Abstract
Antibiotic production in many streptomycetes is influenced by extracellular γ-butyrolactone signalling molecules. In this study, the gene scbA, which had been shown previously to be involved in the synthesis of the γ-butyrolactone SCB1 in Streptomyces coelicolor A3(2), was deleted from the chromosome of Streptomyces lividans 66. Deletion of scbA eliminated the production of the antibiotic stimulatory activity previously associated with SCB1 in S. coelicolor. When the S. lividans scbA mutant was transformed with a multi-copy plasmid carrying the gene encoding the pathway-specific activator for either actinorhodin or undecylprodigiosin biosynthesis, production of the corresponding antibiotic was elevated significantly compared to the corresponding scbA + strain carrying the same plasmid. Consequently, deletion of scbA may be useful in combination with other strategies to construct host strains capable of improved bioactive metabolite production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Actinomycetes produce approximately two-thirds of all known antibiotics of microbial origin. Over 6,000 of these compounds are produced by Streptomyces species and many are commercially important medicinal products used therapeutically as anti-infective (antibiotics, antifungal and antiparasitic), anticancer or immunosuppressant agents (Champness 2000).
Many factors affect the level of production of a particular compound in a specific host. Classically, strain improvement has been achieved by empirical methods usually involving random mutation and subsequent screening for higher-producing progeny. With the information available from the Streptomyces coelicolor A3 (2) genome sequence (http://www.sanger.ac.uk/Projects/S_coelicolor/), it is now possible to attempt more rational approaches to increase yield (Chater et al. 1990). As a model system for strain improvement, we have used the closely related Streptomyces lividans 66, which has been engineered to overproduce either the polyketide actinorhodin (ACT) or the tri-pyrrole undecylprodigiosin (RED). This was achieved by introducing a multi-copy plasmid carrying the gene encoding the pathway-specific regulatory gene for either biosynthetic gene cluster. This leads to the activation of the normally silent act pathway in the S. lividans chromosome (Bruheim et al. 2002), and a substantial increase in the normally poorly expressed red pathway (Takano et al. 1992). Thus, these strains can be viewed as models for strains producing bioactive molecules that have already been subjected to some strain improvement to increase the expression of genes encoding biosynthetic enzymes, but in which some other aspect of host metabolism may still be limiting product formation.
Extracellular γ-butyrolactone signalling molecules are known to be required for streptomycin production and morphological differentiation in Streptomyces griseus (Horinouchi and Beppu 1994) and for virginiamycin production in Streptomyces virginiae (Kondo et al. 1989). Precocious production of ACT and RED was observed when scbA, a homologue of a gene (afsA) involved in γ-butyrolactone biosynthesis in S. griseus, was deleted in S. coelicolor (Takano et al. 2001). Surprisingly, this phenotype is the opposite of that of the S. griseus afsA mutant (which is no longer able to produce streptomycin). To test whether the disruption of scbA could be a generally applicable method for increasing antibiotic production, we examined the effect of making the same mutation in S. lividans overproducing ACT or RED.
Materials and methods
Bacterial strains and plasmids
Escherichia coli strains DH5α (Sambrook et al. 1989) and ET12567 (MacNeil et al. 1992) were used for routine subcloning and conjugation into S. lividans, respectively. Growth was at 37 °C in Luria-Bertani medium and transformation was carried out by standard procedures (Sambrook et al. 1989). E. coli transformants were selected with carbenicillin (100 μg ml−1), apramycin or hygromycin (both at 50 μg ml−1). S. lividans 66 (referred to in this paper by its John Innes Centre strain number 1326) and S. coelicolor M145 were grown and manipulated as described previously (Kieser et al. 2000). Conjugation from E. coli used the helper plasmid pUZ8002 (Paget et al. 1999). S. lividans exconjugants were selected by adding 0.5 mg of nalidixic acid and 1 mg of apramycin in 1 ml of water to each agar plate. Strains were purified and maintained using 20 μg apramycin ml−1 or 50 μg of thiostrepton ml−1.
