amino acid concentrations indicate the total free amino nitrogen content of the medium after supplementation
Succinate addition to batch fermentation
The defined medium was supplemented with succinate after the shift from growth on glucose to growth on ethanol (Fig. 2d–f; Table 3), most of which was consumed within the first 32 h thereafter (Fig. 2f). Succinate consumption improved biomass formation and extracellular xylanase production (Fig. 2d, e). The biomass yield, maximum specific growth rate and intracellular xylanase levels of the recombinant strain increased (Tables 3, 4). The rate of ethanol consumption was not significantly influenced by the uptake of succinate (data not shown).
Quantification in continuous culture
In glucose-limited chemostat culture, the ADH2 promoter regulating heterologous xylanase production by recombinant S. cerevisiae is derepressed during fully respiratory growth at low dilution rates (Du Preez et al. 2001). The effect of amino acid addition to continuous culture could therefore be determined by supplementing the defined feed medium to steady-state, glucose-limited cultures (Table 5). Casamino acids and SD-optimised mixtures of pure amino acids have previously enhanced heterologous protein production by S. cerevisiae (Wittrup and Benig 1994). The supply of an excess of the auxotrophic 7 amino acid mixture, containing His, Leu, Trp, Asp, Glu, Ser and Gly, has improved heterologous xylanase production by an auxotrophic strain recombinant yeast (Görgens et al. 2004).
In the present study, the addition of the four amino acid mixture improved extracellular xylanase production, despite the low level of supplementation, similar to batch cultivation (Table 5). Supplementation with the six amino acid mixture improved both biomass and xylanase production during growth on glucose. In the six amino acid mixture, all of the amino acids were utilised effectively by the prototrophic strain (Table 5). However, xylanase production by the prototrophic strain was inhibited by supplementation of the feed medium with the casamino acids and SD-optimised mixtures, despite the positive effect of casamino acids on biomass formation (Table 5). The auxotrophic 7 aa mixture (see above) inhibited both xylanase and biomass production by the prototrophic transformant in glucose-limited chemostat culture, as was observed elsewhere (Görgens et al. 2004).
Discussion
The supplementation of a chemically defined medium with amino acids or succinate for the improvement of heterologous xylanase production by a prototrophic S. cerevisiae strain was investigated. Amino acids were first screened in shake-flask culture, prior to the testing of combinations in batch and continuous cultivation. Supplementation of the defined medium with suitable amino acid mixtures or succinate significantly improved xylanase production by recombinant S. cerevisiae.
Amino acid supplementation
The screening of all 20 amino acids indicated that heterologous xylanase production could be improved by supplementation with Ala, Arg, Asn, Glu, Gln and Gly. Among these amino acids, Gln and Asn are classified as preferred nitrogen sources because they are consumed readily from defined medium, despite the presence of ammonium (Slaughter et al. 1990; Jiranek et al. 1995; Ter Schure et al. 2000; Wiame et al. 1985; Cooper 1982). Arg and Glu have high molar contents of nitrogen, allowing their preferential utilisation from mixtures (Jiranek et al. 1995; Herraiz and Ough 1993), leading to improved heterologous xylanase and biomass production. The beneficial effect of the selected amino acids was strongly related to the inhibition of extracellular proteases (Kang et al. 2000; Coppella and Dhurjati 1989; Boze et al. 2001; Werten et al. 1999; Goodrick et al. 2001; Sreekrishna et al. 1997), especially during screening at pH 3.0 (Fig. 1a; Ogrydziak 1993; Jones 1991). The selected amino acids Arg, Gly, Ala, Asn and Gln are preferred for storage in the vacuole (Messenguy et al. 1980; Wiame et al. 1985; Grenson 1992; Horák 1997).
