Abstract
The biodegradability of various types of diesel oil (DO), such as straight-run DO, light-cycle DO, hydrocracking DO, Fischer–Tropsch DO and commercial DO, was investigated in biodegradation tests performed in closed-batch systems using two microflorae. The first microflora was an activated sludge from an urban wastewater treatment plant as commonly used in biodegradability tests of commercial products and the second was a microflora from a hydrocarbon-polluted soil with possible specific capacities for hydrocarbon degradation. Kinetics of CO2 production and extent of DO biodegradation were obtained by chromatographic procedures. Under optimised conditions, the polluted-soil microflora was found to extensively degrade all the DO types tested, the degradation efficiencies being higher than 88%. For all the DOs tested, the biodegradation capacities of the soil microflora were significantly higher than those of the activated sludge. Using both microflora, the extent of biodegradation was highly dependent upon the type of DO used, especially its hydrocarbon composition. Linear alkanes were completely degraded in each test, whereas identifiable branched alkanes such as farnesane, pristane or phytane were degraded to variable extents. Among the aromatics, substituted mono-aromatics were also variably biodegraded.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because of their wide use, petroleum products are frequent pollutants of soil and groundwater. This situation results mainly from leakages of underground storage tanks or pipelines and from accidental spills (Rosenberg and Ron 1996; Head and Swannell 1999). In a context of environmental protection, laboratory-scale biotreatability studies are quite useful (Skladany and Baker 1994; Salanitro 2001). On the one hand, tests performed in soil microcosms provide data about the kinetics of pollutant degradation (Miles and Doucette 2001), the level of residual contaminant in the polluted zone and the possible toxic by-products or end-products which could accumulate. On the other hand, tests performed in liquid cultures provide crucial information about the intrinsic biodegradability of pollutants and the biodegradation capacity of the local native microflora.
The intrinsic biodegradability of petroleum has been extensively investigated in batch cultures. The substrates used are generally crude oil, hydrocarbon cuts obtained after distillation or individual hydrocarbons. Among the latter, the most representative low-molecular-weight molecules belonging to normal, branched, cyclic alkanes and aromatics were shown to be readily biodegraded by bacterial isolates (Rosenberg and Ron 1996; Di Lecce et al. 1997; Leahy and Olsen 1997; Paje et al. 1997; Meyer et al. 1999). Even peculiar types of molecules containing quaternary carbon atoms or consecutively methylated carbon chains were degraded by micro-organisms using specialised catabolic pathways (Fall et al. 1979; Bhattacharya et al. 2003) and/or by co-metabolism (Beam and Perry 1974). As a result of the high biodegradability of the low-molecular-weight hydrocarbons, gasoline, the lightest liquid oil cut from oil distillation was extensively biodegraded by a large number of various environmental microflorae (Solano-Serena et al. 1999).
In contrast to low-molecular-weight hydrocarbons, polycyclic aromatics and hydrocarbons included in the asphaltene fraction are usually considered as being only slightly biodegradable because of their insufficient availability to microbial attack (Gibson and Subramanian 1984; Cerniglia 1992; Kanaly and Harayama 2000). Among the pentacyclic triterpanes, hopanes (Ourisson et al. 1979) are so stable that they are commonly used as ubiquitous biomarkers for the assessment of biodegradation levels of crude oil. They were shown to be only slightly biodegraded by specialised microflorae under laboratory conditions (Frontera-Suau et al. 2002).
The biodegradation of diesel oil (DO) is more debatable because it is a middle distillate composed of hydrocarbons ranging from C11 to C25. Mineralisation of DO was reported to be complete in air-sparged liquid cultures (Geerdink et al. 1996) and incomplete in agitated flasks (Olson et al. 1999); and it was generally accepted that degradation of DO hydrocarbons in soil microcosms was incomplete (Chaineau et al. 1995; Gallego et al. 2001; Seklemova et al. 2001). This partial recalcitrance possibly resulted from the low intrinsic biodegradability of DO, a limitation in hydrocarbon transfer or an insufficient amount of available dioxygen. Furthermore, numerous degradation tests were performed in open systems where substantial amounts of substrate might disappear by volatilisation or irreversible adsorption to soil, as indicated by Song et al. (1990).
