Abstract
We examined the ability of a soil bacterium, Klebsiella planticola strain DSZ, to degrade the herbicide simazine (SZ). Strain DSZ is metabolically diverse and grows on a wide range of s-triazine and aromatic compounds. DSZ cells grown in liquid medium with SZ (in 10 mM ethanol) as carbon source mineralized 71.6±1.3% of 0.025 mM SZ with a yield of 4.6±0.3 μg cell dry weight mmol−1 carbon. The metabolites produced by DSZ during SZ degradation included ammeline, cyanuric acid, N-formylurea and urea. We studied the physiological adaptations which allow strain DSZ to metabolize SZ. Using scanning electron microscopy, we detected DSZ cells covering the surfaces of SZ crystals when the herbicide was used at high concentrations (0.1 mM). The membrane order observed by FTIR spectroscopy showed membrane activity at low temperature (4°C) to assimilate the herbicide. Membrane fatty acid analysis demonstrated that strain DSZ adapted to grow on SZ by increasing the degree of saturation of membrane lipid fatty acid; and the opposite effect was detected when both SZ and ethanol were used as carbon sources. This confirms the modulator effect of ethanol on membrane fluidity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
s-Triazine herbicides are used in a variety of weed control programs with major crops, but herbicides containing a s-triazine ring are relatively persistent in the environment. Simazine [SZ; 2-chloro-4,6-bis(ethylamine)-s-triazine] is a synthetic chemical that is widely used as a selective triazine herbicide to control the growth of broad-leaved weeds and annual grasses in field, berry fruit, nuts, vegetable and ornamental crops, turf grass, orchards and vineyards. At higher rates, it is used for non-selective weed control in industrial areas and, before 1992, it was used to control submerged weeds and algae in large aquariums, farm ponds, fish hatcheries, swimming pools, ornamental ponds and cooling towers. The Environmental Protection Agency sets the maximum level of SZ that is allowed in public drinking water supplies. This maximum contaminant level (MCL) for SZ is set at no more than 4 μg SZ l−1 water. Increasing awareness of the harmful effects of environmental pollution leads to much wider research into strategies to clean up the environment. It is now accepted that microbial metabolism provides a safer, more efficient and less expensive alternative to physicochemical methods for pollution abatement (Pandey and Jain 2002). Microorganisms possess a number of adaptative mechanisms for accumulating and transporting xenobiotics into the cell for initial enzymatic catabolism (Sikkema et al. 1992; Whyte et al. 1999).
A significant problem in the biodegradation of s-triazines is the very low solubility of these compounds in water. As a result, their bioavailability decreases and this is responsible for the recalcitrance of these herbicides in soil and water. However, s-triazines are degraded at rates which exceed the rates of water solubility, which shows that some solubilization mechanisms are also used by s-triazine-degrading bacteria (Whyte et al. 1999). Microorganisms may take up insoluble compounds by adhering to the compounds at the water–liquid or water–solid interface (Rosenberg 1991; Sikkema et al. 1994, 1995). To facilitate adhesion to hydrophobic substrates, degrading bacteria may increase their cell surface hydrophobicity by modifying cell surface components (Watkinson and Morgan 1990). Bacteria are also known to adapt to changes in environmental conditions, such as growth temperature or growth in the presence of xenobiotic substrates, by altering the lipid composition of the cytoplasmic membrane in order to maintain or adjust membrane bilayer fluidity (Sikkema et al.1995). Alterations to the fatty acid moieties of membrane lipids are thought to be the most effective means of maintaining the liquid crystalline state in membranes which is essential for optimal membrane function (Danilo et al. 1996; Gerhardt et al. 2000; Heipieper and De Bont 1994; Heipieper et al. 1994).
We studied the biological degradation of the synthetic compound SZ by Klebsiella planticola strain DSZ, a soil-isolated bacterium that is capable of degrading a variety of xenobiotic compounds. We examined the physiological adaptations involved in herbicide utilization, such as modification of membrane fatty acids, ability to use SZ at low temperatures (since the temperature of phase transition from gel to semifluid crystal state begins at 0°C) and the ability of strain DSZ to cover the surfaces of SZ crystals and assimilate the herbicide.
Materials and methods
Bacterial characterization
The bacterium K. planticola strain DSZ was isolated from agricultural fields in Madrid (Spain) that had been exposed to SZ for 36 months (Martín-Montalvo et al. 1997). A pure culture was subjected to biochemical analysis (Api20NE kit; bioMérieux, Marcy l’Etoile, France) and fatty acid methyl ester analysis (Danilo et al. 1996).