The scbA deletion plasmid pIJ6140 (Takano et al. 2001) was based on pKC1132 (Bierman et al. 1992). The multi-copy plasmids pIJ68 (Passantino et al. 1991) and pIJ6014 (Takano et al. 1992), carrying the pathway-specific activator genes actII-orf4 and redD, respectively, were as described previously. The plasmid vector pIJ486 was described by Kieser et al. (2000).
Growth and fermentation conditions
Spores of the transformed strains were initially selected on R2 agar (containing 50 μg thiostrepton ml−1). Spores were streaked on MS (mannitol soya flour medium) agar (containing 50 μg thiostrepton ml−1 ) to generate dense spore suspensions for inoculation into 50 ml of a mixture of two parts YEME (yeast extract-malt extract medium) and one part TSB (tryptone soya broth) liquid medium (again containing 50 μg thiostrepton ml−1 ) in a 250-ml conical spring-flask. After 2 days shaking at 30 °C, the mycelium was collected by centrifugation and resuspended in fresh spring-flasks containing phosphate-limited Evans medium (Evans et al. 1970) with 20 μg thiostrepton ml−1. Incubation was continued at 30 °C for a further 7 days with 1 ml samples being removed for assessment of antibiotic production.
For stirred-tank fermentation experiments, strains were stored as frozen mycelium at −80 °C. The stock cultures were used to inoculate (1% v/v) 100 ml GG1 medium (per litre: 15 g glucose,15 g glycerol, 15 g soy peptone, 3 g NaCl, 1 g CaCO3, 0.005 g thiostrepton) in 500-ml baffled Erlenmeyer flasks. Cultures were grown for 48 h at 28 °C on a rotary shaker at 200 rpm. Eight ml of the culture were used to inoculate 100 ml GYB medium (per litre: 33 g glucose, 15 g yeast extract, 0.005 g thiostrepton) in 500-ml baffled flasks. Cultures were grown for 24 h at 28 °C on a rotary shaker at 200 rpm and 100 ml were used to inoculate 4-l fermenters containing 2.5 l of phosphate-limited Evans medium.
Cultivation conditions (using 3-l Applicon fermenters with a working volume of 1 l) for production of ACT by S. lividans 1326/pIJ68 in a phosphate-limited minimal medium (containing 10 g glucose per litre) were as described previously (Bruheim et al. 2002).
RED production was carried out in 4-l Chemap fermenters with 2.5 l of phosphate limited-Evans minimal medium containing 2 mM NaH2PO4 and 25 g glucose per litre(Butler et al. 2002). For determination of dry cell weight (DCW), 2 ml samples of culture were vacuum-filtered through a pre-weighed Whatman GF/C filter paper, washed with 10 ml distilled water, and dried at 90 °C to constant weight. All measurements were done in triplicate.
Antibiotic and metabolite estimations
ACT was measured as absorbance at 640 nm (ε640=25,320) as described by Bystrykh et al. (1996). RED production was analysed by HPLC following extraction with acidified methanol and using a sample of pure undecylprodigiosin as external standard (Butler et al. 2002).
Isolation of γ-butyrolactones and bioassay analysis
γ-Butyrolactones were isolated from liquid or solid media (SMMS, supplemented liquid minimal medium with 1.5% w/v agar) by extracting the culture supernatant or agar with ethyl acetate. The solvent was then evaporated and the samples were resuspended in methanol for use in bioassays as described previously (Takano et al. 2000).