In fact, commercial DO is a complex hydrocarbon mixture of thousands of individual components. Its hydrocarbon composition depends on the origin of the crude oil used for the distillation process (straight-run DO), on the refining processes of crude oil and on the mixtures added by the refiner for final formulation. In the present study, the aerobic biodegradability of DOs with different hydrocarbon compositions was examined. Accordingly, the DOs used in the present work originate from different refinery processes, to investigate the intrinsic biodegradability of DO over the largest composition range and to address the suitability of these DOs to biodegradation in the environment. In biodegradation tests, we used two different microflorae. The first was an activated sludge from an urban wastewater treatment plant, as usually recommended by standard test procedures. The second was a hydrocarbon-polluted-soil microflora that possibly harboured acclimated species with specialised degradation capacities.
Materials and methods
Culture media and microflorae
The vitamin-supplemented mineral salt medium described by Bouchez et al. (1995) was used as a nutrient solution. Each DO type was added at 400 mg l−1 as a sole carbon source. Two microbial suspensions were used in the degradation tests. The first was a microflora from an urban wastewater treatment plant, obtained by centrifugation of an activated sludge at 15,000 g for 10 min (3 g l−1, dry weight). It was used after re-suspension into nutrient solution at a final concentration of 100 mg l−1 (dry weight). Sludge pellets were stored at −80°C for several months without significant loss of degradative capacity. The second suspension was a microflora from a DO-polluted clay-like gravel. The soil dry mass obtained by weight loss after heat treatment was 73%. The pH value determined according to the NF ISO 10390 standard was 6.05. The hydrocarbon content determined by gas chromatography (GC) with flame ionisation detector (FID) after cyclohexane:acetone (85:15, v/v) extraction was 10 g kg−1 dry soil. Microbial suspensions for biodegradation tests were prepared by dispersing 5 g l−1 of soil sample into the nutrient solution.
Diesel oil characteristics
The DOs involved in commercial DO formulation derive from refining processes. Straight-run DO was obtained from distillation units. Hydrocracking DO and light-cycle DO came from conversion units, hydrocracking and fluid catalytic cracking, respectively, converting heavy fractions to light ones. Fischer–Tropsch DO was a synthetic fuel produced by CO plus H2 condensation (Guibet 1997). Supplemented DO was a hydrocracking DO with a light aromatics cut.
Biodegradation tests
Biodegradation tests were generally performed for 4 weeks at 30°C in 120-ml agitated flasks closed with Teflon-coated stoppers and sealed with aluminium caps. Tests were started by the addition of 5 μl of DO to 10 ml of inoculated culture medium. The overall degradation kinetics were monitored at regular intervals with GC analysis of headspace CO2. Endogenous respiration was similarly monitored in control flasks without DO addition. Experiments were performed in five flasks and abiotic controls supplemented with HgCl2 were run under similar conditions. At the end of the incubation period, 10 ml of dichloromethane were introduced into the flasks, which were stored overnight at −20°C before extraction. The organic phase was evaporated to 1.5 ml and the residual DO was analysed by GC-FID, with dotriacontane (nC32) at 50 mg l−1 in dichloromethane as an internal standard. The final CO2 production was determined in the flask headspace by GC after acidification of the medium with 200 μl of 4 N HCl.
The DO degradation yield was calculated as the ratio of the amount of substrate degraded in test flasks to the amount of substrate recovered in abiotic controls. The net CO2 production was the difference between the final quantities of CO2 in the test flask and in the hydrocarbon-free flask. The mineralisation yield was the carbon ratio of the net CO2 produced to the DO consumed.