Chemicals
SZ (99% purity), and [U-ring 14C] SZ were purchased from Sigma-Aldrich (St. Louis, Mo.). All chemical compounds were of the highest purity commercially available.
Growth conditions and preparation of membrane vesicles
Cells were grown aerobically at 30°C in MB medium (Martín-Montalvo et al. 1997). We sterilized the carbon sources separately and added them to give 5 mM m-hydroxybenzoate (m-HBA), 5 mM p-HBA, 5 mM 4-hydroxyphenylacetate (4-HPA), 5 mM 3,4-dihydroxybenzoate (3,4-DHBA), 5 mM 2,5-DHBA, 0.5 mM propachlor (PCH), 0.4 mM alachlor (ACH), 10 mM glucose, 0.023 mM atrazine (Atz), 0.025–0.1 mM SZ, 0.04 mM melamine and 0.039 mM cyanuric acid.
Vesicles were prepared using a modification of the procedures for gram-negative bacteria described by Gerhardt et al. (2000). Purified membranes were collected by centrifugation (20,600 g, 60 min, 4°C) and suspended by means of a Potter–Elvehjem glass homogenizer in 50 mM potassium phosphate (pH 7.0) containing 10 mM MgSO4. Aliquots of 250 μl containing membrane protein levels of 5–8 mg ml−1 were frozen and stored in liquid nitrogen.
Membrane phase transition temperature
Membrane phase transition temperatures were measured by Fourier transform infrared (FTIR) spectroscopy (Crowe et al. 1989). Membranes were collected by centrifugation (46,000 g, 30 min, 4°C) and resuspended to a level of 28 mg ml−1 protein in 50 mM potassium phosphate buffer, pH 7.0, containing 10 mM MgSO4. A 30-μl aliquot of the membrane suspension was placed between glass plates and then inserted into a temperature controller. The CH2 symmetric stretching frequency (wave number range 2,850–2,854 cm−1) at different temperatures was then measured using a Hewlett–Packard model 5459 FTIR spectrometer.
Metabolism of [U-ring 14C] SZ
Mineralization of [U-ring 14C] SZ by strain DSZ was determined in MB medium with 0.025–0.07 mM unlabeled SZ (in 10 mM ethanol; EtOH) and supplemented with 2 μCi of [U-ring 14C] SZ. Experiments were carried out in triplicate. Cultures were grown at 30°C for 3 h in 250-ml biometer flasks sealed with Teflon stoppers. 14CO2 from mineralization was trapped in a vial with 1 ml of 1 N NaOH solution. Radioactivity was finally measured by scintillation counting with a Hewlett–Packard model 2500TR scintillation spectrometer.
Chromatographic and mass spectral identification of intermediates
We identified intermediates by non-growing cell experiments as described by Martin et al. (1999, 2000). Gas chromatographic–mass spectral (GC-MS) analyses were done with a Hewlett–Packard model 5890 series II gas chromatograph equipped with a VA-5 capillary column (30 m, 0.25 mm i.d.), programmed from 80°C to 290°C (15°C min−1) and connected to a Hewlett–Packard 5989A quadrupole mass detector. Strain DSZ cells were grown in MB medium with 0.025 mM SZ in EtOH (10 mM), these cultures were centrifuged at 10,000 g for 10 min at 4°C and the pellets were then washed twice with 10 mM phosphate buffer (pH 7.2) and resuspended in the same buffer. SZ (0.025 mM) was added to the cell suspensions and incubated at 30°C. Samples were taken at 3 h and 24 h and identification of intermediates was performed by GC-MS analysis. Solid-phase extractions were done with Varian C18 and SCX cartridges, acetone was used as the eluent and 2-μl aliquots were injected into the column. Metabolites were identified by comparing their electron impact mass spectra with those of standard samples and by coelution in GC.
Analytical methods
SZ and cyanuric acid were measured by HPLC analysis, performed with a Waters model 616PDA996 photodiode array detector equipped with a Millennium 20/10 for data analysis. Separation was done in a Novapack C-18 (3.9×150.0 mm) column, using a mobile phase consisting of 40% acetonitrile in water at a flow rate of 0.5 ml min−1. SZ was monitored at 214 nm and cyanuric acid at 223 nm. The injection volume was 10 μl. SZ and cyanuric acid were identified by coelution with standards in HPLC analysis.