Results
Construction of a S. lividans scbA deletion mutant
The deletion mutant allele of scbA from S. coelicolor (Takano et al. 2001) was introduced into S. lividans 1326 by conjugation of pIJ6140 from E.coli ET12567. Integration of the non-replicating plasmid was selected using apramycin. Purified apramycin-resistant exconjugants were grown without antibiotic selection for three rounds of sporulation (on MS agar). Colonies were screened for sensitivity to apramycin, indicating loss of the plasmid by a second homologous recombination event. Four apramycin-sensitive colonies were identified among 3,000 colonies screened. PCR analysis of chromosomal DNA produced amplified DNA fragments consistent with the wild-type chromosomal arrangement for three colonies, whereas the fourth colony yielded a smaller DNA fragment consistent with the in-frame deletion allele (ΔscbA). Southern hybridisation experiments on chromosomal DNA digested either with NcoI or a mixture of BglII and PstI produced hybridizing bands consistent with the wild-type from the first three colonies and with the ΔscbA mutant from the fourth colony. The mutant was designated S. lividans M707.
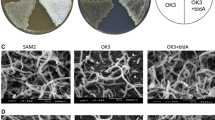
No γ-butyrolactone with antibiotic stimulatory activity on S. coelicolor M145 was detected in ethyl acetate extracts of M707 grown on agar medium (Fig. 1), whereas stimulatory activity (visualised as a ring of pigmented mycelial growth in Fig. 1) was produced by S. lividans 1326 (scbA +). Thus, the phenotype of the ΔscbA mutation in S. lividans with respect to the production of antibiotic stimulatory factors was indistinguishable from that observed previously in S. coelicolor (Takano et al. 2001).
Production of antibiotics in shake flask fermentations using S. lividans M707
Analysis of growth and antibiotic production of an scbA mutant of S. coelicolor M145 had previously shown precocious production of both ACT and RED (Takano et al. 2001). We therefore examined whether increased levels of antibiotic production could be obtained in scbA mutants of S. lividans that had been engineered to over-produce either ACT or RED by cloning the relevant pathway-specific activator gene on a multi-copy plasmid.
Protoplasts of S. lividans strain M707 were transformed with pIJ68 (actII-orf4), pIJ6014 (redD) or pIJ486 (vector control), selecting in each case for the plasmid-borne thiostrepton-resistance gene. The effect of the scbA mutation on ACT production was assessed initially in shake-flask fermentation experiments using Evans liquid medium. No ACT was detected in either 1326 or M707 containing the control vector pIJ486 (data not shown). M707/pIJ68 produced ACT at an eight-fold higher concentration (12.1±1.0 mg ACT per g of mycelial DCW) than did 1326/pIJ68 (1.5±0.8 ACT per g of DCW), at a faster rate, and for a longer period of time (Fig. 2).
RED production in shake-flasks was also assessed (Fig. 3). RED production was approximately three times higher in M707/pIJ6014 than in 1326/pIJ6014 (biomass levels were not significantly different, data not shown). As for ACT production, RED synthesis occurred at a faster rate for a longer time period in the ΔscbA mutant. M707 and 1326 carrying the control vector (pIJ486) did not produce RED under the experimental conditions used (data not shown). Replicate experiments produced essentially the same levels of RED (±10%) as those shown in Fig. 3.
Production of antibiotics in stirred-tank fermenters using S. lividans M707
The strains were further tested for their ability to produce antibiotics in stirred-tank batch fermentations. M707/pIJ6014 and 1326/pIJ6014 were cultured in 4-l stirred-tank fermenters in phosphate-limited Evans medium (Fig. 4). The two strains showed similar growth kinetics and reached a maximum biomass concentration of approximately 5 g per litre. The onset of RED production coincided with the beginning of stationary phase. While the initial rates of RED synthesis were similar for both strains, the production phase of M707/pIJ6014 continued for an additional 20 h, resulting in approximately 50% more RED production in the ΔscbA mutant.
Similarly, in three independent experiments (data not shown), an approximately two-fold improvement in ACT production was observed in 1 l fermentations with M707/pIJ68 (10±0.5 g ACT per litre) compared to 1326/pIJ68 (5±0.5 g per litre).