GC analyses
CO2 was measured using a GC equipped with a thermal conductivity detector and a Porapak Q column (80/100 mesh, 2 m), using an external standard method (Solano-Serena et al. 1999).
DOs were analysed with a 3400 GC (Varian, USA) equipped with a FID and a DB-5 column (60 m), using helium as the carrier gas. The detector temperature was 310°C. The column temperature was first set at 50°C for 10 min and then increased to 310°C at 2°C min−1. The injector temperature was initially 50°C for 0.2 min and then increased to 280°C at 180°C min−1. Quantification of DO was obtained using JMBS software (BORWIN) by integrating the global area of the separated peaks and unresolved-mass complex (UMC) to the baseline.
Fractions containing saturated and aromatic hydrocarbons of DOs were separated by liquid adsorption chromatography (LC). Mini-columns of Pasteur pipettes containing regenerated silica (1 g of SiO2, 60:70–230 mesh from Merck) were used. At the top of the column, 0.5 g of Na2SO4 was added and chromatography was carried out using 20 mg of DO. Saturated alkanes (linear, branched and cyclic alkanes) were eluted with 1.95 ml of hexane and 0.65 ml of a hexane:dichloromethane mixture (4:1, v/v). Aromatics were eluted with 3.25 ml of a hexane:dichloromethane mixture (1:1, v/v). Fractions were then quantified by GC-FID using a Varian 3400 GC and a DB-5 column (60 m), as previously described for DOs.
The structural hydrocarbon classes of DOs were determined by HPLC with refractometric identification (RI), using a Prostar system (Varian, USA) and a silica-NH2 column. Operating conditions were similar to the NF EN 12916 standard.
Results
Composition of DOs
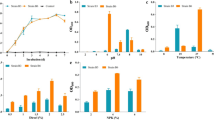
The composition of the DOs used was determined using two methods. Saturated alkanes (fraction S) and aromatics (fraction A) were separated by LC and each fraction was quantified by GC-FID (Fig. 1). In fraction S (Fig. 1A), all the linear alkanes were identified. Branched hydrocarbons were not identifiable but they were globally quantified, assuming that their responses in the FID were identical to those of linear alkanes. The aromatics content present in fraction A was evaluated using the same method as that used for branched alkanes (Fig. 1B).
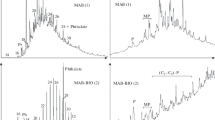
DOs were also analysed using HPLC with RI detection (Fig. 2). Aliphatic hydrocarbons were first eluted at a retention time (RT) of 9 min. Mono-aromatics and tri-aromatics were eluted as single peaks at RTs of 12 min and 16 min, respectively. Di-aromatics were resolved into several peaks ranging from 17 min to 26 min RT, depending on the substituents of their aromatic rings.
HPLC pattern of light-cycle DO. Hydrocarbon classes are indicated: a saturated hydrocarbons, b mono-aromatics, c di-aromatics, d tri-aromatics. A silica-NH2 column was used as described in the Materials and methods. The heptane flow was 0.5 ml min−1
Data from HPLC separation with RI detection was in agreement with those from LC separation with GC-FID quantification. Table 1 summarises the main characteristics of the DOs used. Fischer–Tropsch DO was mainly composed of linear hydrocarbons, while hydrocracking DO and light-cycle DO were principally constituted of aromatics. Straight-run DO and commercial DO contained large amounts of branched and cyclic alkanes.