Confocal scanning laser microscopy analysis of strain DSZ
A Bio-Rad MRC 1024 confocal scanning laser microscope (CSLM) with standard confocal configuration was used to non-destructively obtain images of strain DSZ grown on liquid medium containing 0.1 mM SZ. The CSLM was operated as described by Martin et al. (2000). SYTO-13 (Molecular Probes, Eugene, Ore.) was used as a nucleic acids stain to positively stain strain DSZ (Martin et al. 2000). Optical thin sections in the xy plane and xz sagital images were obtained.
Results
Identification
Strain DSZ was isolated from a herbicide-contaminated soil for its ability to grow on SZ as carbon source by enrichment culture (Martín-Montalvo et al. 1997). Biochemical analysis (Api20NE kit) showed that strain DSZ has a 0.982 similarity to K. planticola. Fatty-acid analysis of DSZ showed an excellent match (similarity index = 0.661) with K. planticola. The range of growth substrates metabolized by K. planticola DSZ was determined in MB medium containing 4-HPA, 3,4-HPA, 2,5-HBA, m-HBA, p-HBA, EtOH, PCH, ACH, SZ, Atz, or cyanuric acid as carbon source. Also, we tested the capacity of DSZ to use SZ and melamine as the sole nitrogen source and glucose as the carbon source. All the compounds tested were substrates; and SZ and melamine were shown to support growth as the sole nitrogen source.
Metabolism of SZ
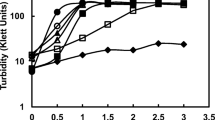
K. planticola strain DSZ was grown in liquid minimal MB medium containing 0.025 mM SZ as the sole carbon source. After 30 days of growth at 30°C, the culture reached an optical density at 680 nm (OD680) of 0.2; and the molar growth yield was 1.9±0.1 μg cell dry weight mmol−1 carbon. This may be due to the low water solubility (6 mg l−1) of SZ, which implies a low bioavailability of the herbicide. Above this concentration, SZ forms microprecipitates (see below) and resolubilization may limit biodegradation. One of the main problems in studying the microbial degradation of xenobiotics is their high hydrophobicity and low bioavailability. To enhance bioavailability, EtOH was used to dissolve the herbicide, which increased its solubility (700 mg l−1, 20°C). The enhancement of SZ utilization was seen when strain DSZ was grown in MB medium with 0.025 mM SZ dissolved in 10 mM EtOH (Fig. 1a). After 20 days of growth, the culture reached an OD680 of 0.58, equivalent to 9×108 cells ml−1. The final cell dry weight was 318 mg l−1 and the molar growth yield was calculated to be 4.6±0.3 μg cell dry weight mmol−1carbon. Strain DSZ had a doubling time of 5 h when grown on SZ + EtOH as carbon source.
a Degradation of 0.025 mM SZ in MB medium by strain DSZ (filled circles). SZ remaining in the medium is shown in relation to biomass formation (open circles). b Kinetics of mineralization of [U-ring 14C] SZ by strain DSZ, expressed as 14CO2 production. Cells were grown on 0.01–0.07 mM SZ, in 10 mM EtOH
Mineralization of [U-ring 14C] SZ was described by a logistic model (Fig. 1b). SZ-grown whole cells produced 14CO2 from [U-ring 14C] SZ, with a maximum mineralization rate of 1.8(±0.02)×10−4 nmol CO2 μg−1 protein h−1. DSZ mineralized approximately 71.6±1.3% of the [U-ring 14C] SZ (0.025 mM). No significant 14CO2 was released in control experiments without cells; and no counts were obtained in controls without radioactivity.
Identification of metabolites
Since the above metabolic test data demonstrated the ability of K. planticola strain DSZ to grow on SZ (0.025 mM) dissolved in EtOH (10 mM) as carbon source, attempts were then made to identify the corresponding metabolites in the catabolic pathway. The metabolites produced by the degrading bacteria were ammeline, cyanuric acid, N-formylurea, urea and acetic acid (Fig. 2). GC-MS analysis of the culture medium, after 24 h of incubation, showed the accumulation of ammeline (Rf = 17.32 min) with a molecular ion at m/z 127 (M+1) and a fragment ion at m/z 43 [CONH]+ in the culture medium (Fig. 2a). Ammeline was estimated at only 4% of the initial SZ added to the medium. HPLC-MS was also used to identify SZ degradation-pathway intermediates; and a metabolite in the liquid culture with a molecular ion at m/z 130 (M+1) was identified as cyanuric acid.