Supplementation with succinate
The availability of the TCA cycle intermediate succinate, limited biomass formation and protein production during growth on ethanol. Most of the succinate supplemented to the defined medium was consumed during batch growth on ethanol (Fig. 2f). Improved xylanase and biomass production were thus related to the actual consumption of succinate, and not to its buffering capacity (Adams et al. 1989), which dominated a previous investigation into its use as a medium supplement (Cha et al. 1998). The addition of succinate to cultures without pH control (Cha et al. 1998) will halt the characteristic reduction in pH during the course of the cultivation. This will minimise the extracellular proteolysis of the gene product at low pH (Ogrydziak 1993; Jones 1991) and result in apparently higher production levels. In the present investigation, the influence of the succinate buffering capacity on xylanase production was avoided by careful maintenance of the pH of control cultures. The positive effect of succinate consumption contradicts previous claims that extracellular TCA intermediates cannot support S. cerevisiae growth (Kaclikova et al. 1992).
The present fermentation results indicated that the availability of exogenous amino-nitrogen had a positive effect on protein production and biomass formation during growth on ethanol, as was previously observed (Chen et al. 1993; Gu et al. 1991; Albers et al. 1996). The positive effect of amino acid supplementation on xylanase production was observed only when a balanced mixture of individual amino acids was supplemented. The production of heterologous proteins may therefore be partially limited by the availability of metabolic precursors in carbon- or nitrogen metabolism during growth on ethanol or glucose, requiring empirical medium optimisation for new production strains. Alternatively, the supply of exogenous amino acids may also have improved the supply of tRNA loaded with particular amino acids, and thereby improve xylanase production. During growth on glucose in continuous culture, the presence of the amino acids, His, Ile, Leu, Met, Phe, Ser, Thr, Tyr and Val in the auxotrophic 7 amino acid, casamino acid and SD-optimised mixtures, inhibited xylanase production (Table 5). Some of the supplemented amino acids had a strong inhibitory effect on either biomass formation or heterologous protein production, and supplementation of yeast cultures with Cys, His, Ile, Leu, Met, Phe, Ser, Thr, Trp, Tyr and Val should probably be avoided. The disparity between the reported stimulation of heterologous protein production by the latter two mixtures (Wittrup and Benig 1994) and the inhibition of xylanase production observed in the present study, emphasises the need for empirical optimisation of defined media for each yeast production system.
References
Adams MR, Bryan JJ, Thurston PJ (1989) A medium designed for monitoring pitching yeast contamination in beer using a conductimetric technique. Lett Appl Microbiol 8:55–58
Albers E, Larsson C, Lidén G, Niklasson C, Gustafsson L (1996) Influence of nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl Environ Microbiol 62:3187–3195
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23:257–270
Barkholt V, Jensen AL (1989) Amino acid analysis determination of cysteine plus half-cysteine in proteins after hydrochloric acid hydrolysis with a disulfide compound as additive. Anal Biochem 177:318–322
Blechl AE, Thrasher KS, Vensel WH, Greene FC (1992) Purification and characterization of wheat α-gliadin synthesized in the yeast Saccharomyces cerevisiae. Gene 116:119–127
Boze H, Celine L, Patrick C, Fabien R, Christine V, Yves C, Guy M (2001) High-level secretory production of recombinant porcine follicle-stimulating hormone by Pichia pastoris. Process Biochem 36:907–913
Cha HJ, Kim M-H, Kim SH, Yeo JS, Chae HJ, Yoo YJ (1998) Enhancement, by succinate addition, of the production of cloned glucoamylase from recombinant yeast using a SUC2 promoter. Process Biochem 33:257–261