Degradation of commercial DO by two different microflorae
The two microflorae tested were an activated sludge and the microflora from a polluted soil. Since no standard procedure was available for the use of soil microflorae in biodegradability tests, the experimental conditions had to be defined first. Preliminary experiments indicated that the best procedure for seeding was to use soil aliquots as inocula of test cultures. Figure 3A shows the effect of the initial amount of commercial DO on mineralisation kinetics. The final CO2 produced in test flasks increased linearly with the initial amount of DO introduced (Fig. 3B). Nevertheless, a substantial amount of CO2 was produced in the absence of DO, indicating that the soil amount used for seeding had to be reduced to minimise endogenous respiration. Endogenous respiration was adequately limited by using 5 g l−1 of soil. The kinetics of substrate mineralisation determined after deduction of endogenous CO2 were found to be similar using 5 g l−1 or 50 g l−1 of soil. However, using 1 g l−1 of soil as an inoculum, the mineralisation kinetics were substantially slowed down and did not ensure reproducible end-point values (data not shown).
Effect of the initial commercial DO concentration. A Effect on the biodegradation kinetics of CO2 production. B Correlation between final CO2 production and initial DO. Initial DO concentrations were: 0 mg l−1 (open triangles), 40 mg l−1 (open squares), 80 mg l−1 (open circles), 160 mg l−1 (filled triangles), 240 mg l−1 (filled circles), 400 mg l−1 (filled squares). The tests were performed in 120-ml flasks with 10 ml of medium and were inoculated using 5 g l−1 of soil. The incubation time was 30 days. L Litres
At the end of the incubation period, residual DO hydrocarbons were analysed by GC-FID (Fig. 4). All linear alkanes were completely degraded within 3 days (data not shown) by the polluted-soil microflora. Some identifiable branched alkanes, such as farnesane, pristane and phytane, were also totally degraded. However, the UMC was only partially degraded and, in particular, the long-chain hydrocarbons of the UMC were still present. Activated sludge seemed to be less efficient than polluted soil. Even though n-alkanes were completely removed, some identifiable branched alkanes, such as farnesane, pristane and phytane, were detected by GC at the end of the incubation period (Fig. 4C). Compared with the residual hydrocarbons from the tests carried out with the soil microflora, the amount of UMC hydrocarbons was higher and hydrocarbons of lower-molecular-weight were still present.
As shown by the DO compositions determined by HPLC (Table 2), there was an increase in the relative abundance of saturated compounds (83% and 73% for the polluted soil and the activated sludge, respectively) compared with that of the initial content (64%), which accounted for the recalcitrance of branched and cyclic alkanes. Aromatics were more extensively degraded than branched and cyclic alkanes, especially with the soil microflora. Mono-aromatics were more abundant than di- and tri-aromatics in the final solution, suggesting the presence of highly alkylated mono-aromatics with low degradation properties.
Biodegradability of various DO types
The degradation performances of both microflorae were investigated for each DO type (Table 3). Abiotic recovery rates in all assays were higher than 75%, except for the light-cycle DO which contained many volatile compounds. Each DO type was biodegraded to a large extent by the polluted-soil microflora (88–95%). For all type of DO tested, this microflora was more efficient than the one from activated sludge. Therefore, the effect of the type of DO on biodegradation performances was much more pronounced using activated sludge, since its biodegradation rates varied from 45% to 82%. Supplementation of hydrocracking DO with light mono-aromatics increased the biodegradation rate from 61% to 82%, probably because the aromatics added were slightly water-soluble and thus easily biodegradable. Fischer–Tropsch DO contained a large amount of linear and slightly branched alkanes and was also extensively biodegraded. Similarly, the biodegradability of commercial DO was higher than that of straight-run DO because of a higher content in linear alkanes.
The mineralisation yields of DOs by the microflorae are shown in Table 3. For all types of DO, the mineralisation yields were higher than 50%. Taking into account that a significant fraction of the carbon source is used for cell biomass, this shows that only limited amounts of metabolic intermediates accumulated during the degradation of DOs.
Discussion
In soils contaminated by petroleum compounds, the extent of natural attenuation mainly depends on the hydrocarbons present in the contaminated matrix (Husemann 1995). DO is a potential pollutant of soils and its composition may vary according to the processes used for its preparation. Hence, the biodegradability of various types of DO was studied in the present work.