a SZ metabolites detected in exponential growth phase culture supernatants of K. planticola DSZ by GC-MS. A SZ, B ammeline. b Metabolites detected in the cytosolic fraction of K. planticola DSZ by GC-MS. C Acetic acid, (which appeared in the samples as a result of EtOH catabolism), D urea, E N-formylurea, A SZ
When the cytosolic fraction was analyzed by GC-MS, three other peaks of possible metabolites were detected (Fig. 2b). One of the compounds with a retention time of 4.30 min was identified as acetic acid, which appeared in the samples as a result of EtOH catabolism. A metabolite with m/z 59 (M+1) was identified as urea (Rt = 5.73 min) and another metabolite with m/z 88 (M+1) was identified as N-formylurea (Rt = 18.90 min), a degradation product from cyanuric acid. The identified metabolites, N-formylurea and urea, were estimated as only 5% and 4%, respectively, of the initial SZ added to the culture.
CSLM analysis of strain DSZ
We examined physiological adaptations which allow strain DSZ to assimilate SZ at concentrations higher than the water solubility concentration (0.1 mM). Strain DSZ grew as freely suspended cells in SZ medium. We observed that, at 0.1 mM SZ, strain DSZ cells completely surrounded and adhered to the surfaces of SZ crystals (Fig. 3). At lower SZ concentrations, 0.01, 0.025 and 0.05 mM, no formation of SZ crystals was observed. Whyte et al. (1999) described a similar cell adhesion which allowed the psychrotroph Rhodococcus sp. strain Q15 to assimilate alkanes at low temperature. Microorganisms are also known to adhere to hydrocarbons at the water–hydrocarbon liquid or solid interface in order to degrade insoluble hydrocarbons (Herbert 1986; Sikkema et al. 1995).
Analysis of cell membrane fatty acid composition in strain DSZ
This analysis was performed to identify the membrane fatty acid profile and to evaluate the effects of SZ in the degree of saturation of membrane fatty acids. K. planticola strain DSZ cells were grown on MB supplemented with either glucose, SZ, EtOH, or SZ-EtOH to determine changes in the fatty acid composition of the extractable lipids in response to changes in carbon source (Fig. 4). The acyl chain lengths of the observed fatty acids ranged from C14 to C20, but C16 and C18 fatty acids were generally the most prominent fatty acids, regardless of the carbon source. The fatty acid profile of the bacterial strain grown on SZ shows that using the herbicide as carbon source greatly decreased the presence of the C16 fatty acids compared with that in glucose-grown cells. A small amount of hexadecanoic (C16:0) fatty acid was measured in SZ-grown cells, no cis-9-hexadecenoic (C16:1) fatty acid was detected in these samples and the octadecenoic (C18:0) fatty acid present was twice that of the control in glucose (Fig. 4a). When bacteria were grown in EtOH, as the sole source of carbon, there was a decrease (20%) in the amount of cis-9-octadecenoic (C18:1) fatty acid. So the use of SZ or EtOH as sole carbon source reduced the percentage of the unsaturated fatty acids. The fatty acid profiles of strain DSZ cells grown on SZ-EtOH (Fig. 4b) showed a contrasting effect of carbon source in the membrane fatty acid composition, with a decrease in saturated fatty acids. Compared with glucose-grown cells, the relative amount of C18:0 fatty acid was 31% below the control. These data confirm that EtOH modulates the effect of xenobiotics on bacterial membranes (Fetzner and Lingens 1994; Ingram 1990).