Discussion
Eliminating the ability of S. lividans to synthesise γ-butyrolactone signalling molecules capable of stimulating antibiotic production in S. coelicolor resulted in improved production of both ACT and RED in strains already engineered to produce substantial amounts of each antibiotic. The molecular events involved in the control of antibiotic biosynthesis by γ-butyrolactones (and their associated binding proteins) in S. coelicolor, S. lividans, and other streptomycetes are not known (e.g. Takano et al. 2001; Stratigopoulos and Cundliffe 2002). S. coelicolor scbA mutants show markedly reduced levels of transcription of the putative repressor protein ScbR (Takano et al. 2001), but there is currently no evidence to suggest that this mutation results in elevated levels of transcription of the act and red genes. Conceivably, the scbA mutation might reduce the level of expression of metabolic pathways that compete with the act and red biosynthetic enzymes for metabolic precursors. In this context, it is interesting to note that deletion of scbA in S. coelicolor results in reduced expression of a type I polyketide synthase gene cluster (Kotowska et al. 2002; E. Takano, unpublished results). The product of this gene cluster is not known, but the overproduction of ACT and RED observed in the scbA mutant may at least partially reflect its reduced expression and elevated levels of metabolic precursors. This interpretation would also be consistent with results obtained for S. coelicolor, in which deletion of actII-orf4 resulted in enhanced RED production, and deletion of redD resulted in increased ACT synthesis (B. Floriano and M. Bibb, unpublished results). This effect was subsequently confirmed for RED production in fermenters by comparing the levels produced by S. coelicolor M145 (28 mg per litre) with those of its ΔactII-orf4 derivative M511 (120 mg per litre; S. Jovetic and F. Marinelli, unpublished results). Alternatively, it is conceivable that scbA plays a role in regulating aspects of primary metabolism that influence levels of secondary metabolite production.
Irrespective of the physiological role of scbA, it may be useful to combine mutations in this gene with other approaches that have been used to enhance antibiotic production (Butler et al. 2002; Chen et al. 2000; Lombo et al. 2001; Minas et al. 1998; Pfeifer et al. 2001), thereby improving S. lividans as a host for the production of a wide range of secondary metabolites.
References
Bierman M, Logan R, O'Brien K, Seno ET, Rao NR, Schoner B (1992) Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43–49
Bruheim P, Sletta H, Bibb MJ, White J, Levine DW (2002) High yield actinorhodin production in fed-batch culture by a Streptomyces lividans strain over-expressing the pathway-specific activator gene actII-ORF4. J Ind Microbiol Biotechnol 28:103–11
Butler MJ, Bruheim P, Jovetic S, Marinelli F, Postma P, Bibb MJ (2002) Engineering of primary carbon metabolism for improved antibiotic production in Streptomyces lividans. Appl Env Microbiol 68:4731–4739
Bystrykh LV, Fernandez-Moreno MA, Herrema JK, Malpartida FM, Hopwood DA, Dijkhuizen L (1996) Production of actinorhodin-related "blue pigments" by Streptomyces coelicolor A3(2). J.Bacteriol. 178:2238–2244
Champness W (2000) Actinomycete development, antibiotic production and phylogeny: questions and challenges. In: Brun YV, Skimkets LJ (eds) Prokaryotic development. American Society for Microbiology , Washington DC, pp11–31
Chater KF (1990) The improving prospects for yield increase by genetic engineering in antibiotic-producing streptomycetes. Biotechnology 8:115–121
Chen G, Wang G-Y, Li X, Waters B, Davies JE (2000) Enhanced production of microbial metabolites in the presence of dimethyl sulfoxide. J Antibiotics 53:1145–1153