The tests used for the determination of biodegradability of commercial products, such as those of OECD, are not suitable for petroleum products since they were designed for water-soluble compounds with low volatility properties. An easy-to-use test was developed for oil products by CONCAWE, i.e. the oil companies’ European organisation for the environment, health and safety (Battersby et al. 1999). Although highly reproducible, this test, based on CO2 production from low amounts of substrate, does not allow the measurement of residual hydrocarbons. The methodology used in the present study is based on both the determination of residual hydrocarbons and the production of CO2. Residual hydrocarbons are obtained by means of GC-FID, using an internal standard to prevent overestimation of the degree of biodegradation, as recommended for crude oil by Vinas et al. (2002). Our analytical procedure was quite satisfactory, as shown by the hydrocarbon recoveries measured in abiotic flasks (higher than 75% except for light cycle DO). Moreover, the mineralisation yield was estimated in order to know whether metabolic intermediates accumulated or not.
Using activated-sludge microflora, the biodegradation rates determined for each DO type were lower than those determined using polluted-soil microflora and showed more variability. This indicates that activated-sludge microflorae from wastewater treatment plants may be not appropriate for measuring the biodegradation of oil products and that adapted microflorae are probably more suitable, as indicated by Battersby et al. (1999). Moreover, the microflora from polluted soil used in the present study seems to be adapted to the degradation of recalcitrant hydrocarbons. In the environment, hydrocarbon-degrading micro-organisms are found ubiquitously (Jones and Edington 1968; Rosenberg 1992). The extended degradation capacities of polluted-soil microflora compared with those of the activated-sludge microflora account for the probable occurrence of a selective adaptation of the native microflora (Liu and Suflita 1993; Atlas 1995).
The relative recalcitrance of hydrocarbon types was illustrated in the tests performed with both microflorae. For each type of DO tested, linear alkanes were totally degraded within 1–2 days (data not shown). Recalcitrant hydrocarbons include mainly branched alkanes and aromatics (McKenna and Kallio 1971; Pirnik et al. 1974; Fall et al. 1979; Nakajima et al. 1985; Rontani et al. 1986). Moreover, mineralisation yields were higher than 50% in each case (Table 3). Considering that the mineralisation value of extensively degraded hydrocarbons such as n-hexadecane was around 70% (Battersby et al. 1999), it could be deduced that, apart from CO2 production, biomass formation may account for a large part of the carbon balance and that the accumulation of metabolic intermediates from DO was rather limited.
There is little data in the literature on the biodegradability of DO in closed liquid systems. Using an adapted bacterial consortium produced by subculturing, Richard and Vogel (1999) found that 90% of a commercial DO was biodegraded within 50 days. A similar extent of degradation was obtained by Marquez-Rocha et al. (2001) within 2 weeks. In the present study, the data obtained with commercial DO using a soil sample as an inoculum are in agreement with those results. Furthermore, the level of biodegradation (higher than 88%) obtained with all the DO types tested indicates that DO with its variable hydrocarbon composition is a highly biodegradable product. The biodegradation test developed in this work constitutes a supplementary and useful tool to study efficiency in pollutant attenuation.
Various methods have been proposed to characterise the hydrocarbon-degrading microflorae of soil (Theron and Cloete 2000; Widada et al. 2002; Margesin et al. 2003). Numerous studies have been used to investigate the composition of the microflorae involved in hydrocarbon degradation, such as culturing and molecular techniques based on 16S rRNA analysis or the characterisation of metabolic genes. In this study, we demonstrate an important difference in the biodegradation capacities of two microflorae (activated sludge, polluted soil); and experiments are in progress in our laboratory to determine the community structure of soil microflora.