a Comparison of C16 and C18 fatty acid profiles of K. planticola strain DSZ cells grown on glucose (open columns), 0.025 mM SZ (horizontally striped columns), 10 mM EtOH (diagonally striped columns) and 0.025 mM SZ + 10 mM EtOH (checkered columns). b Comparison of saturation degree (dotted columns) and C18/C16 ratio (diagonally striped columns) of K. planticola strain DSZ cells grown on glucose, 10 mM EtOH, 0.025 mM SZ, and 0.025 mM SZ + 10 mM EtOH. Values are means ±SD for quadruplicate samples
To elucidate the adaptation mechanisms of membrane fatty acids in strain DSZ in response to growth on different carbon sources, we investigated and compared the degree of saturation (i.e., saturated/unsaturated ratio) of fatty acids and the C18/C16 ratio (Fig. 4b). The comparison indicated that the fatty acids changed from relatively saturated to relatively unsaturated fatty acids when SZ-EtOH was used as carbon source. Moreover, the degree of saturation increased (34%) when SZ was used as sole carbon source, as a consequence of the decrease in unsaturated fatty acids. The C18/C16 ratio showed a decrease (23.2%) when DSZ cells were grown in EtOH, as compared with the bacteria grown in glucose, as a consequence of a C18 fatty acids decrease (Fig. 4). When SZ was used as carbon source, there was a dramatic increase in the ratio (more than four times that of the control), suggesting that C16 fatty acids were strongly involved in the adaptation mechanisms of membrane fatty acids in strain DSZ in response to the use of the herbicide as the growth substrate. The use of both SZ and EtOH as substrates clearly showed the modulator effect of EtOH; and the C18/C16 ratio obtained was only slightly lower than that of the control (Fig. 4).
Measurement of membrane order
With a view to using K. planticola strain DSZ in environmental bioremediation processes, the membrane order was measured. Membrane phase transitions determined by changes in the vibrational frequency of the CH2 symmetric stretch band in FTIR spectra revealed a broad membrane lipid phase transition (Fig. 5). Data obtained with DSZ membranes from cells grown on SZ showed that the lower boundary of the phase transition is 0°C and the upper boundary is 24°C. When sucrose or KCl to 500 mOsm was added, no effect on the phase transition temperature was detected. These results suggest that SZ uptake was affected by the physical state of the membrane, and Fig. 5 shows that SZ could be transported into DSZ cells at 0–20°C.
Discussion
We studied the biological degradation of the synthetic compound SZ, prevalent in soils and waters as a consequence of excessive use of pesticides, especially in areas of intensive agriculture. K. planticola DSZ is able to use different xenobiotic compounds as carbon source—among them some herbicides such as PCH, ACH, Atz and SZ. K. planticola DSZ grows on SZ, which can be used as nitrogen and carbon sources at concentrations between 0.01 mM and 0.1 mM in liquid cultures. Other bacteria isolated from contaminated soils have been found to consume triazines when applied in a very wide range of concentrations, from 0.1 mg l−1 to 100 mg l−1 (Seffernick et al. 2001; Strong et al. 2002; Struthers et al. 1998). Many reports mention atrazine; and the solubility of this herbicide in H2O is 33 mg l−1, five times that of SZ (6 mg l−1). Strong et al. (2002) and Struthers et al. (1998) found an inhibiting effect of inorganic nitrogen on the degradation of triazines. We found that DSZ retains its capacity to metabolize SZ, even in the presence of inorganic nitrogen in the culture medium when this herbicide is used as carbon source.
Some reports state that SZ can be oxidized biologically but very slowly (Lai et al. 1995), which was confirmed by our findings. When DSZ used only SZ as carbon source, very little biomass was formed. With EtOH in the medium, both the formation of biomass and the use of SZ were notably higher (see Fig. 1). This is fairly frequent in other soil bacteria capable of degrading triazines, such as Agrobacterium radiobacter J14a (Struthers et al. 1998), which produces a significant increase in biomass when it uses other compounds such as citrate, saccharin or glucose as carbon source. The destructive effects of EtOH on bacterial growth, cellular viability and metabolism come mainly from the increased permeability of the cell membrane, growth being the cellular activity that is most sensitive to inhibition by EtOH. There are, however, various microorganisms such as DSZ that can grow, using EtOH as carbon source, maybe as a result of evolutionary changes or adaptation of the composition of the cellular membrane (Ingram 1990). For example, bacteria of the genus Pseudomonas are capable of oxidizing EtOH to form acetic acid. Descriptions of the molecular mechanism of EtOH oxidation and its regulation in P. aeruginosa ATCC 17933 were published recently (Kretzschmar et al. 2002; Gorisch 2003).