Chen Y, Kirk N, Piper PW (1993) Effects of medium composition on MFα1 promoter-directed secretion of a small protease inhibitor in Saccharomyces cerevisiae batch fermentation. Biotechnol Lett 15:223–228
Choi W-A, Oh GH, Kang HA, Chung BH (2000) Improvement of intact human lipocortin-I production in Saccharomyces cerevisiae by inhibiting proteolysis. J Biosci Bioeng 89:77–80
Chung BH, Park KS (1998) Simple approach to reducing proteolysis during secretory production of human parathyroid hormone in Saccharomyces cerevisiae. Biotechnol Bioeng 57:245–249
Cooper TG (1982) Nitrogen metabolism in Saccharomyces cerevisiae. In Strathern JN, Jones EW, Broach JR (eds), The molecular and cellular biology of the yeast Saccharomyces. Cold Spring Harbour Laboratory, pp 39–100
Coppella SJ, Dhurjati P (1989) α-Factor directed expression of the human epidermal growth factor in Saccharomyces cerevisiae. Biotechnol Bioeng 33:976–983
Donald KAG, Carle A, Gibbs MD, Bergquist PL (1994) Production of a bacterial thermophilic xylanase in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 42:309–312
Dubois E, Messenguy F (1997) Integration of the multiple controls regulating the expression of the arginase gene CAR1 of Saccharomyces cerevisiae in response to different nitrogen signals: role of Gln3p, ArgRp-Mcm1p, and Ume6p. Mol Gen Genet 253:568–580
Du Preez JC, Mare JE, Albertyn J, Kilian SG (2001) Transcriptional repression of ADH2-regulated β-xylanase production by ethanol in recombinant strains of Saccharomyces cerevisiae. FEMS Yeast Res 1:233–240
Goodrick JC, Xu M, Finnegan R, Schilling BM, Schiavi S, Hoppe H, Wan NC (2001) High-level expression and stabilization of recombinant human chitinase produced in a continuous constitutive Pichia pastoris expression system. Biotechnol Bioeng 74:492–497
Görgens JF, Van Zyl WH, Knoetze JH, Hahn-Hägerdal B (2001) The metabolic burden of the PGK1 and ADH2 promoter systems for heterologous xylanase production by Saccharomyces cerevisiae in defined medium. Biotechnol Bioeng 73:238–245
Görgens JF, Planas J, van Zyl WH, Knoetze JH, Hahn-Hägerdal B (2004) Comparison of three expression systems for heterologous xylanase production by S. cerevisiae in defined medium. Yeast 21:1205–1217
Greasham RL, Herber WK (1997) Design and optimization of growth media. In: Rhodes, PM, Stanbury PF (eds) Applied microbial physiology—a practical approach. Oxford University Press, Oxford, pp 53–74
Grenson M (1992) Amino acid transporters in yeast: structure, function and regulation. In: Molecular aspects of transport proteins. Elsevier, Amsterdam, pp 219–245
Gu MB, Park MH, Kim D-I (1991) Growth rate control in fed-batch cultures of recombinant Saccharomyces cerevisiae producing hepatitis B surface antigen (HBsAg). Appl Microbiol Biotechnol 35:46–50
Herraiz T, Ough CS (1993) Formation of ethyl esters of amino acids by yeasts during the alcoholic fermentation of grape juice. Am J Enol Viticul 44:41–48
Horák J (1997) Yeast nutrient transporters. Biochim Biophys Acta 1331:41–79
Jin S, Ye K, Shimizu K (1997) Metabolic flux distributions in recombinant Saccharomyces cerevisiae during foreign protein production. J Biotechnol 54:161–174
Jiranek V, Langridge P, Henschke PA (1995) Amino acid and ammonium utilization by Saccharomyces cerevisiae wine yeasts from a chemically defined medium. Am J Enol Vitic 46:75–83
Jones EW (1991) Tackling the protease problem in Saccharomyces cerevisiae. In: Guthrie C, Fink GR (eds) Methods in enzymology, guide to yeast genetics and molecular biology, vol 194. Academic Press, San Diego, pp 428–452
Kaclikova E, Lachowicz TM, Gbelska Y, Subik J (1992) Fumaric acid overproduction in yeast mutants deficient in fumarase. FEMS Microbiol Lett 91:101–106
Kang HA, Choi E-S, Hong W-K, Kim J-Y, Ko S-M, Sohn J-H, Rhee SK (2000) Proteolytic stability of recombinant humans serum albumin secreted in the yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol 53:575–582