Evans CGT, Herbet D, Tempest DW (1970) The continuous culture of microorganisms 2. Construction of a chemostat. In: JR Norris, Ribbons DW (eds) Methods in microbiology, vol 2. Academic , London, pp277–327
Horinouchi S, Beppu T (1982) Autoregulators. In: Vining L (ed) Genetics and biochemistry of antibiotic production. Butterworth-Heinemann, Newton, Massachusetts, pp 103–119
Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA (2000) Practical Streptomyces genetics. John Innes Foundation, Norwich
Kondo K, Higuchi Y, Sakuda S, Nihira T, YamadaY (1989) New virginiae butanolides from Streptomyces virginiae. J Antibiot 42:769–778
Kotowska M, Pawlik K, Butler AR, Cundliffe E, Takano E, Kuczek K (2002) Type II thioesterase from Streptomyces coelicolorA3 (2). Microbiology 148:1777–83
Lombo F, Pfeifer B, Leaf T, Ou S, Kim YS, Cane DE, Licari P, Khosla CH (2001) Enhancing the atom economy of polyketide biosynthetic processes through metabolic engineering. Biotechnol Prog 17:612–617
MacNeil DJ, Gewain KM, Ruby CL, Dezeny G, Gibbons PH, MacNeil T (1992) Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilising a novel integration vector. Gene 111:61–68
Minas W, Brunker P, Kallio PT, Bailey JE (1998) Improved erythromycin production in a genetically engineered industrial strain of Saccharopolyspora erythraea. Biotechnol Prog 14:51–566
Paget MSB, Chamberlin L, Atrih A, Foster SJ, Buttner MJ (1999) Evidence that the extracytoplasmic function sigma factor, σE, is required for normal cell wall structure in Streptomyces coelicolor A3(2). J Bacteriol 181:201–211
Passantino R, Puglia A-M, Chater KF (1991) Additional copies of the actII regulatory gene induce actinorhodin production in pleiotropic bld mutants of Streptomyces coelicolor A3 (2).J Gen Microbiol 137:2059–2064
Pfeifer BA, Admiraal SJ, Gramajo H, Cane DE, Khosla CH (2001) Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science 291:1790–2
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning : a laboratory manual, 2nd edn. Cold Spring Harbor , Cold Spring Harbor, New York
Stratigopoulos G, Cundliffe E (2002) Inactivation of a transcriptional repressor during empirical improvement of the tylosin producer, Streptomyces fradiae. J Ind Microbiol Biotechnol 28:219–224
Takano E, Gramajo HC, Strauch E, Andres N, White J, Bibb MJ (1992) Transcriptional regulation of theredD transcriptional activator gene accounts for growth-phase-dependent production of the antibiotic undecylprodigiosin in Streptomyces coelicolor A3 (2). Molecular Microbiology 6:2797–2804
Takano E, Nihira T, Hara Y, Jones JJ, Gershater CJL, Yamada Y, Bibb MJ (2000) Purification and structural determination of SCB1, a γ-butyrolactone that elicits antibiotic production in Streptomyces coelicolor A3(2). J Biol Chem 275:11010–11016
Takano E, Chakraburtty R, Nihira T, Yamada Y, Bibb MJ (2001) A complex role for the γ-butyrolactone SCB1 in regulating antibiotic production in Streptomyces coelicolor A3 (2). Molecular Microbiology 41:1015–1028
Acknowledgements
This work was supported by European Union Cell Factory grant B104-CT96–0332 (coordinated by Dr. R. Luiten) and Human Frontiers Science Program Grant RG0330/1998-M to Mervyn Bibb and Biotechnology and Biological Sciences Research Council grant 208/P14580. We are grateful to Professor K.F. Chater for helpful discussions and comments on the manuscript. These experiments were carried out in accordance with the laws of the United Kingdom and the European Union.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Butler, M.J., Takano, E., Bruheim, P. et al. Deletion of scbA enhances antibiotic production in Streptomyces lividans . Appl Microbiol Biotechnol 61, 512–516 (2003). https://doi.org/10.1007/s00253-003-1277-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1277-8