One problem that is general in triazine utilization is that a portion of the ring remains unbroken in the medium. A number of publications report the metabolization or co-metabolization of SZ by various soil microorganisms (Ernst and Rehm 1995), but there is very rarely confirmation of ring rupture. K. planticola DSZ degrades SZ and 14CO2 is produced simultaneously from [U-ring 14C] SZ. In the course of this mineralization, we identified small amounts of ammeline, urea and N-formylurea (Fig. 2). It is now established that bacteria metabolize melamine and triazine herbicides via enzyme-catalyzed hydrolytic reactions (Strong et al. 2002). The enzymatic basis of atrazine mineralization has been most extensively studied in Pseudomonas sp. strain ADP (Martinez et al. 2001). In this strain, three enzymatic steps (yielding hydroxyatrazine, N-isopropylammelide, N-isopropylammeline as intermediates) mediate displacement of the three substituents on the s-triazine ring. All six genes encoding atrazine catabolism in Pseudomonas sp. strain ADP were localized to plasmid pADP-1, which was completely sequenced (Martinez et al. 2001). Another bacterium isolated from soils contaminated by triazines, K. pneumoniae AZ (Seffernick et al. 2002), metabolizes Cl-diamino-2-triazines by a different route, but no details have been given of the biochemical process.
One difficulty arising in the biodegradation of s-triazines and hydrocarbonate contaminants is their poor solubility in the aqueous phase. DSZ takes soluble SZ in the aqueous phase into its membrane by a passive transport process; and there it is held by its lipophillic nature. But, on using concentrations of SZ above the level of solubility, we found that the cells were mainly adhering to the crystal surface, which means that DSZ was using the solid phase of SZ for its growth (Fig. 3). A similar phenomenon was observed by Whyte et al. (1999) in Rhodococcus sp. strain Q15, which uses alkanes at low temperatures: during their growth at 5°C in octacosane they were found adhered to the crystals of this alkane. One possible explanation might be that many bacteria are capable of emulsifying hydrocarbons in solution, producing biosurfactants (Desai et al.1997; Neu et al. 1988). These amphipathic compounds lower the surface tension because they accumulate at the interphase of immiscible fluids or at the interphase of a fluid and a solid, enlarging the surface area of the insoluble compounds and thus increasing their bioavailability and the consequent degradation of the hydrocarbon (Desai et al. 1997; Neu et al. 1988). In addition, bacteria can augment their surface hydrophobicity by modifying their surface components; and this promotes their adhesion to hydrophobic compounds (Watkinson and Morgan 1990).
One of the ways that seems more effective for maintaining the fluidity that is essential for the right functioning of the membranes is the alteration of the movements of the membrane lipids (Herbert 1986). Bacteria are known to adapt to environmental changes such as a change in the growth temperature or growing in the presence of hydrocarbons or aromatic substrates by altering the lipid composition of the cytoplasmic membrane to maintain or adjust the fluidity of the double layer (Keweloh et al. 1990; Sikkema et al. 1995). The effect of the hydrophobic growth substrates is similar to that produced during growth at high temperature and is contrary to the changes observed during growth at low temperature. In the latter condition, the bacteria modulate the viscosity of the lipids to maintain or raise the level of fluidity, reducing saturation either by shortening the aliphatic chains, by increasing the amount of branched fatty acids, or by reducing their saturation. However, the presence of aromatic compounds or substances such as long-chain alcohols raises the level of saturation (Keweloh et al. 1991; Kitagawa et al. 1990). These compounds are toxic for the organisms because they spread mainly in the membranes, raising their fluidity to a specific permeability rate (Heipieper et al. 1991, 1994; Sikkema et al. 1992, 1994).
Membrane fatty acid analysis indicated that SZ raises the degree of saturation of the membrane fatty acid, as was reported also in the case of cells of Ochrobactrum anthropi grown in atrazine (Danilo et al. 1996). The effect of EtOH on the membranes of DSZ cells, at low concentrations (10 mM) was similar; and a slight increase in the degree of saturation was observed when compared with the control (grown on glucose). When DSZ cells were grown on SZ-EtOH (0.025 mM SZ + 10 mM EtOH), the opposite effect was observed: the degree of saturation of the membrane fatty acids decreased in order to maintain optimal membrane fluidity. An explanation of this effect may be the inhibition of the soluble enzymes for the biosynthesis of the saturated fatty acids found in E. coli which was caused by EtOH (Buttke and Ingram 1978, 1980). On account of this inhibition, the organism might be forced to synthesize unsaturated fatty acids.