La Grange DC, Pretorius IS, Van Zyl WH (1996) Expression of a Trichoderma reesei β-xylanase gene (XYN2) in Saccharomyces cerevisiae. Appl Environ Microbiol 62:1036–1044
Mendoza-Vega O, Sabatie J, Brown SW (1994) Industrial production of heterologous proteins by fed-batch cultures of the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 15:369–410
Messenguy F, Colin D, Ten Have J-P (1980) Regulation of compartmentalisation of amino acid pools in Saccharomyces cerevisiae and its effects on metabolic control. Eur J Biochem 108:439–447
Miller GL, Blum R, Glennon WE, Burton AL (1960) Measurement of carboxymethylcellulase activity. Anal Biochem 2:127–132
Nuyens F, Van Zyl WH, Iserentant D, Verachtert H, Michiels C (2001) Heterologous expression of the Bacillus pumilus endo-β-xylanase (xynA) gene in the yeast Saccharomyces cerevisiae. Appl Microbiol Biotechnol 56:431–434
Ogrydziak DM (1993) Yeast extracellular proteases. Crit Rev Biotechnol 13: 1–55
Pérez-González JA, De Graaff LH, Visser J, Ramón D (1996) Molecular cloning and expression in Saccharomyces cerevisiae of two Aspergillus nidulans xylanase genes. Appl Environ Microbiol 62:2179–2182
Rothstein R (1991) Targeting, disruption, replacement, and allele rescue: integrative DNA transformation in yeast. In: Guthrie C, Fink GR (eds) Methods in enzymology, guide to yeast genetics and molecular biology, vol 194. Academic Press, San Diego, pp 302–318
Slaughter JC, McKernan G, Saita M (1990) Intracellular asparagine pool as a factor in control of ammonium uptake by Saccharomyces cerevisiae. Mycol Res 94:1009–1012
Sreekrishna K, Brankamp RG, Kropp KE, Blankenship DT, Tsay J-T, Smith PL, Wierschke JD, Subramaniam A, Birkenberger LA (1997) Strategies for optimal synthesis and secretion of heterologous proteins in methylotropic yeast Pichia pastoris. Gene 190:55–62
Ter Schure EG, Van Riel NAW, Verrips CT (2000) The role of ammonia metabolism in nitrogen catabolite repression in Saccharomyces cerevisiae. FEMS Microbiol Rev 24:67–83
Thomas KC, Ingledew WM (1990) Fuel alcohol production: effects of free amino nitrogen on fermentation of very-high-gravity wheat mashes. Appl Environ Microbiol 56:2046–2050
Thomas KC, Ingledew WM (1992) Relationship of low lysine and high arginine concentrations to efficient ethanolic fermentation of wheat mashes. Can J Microbiol 38:626–634
Toman PD, Chisholm G, McMullin H, Giere LM, Olsen DR, Kovach RJ, Leigh SD, Fong BE, Chang R, Daniels GA, Berg RA, Hitzeman RA (2000) Production of recombinant human type I procollagen trimers using a four-gene expression system in the yeast Saccharomyces cerevisiae. J Biol Chem 275:23303–23309
Verduyn C, Postma E, Scheffers WA, Van Dijken JP (1992) Effect of benzoic acid metabolism on metabolic fluxes in yeast: a continuous culture study on the regulation of respiration and alcoholic fermentation. Yeast 8:501–507
Werten MWT, Van den Bosch TJ, Wind RD, Mooibroek H, De Wolf FA (1999) High-yield secretion of recombinant gelatins by Pichia pastoris. Yeast 15:1087–1096
Wiame J-M, Grenson M, Arst HN (1985) Nitrogen catabolite repression in yeasts and filamentous fungi. Adv Microb Phys 26:1–88
Wittrup KD, Benig V (1994) Optimisation of amino acid supplements for heterologous protein secretion in Saccharomyces cerevisiae. Biotechnol Tech 8:161–166
Acknowledgements
Ms Jenny Ågren (Lund University) and Mr Henk Blignault (Stellenbosch University) are gratefully acknowledged for technical assistance during screening of the medium components.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Görgens, J.F., van Zyl, W.H., Knoetze, J.H. et al. Amino acid supplementation improves heterologous protein production by Saccharomyces cerevisiae in defined medium. Appl Microbiol Biotechnol 67, 684–691 (2005). https://doi.org/10.1007/s00253-004-1803-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1803-3