References
Atlas RM (1995) Bioremediation of petroleum pollutants. Int Biodeterior Biodegrad 21:317–327
Battersby NS, et al (1999) An “inherent” biodegradability test for oil products: description and results of an international ring test. Chemosphere 38:3219–3225
Beam HW, Perry JJ (1974) Microbial degradation of cycloparaffinic hydrocarbons via co-metabolism and commensalism. J Gen Microbiol 82:163–169
Bhattacharya D, Sarma PMS, Krishnan S, Mishra S, Lal B (2003) Evaluation of genetic diversity among Pseudomonas citronellolis strains isolated from oily sludge-contaminated sites. Appl Environ Microbiol 69:1435–1441
Bouchez M, Blanchet D, Vandecasteele JP (1995) Degradation of polycyclic aromatic hydrocarbons by pure strains and by defined strain associations: inhibition phenomena and cometabolism. Appl Microbiol Biotechnol 43:156–164
Cerniglia CE (1992) Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 3:351–368
Chaineau CH, Morel JL, Oudot J (1995) Microbial degradation in soils microcosms of fuel oil hydrocarbons from drilling cuttings. Environ Sci Technol 29:1615–1621
Di Lecce CD, Accarino M, Bolognese F, Galli E, Barbieri P (1997) Isolation and metabolic characterization of a Pseudomonas stutzeri mutant able to grow on the three isomers of xylene. Appl Environ Microbiol 63:3279–3281
Fall RR, Brown JL, Schaeffer TL (1979) Enzyme recruitment allows the biodegradation of recalcitrant branched hydrocarbons by Pseudomonas citronellis. Appl Environ Microbiol 38:715–722
Frontera-Suau R, Bost F, Mc Donnald T, Morris PJ (2002) Aerobic biodegradation of hopanes and other biomarkers by crude oil-degrading enrichment cultures. Environ Sci Technol 36:4585–4592
Gallego JL, Loredo J, Llamas JF, Vazquez F, Sanchez J (2001) Bioremediation of diesel-contaminated soils: evaluation of potential in situ techniques by study of bacterial degradation. Biodegradation 12:325–335
Geerdink MJ, Loosdrecht MCM van, Luyben KCAM (1996) Biodegradability of diesel oil. Biodegradation 7:73–81
Gibson DT, Subramanian V (1984) Microbial degradation of aromatic hydrocarbons. In: Gibson DT (ed) Microbial degradation of organic compounds. McGraw–Hill, New York, pp 181–252
Guibet JC (1997) Technologies, energie, environnement. (Carburants et moteurs, vol 2) Technip, Paris
Head IM, Swannell RP (1999) Bioremediation of petroleum hydrocarbon contaminants in marine habitats. Curr Opin Biotechnol 10:234–239
Husemann MH (1995) Predictive model for estimating the extent of petroleum hydrocarbon biodegradation in contaminated soils. Environ Sci Technol 29:7–18
Jones JG, Edington MA (1968) An ecological survey of hydrocarbons-oxidizing micro-organisms. J Gen Microbiol 52:381–390
Kanaly RA, Harayama S (2000) Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol 182:2059–2067
Leahy JG, Olsen RH (1997) Kinetics of toluene degradation by toluene-oxidizing bacteria as a function of oxygen concentration, and the effect of nitrate. Microb Ecol 23:23–30
Liu S, Suflita JM (1993) Ecology and evolution of microbial populations for bioremediation. Trends Biotechnol 11:344–352
Margesin R, Labbé D, Schinner F, Greer CW, Whyte LG (2003) Characterization of hydrocarbon-degrading microbial populations in contaminated and pristine alpine soils. Appl Environ Microbiol 69:3085–3092
Marquez-Rocha FJ, Hernandez-Rodriguez V, Lamela MT (2001) Biodegradation of diesel oil in soil by a microbial consortium. Water Air Soil Pollut 128:313–320
McKenna EJ, Kallio RE (1971) Microbial metabolism of the isoprenoid alkane pristane. Proc Natl Acad Sci USA 68:1552–1554