The biotransformation or mineralization of triazines is a slow process that occurs basically in the cellular cytoplasm, but there is a previous almost instant absorption of the contaminant into the membrane. The hydrophobicity of triazines and other xenobiotics implies a high lipoid affinity, which means that the cellular membrane is probably the first and most important site of the toxic action of liposoluble xenobiotics. The ability of strain DSZ to adhere to solid SZ may be the key mechanism by which this microorganism takes in and degrades SZ at high concentrations. The adhesion observed may be a consequence of the increased cell surface hydrophobicity of strain DSZ observed during growth on SZ. The membrane fatty acid analysis indicated that DSZ cells adapted to growth on SZ by increasing the C18/C16 ratio (more than four-fold), as determined by the increase in C18 fatty acid, and the decrease in C16 fatty acid (Fig. 2). Moreover, the FTIR spectra showed that the fluidity of the membrane at low temperature allowed the solubilization of the herbicide into the membrane and then its assimilation by the cells.
In conclusion, the elimination of SZ by strain DSZ is more effective when it is carried out with EtOH in the culture medium. This offers advantages for its possible use in systems of decontamination on an industrial scale. EtOH is cheaper than other carbon sources; and SZ is readily soluble in this solvent. The toxic effects of SZ are reduced, as shown by the data on the variations in the fatty acids in the membrane. DSZ strain could use SZ at low temperatures since the temperature of phase transition from gel to semifluid crystal state begins at 0°C, so temperature would not restrain the degradation of SZ. This is of special interest when considering the use of DSZ in the decontamination of soil.
References
Buttke TM, Ingram LO (1978) Effects of ethanol on the Escherichia coli plasma membrane. Mechanism of ethanol-induced changes in lipid composition of Escherichia coli: inhibition of saturated fatty acid synthesis in vivo. Biochemistry 17:637–644
Buttke TM, Ingram LO (1980) Mechanism of ethanol-induced changes in lipid composition of Escherichia coli: inhibition of saturated fatty acid synthesis in vitro. Arch Biochem Biophys 203:565–571
Crowe JH, Hoekstra FA, Crowe LM, Anchordoguy TJ, Drobnis E (1989) Lipid phase transitions measured in intact cells with Fourier transform infrared spectroscopy. Cryobiology 26:76–84
Danilo L, De Socio G, Frassanito R, Rotilio D (1996) Effects of atrazine on Ochrobactrum anthropi membrane fatty acids. Appl Environ Microbiol 62:2644–2646
Desai JD, Banat IM (1997) Microbial production of surfactants and their commercial potential. Microbiol Mol Biol Rev 61:47–64
Ernst C, Rehm HJ (1995) Utilization of chlorinated s-triazines by a new strain of Klebsiella pneumoniae. Appl Microbiol Biotechnol 42:763–768
Fetzner S, Lingens F (1994) Bacterial dehalogenases: biochemistry, genetics, and biotechnological applications. Microbiol Rev 58:641–685
Gerhardt PNM, Smith TL, Smith GM (2000) Osmotic and chill activation of glycine betaine porter II in Listeria monocytogenes membrane vesicles. J Bacteriol 182:2544–2550
Gorisch H (2003) The ethanol oxidation system and its regulation in Pseudomonas aeruginosa. Biochim Biophys Acta 1647:98–102
Heipieper HJ, De Bont JAM (1994) Adaptation of Pseudomonas putida S12 to ethanol and toluene at the level of fatty acid composition of membranes. Appl Environ Microbiol 60:4440–4444
Heipieper HJ, Keweloh H, Rehm HJ (1991) Influence of phenols on growth and membrane permeability of free and immobilized Escherichia coli. Appl Environ Microbiol 57:1213–1217
Heipieper HJ, Weber FJ, Sikkema J, Keweloh H, Bont JAM de (1994) Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol 12:409–415
Herbert RA (1986) The ecology and physiology of psychrophilic microorganisms. In: Herbert RA, Codd GA (eds) Microbes in extreme environments. Academic, New York, pp 1–23
Ingram LO (1990) Ethanol tolerance in bacteria. Crit Rev Biotechnol 9:305–319
Keweloh H, Weyrauch G, Rehm HJ (1990) Phenol induced membrane changes in free and immobilized Escherichia coli. Appl Microbiol Biotechnol 33:66–71
Keweloh H, Diefenbach R, Rehm H-J (1991) Increase of phenol tolerance of Escherichia coli by alterations of the fatty acid composition of the membrane lipids. Arch Microbiol 157:49–53
Kitagawa S, Kametani F, Tsuchiya K, Sakurai H (1990) ESR analysis with long-chain alkyl spin labels in bovine blood platelets-relationship between the increase in membrane fluidity alcohols and phenolic compounds and their inhibitory effects on aggregation. Biochim Biophys Acta 1027:123–129
Kretzschmar U, Ruckert A, Jeoung JH, Gorisch H (2002) Malate: quinone oxidoreductase is essential for growth on ethanol or acetate in Pseudomonas aeruginosa. Microbiology 148:3839–3847
Lai MS, Weber AS, Jensen JN (1995) Oxidation of simazine, biological oxidation of simazine and its chemical oxidation by products. Water Environ Res 67:347–354
Leahy JG, Colwell RR (1990) Microbial degradation of hydrocarbons in the environment. Microbiol Rev 54:305–315
Martin M, Mengs G, Allende JL, Fernandez J, Alonso R, Ferrer E (1999) Characterization of two novel propachlor degradation pathways in two species of soil bacteria. Appl Environ Microbiol 65:802–806
Martin M, Mengs G, Plaza E, Garbi C, Sanchez M, Gutierrez F, Ferrer E (2000) Propachlor removal by a soil isolated Pseudomonas strain in immobilized cells system. Appl Environ Microbiol 66:34–39
Martinez B, Tomkins J, Wackett LP, Wing R, Sadowsky MJ (2001) Complete nucleotide sequence and organization of the atrazine catabolic plasmid pADP-1 from Pseudomonas sp. strain ADP. J Bacteriol 183:5684–5697
Martín-Montalvo D, Ferrer E, Mengs G, Allende JL, Alonso R, Martín M (1997) Simazine degradation by immobilized and suspended soil bacterium. Int Biodeterior Biodegrad 40:93–99
Neu TR, Poralla K (1988) An amphiphilic polysaccharide from an adhesive Rhodococcus strain. FEMS Microbiol Lett 49:389–392
Pandey G, Jain RK (2002) Bacterial chemotaxis toward environmental pollutants: role in bioremediation. Appl Environ Microbiol 68:5789–5795
Rosenberg M (1991) Basic and applied aspects of microbial adhesion at the hydrocarbon:water interface. Crit Rev Microbiol 18:159–173
Seffernick JL, De Souza ML, Sadowsky MJ, Wackett LP (2001) Melamine deaminase and atrazine chlorohydrolase: 98 percent identical but functionally different. J Bacteriol 183:2405–2410
Seffernick JL, Shapir N, Schoeb M, Johnson G, Sadowsky MJ, Wackett LP (2002) Enzymatic degradation of chlorodiamino-s-triazine. Appl Environ Microbiol 68:4672–4675
Sikkema J, Poolman B, Konings WN, De Bont JAM (1992) Effects of the membrane action of tetralin on the functional and structural properties of artificial and bacterial membranes. J Bacteriol 174:2986–2992
Sikkema J, De Bont JAM, Poolman B (1994) Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem 269:8022–8028
Sikkema J, De Bont JAM, Poolman B (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59:201–222
Strong LC, Rosendahl C, Johnson G, Sadowsky M, Wackett LP (2002) Arthrobacter aurescens TC1 metabolizes diverse s-triazine ring compounds. Appl Environ Microbiol 68:5973–5980
Struthers JK, Jayachandran K, Moorman TB (1998) Biodegradation of atrazine by Agrobacterium radiobacter J14a and use of this strain in bioremediation of contamined soil. Appl Environ Microbiol 64:3368–3375
Thomas JM, Yordy JR, Amador JA, Alexander M (1986) Rates of dissolution and biodegradation of water-insoluble organic compounds. Appl Environ Microbiol 52:290–296
Watkinson R, Morgan P (1990) Physiology of aliphatic hydrocarbon-degrading microorganisms. Biodegradation 1:79–92
Whyte LG, Slagman SJ, Pietrantonio F, Bourbonniere L, Koval SF, Lawrence JR, Inniss WE, Greer CW (1999) Physiological adaptations involved in alkaline assimilation at a low temperature by Rhodococcus sp. strain Q15. Appl Environ Microbiol 65:2961–2968
Acknowledgements
This research was supported by the Ministerio de Ciencia y Tecnología, Spain (project AGL2002-04003-C03-AGR) and the Comunidad Autonoma de Madrid (grant 08.8/0006/2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sánchez, M., Garbi, C., Martínez-Álvarez, R. et al. Klebsiella planticola strain DSZ mineralizes simazine: physiological adaptations involved in the process. Appl Microbiol Biotechnol 66, 589–596 (2005). https://doi.org/10.1007/s00253-004-1735-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1735-y