Meyer S, Moser R, Neef A, Stahl U, Kämpfer P (1999) Differential detection of key enzymes of polyaromatic-hydrocarbon-degrading bacteria using PCR and gene probes. Microbiology 145:1731–1741
Miles RA, Doucette WJ (2001) Assessing the aerobic biodegradability of 14 hydrocarbons in two soils using a simple microcosm/respiration method. Chemosphere 45:1085–1090
Nakajima K, Sato A, Takahara Y, Iida T (1985) Microbial oxidation of isoprenoid alkanes, phytane, norpristane and farnesane. Agric Biol Chem 49:1993–2002
Olson JJ, Mills G, Herbert BE, Morris PJ (1999) Biodegradation rates of separated diesel components. Environ Toxicol Chem 18:2448–2453
Ourisson G, Albrecht P, Rohmer M (1979) The hopanoids. Paleochemistry and biochemistry of a group of natural products. Pure Appl Chem 51:709–729
Paje MLF, Neilan BA, Couperwhite I (1997) A Rhodococcus species that thrives on medium saturated with liquid benzene. Microbiology 143:2975–2981
Pirnik MP, Atlas RM, Bartha R (1974) Hydrocarbon metabolism by Brevibacterium erythrogenes: normal and branched alkanes. J Bacteriol 119:868–878
Richard JY, Vogel TM (1999) Characterization of soil bacterial consortium capable of degrading diesel fuel. Int Biodeterior Biodegrad 44:93–100
Rontani JF, Bertrand JC, Blanc F, Giusti G (1986) Gas chromatography and gas chromatography/mass spectrometry applied to the determination of a new pathway of pristane degradation by a marine mixed bacterial population. Mar Chem 18:9–16
Rosenberg E (1992) The hydrocarbon-oxidizing bacteria. Springer, Berlin Heidelberg New York, pp 446–459
Rosenberg E, Ron EZ (1996) Bioremediation of petroleum contamination. Cambridge University, Cambridge, pp 100–125
Salanitro JP (2001) Bioremediation of petroleum hydrocarbons in soil. Adv Agron 72:53–105
Seklemova E, Pavlova A, Kovacheva K (2001) Biostimulation-based bioremediation of diesel fuel: field demonstration. Biodegradation 12:311–316
Skladany GJ, Baker KH (1994) Laboratory biotreatability studies. McGraw–Hill, New York, pp 97–172
Solano-Serena F, Marchal R, Ropars M, Lebeault JM, Vandecasteele JP (1999) Biodegradation of gasoline: kinetics, mass balance and fate of individual hydrocarbons. J Appl Microbiol 86:1008–1016
Song HC, Wang X, Bartha R (1990) Bioremediation potential of terrestrial fuel spills. Appl Environ Microbiol 56:652–656
Theron J, Cloete TE (2000) Molecular techniques for determining microbial diversity and community structure in natural environments. Crit Rev Microbiol 26:37–57
Vinas M, Grifoll M, Sabate J, Solanas AM (2002) Biodegradation of a crude oil by three microbial consortia of different origins and metabolic capabilities. J Ind Microbiol Biotechnol 28:252–260
Widada J, Nojiri H, Kasuga K, Yoshida T, Habe H, Omori T (2002) Molecular detection and diversity of polycyclic aromatic hydrocarbon-degrading bacteria isolated from geographically diverse sites. Appl Microbiol Biotechnol 58:205–209
Acknowledgements
We thank Véronique Bardin and Bernard Chaussepied for technical assistance in GC and HPLC, Vincent Genet for experimental work, Susan Cure for reading the manuscript and Denis Le Paslier for helpful discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Penet, S., Marchal, R., Sghir, A. et al. Biodegradation of hydrocarbon cuts used for diesel oil formulation. Appl Microbiol Biotechnol 66, 40–47 (2004). https://doi.org/10.1007/s00253-004-1660-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1660-0