Abstract
The presentation of identical peptides by different major histocompatibility complex class I (MHC-I) molecules, termed promiscuity, is a controversial feature of T cell-mediated immunity to pathogens. The astounding diversity of MHC-I molecules in human populations, presumably to enable binding of equally diverse peptides, implies promiscuity would be a rare phenomenon. However, if it occurs, it would have important implications for immunity. We screened 77 animals for responses to peptides known to bind MHC-I molecules that were not expressed by these animals. Some cases of supposed promiscuity were determined to be the result of either non-identical optimal peptides or were simply not mapped to the correct MHC-I molecule in previous studies. Cases of promiscuity, however, were associated with alterations of immunodominance hierarchies, either in terms of the repertoire of peptides presented by the different MHC-I molecules or in the magnitude of the responses directed against the epitopes themselves. Specifically, we found that the Mamu-B*017:01-restricted peptides Vif HW8 and cRW9 were also presented by Mamu-A2*05:26 and targeted by an animal expressing that allele. We also found that the normally subdominant Mamu-A1*001:01 presented peptide Gag QI9 was also presented by Mamu-B*056:01. Both A2*05:26 and B*056:01 are molecules typically or exclusively expressed by animals of Chinese origin. These data clearly demonstrate that MHC-I epitope promiscuity, though rare, might have important implications for immunodominance and for the transmission of escape mutations, depending on the relative frequencies of the given alleles in a population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CD8+ T lymphocytes (CTL) are important in control of AIDS virus replication, both for controlling acute phase viremia (Borrow et al. 1994, 1997; Matano et al. 1998) and for establishing low set point viremia in humans and rhesus macaques that spontaneously control viral replication (elite controllers) (Carrington and O'Brien 2003; Friedrich et al. 2007). Hence, recognition of virally derived peptides presented by particular major histocompatibility complex class I (MHC-I) molecules is an important event in immunity to AIDS viruses.
MHC-I molecules bind short peptides and present them at the surface of cells. These peptides can be derived either from self or non-self proteins, such as those from viruses and other intracellular pathogens. Thus, in this role, MHC-I molecules are the mediators of immune recognition of infected or altered-self (tumor) cells by circulating CTL. Each MHC-I allele encodes a molecule that binds peptides with exquisite specificity. Peptides bound by a given MHC-I molecule cluster into motifs defined mostly by conservation of amino acids at positions 2 and the C terminus (generally position 9, 10, or 11, with some exceptions) (Falk et al. 1991; Allen et al. 1998; Loffredo et al. 2007a, 2004; Mothe et al. 2002; Sette et al. 2005; Sidney et al. 2000; Vogel et al. 2002). Several studies of the three-dimensional structures of MHC-I molecules bound to peptides show that pockets within the peptide binding region of these molecules are responsible for the existence of these “anchor” residues (Madden et al. 1992; Matsumura et al. 1992). Because of this specificity, it is not surprising that very closely related alleles might encode MHC-I proteins with overlapping or nearly identical peptide-binding repertoires, as is the case with B*003:01:01 and B*008:01 (formerly known as B*03 and B*08, respectively) in rhesus macaques (Loffredo et al. 2009) and alleles of the HLA-B7 supertype in humans (Leslie et al. 2006). However, the promiscuous binding of identical peptides by molecules with either divergent binding regions or similar ones acquired through convergent evolution is a far more controversial issue in immunology.
MHC-I polymorphism is selected for by differences in peptide binding (Hughes and Nei 1988; Hughes et al. 1990). Thus, each MHC-I allele encodes a protein that binds unique sets of peptides. However, recent reports have shown that different MHC-I proteins can bind the same peptides in infections with HIV-1 (Frahm et al. 2007) or Mycobacterium tuberculosis (Axelsson-Robertson et al. 2010). This is counterintuitive to our understanding of how these MHC-I molecules have evolved. While the phenomenon has been reported in HIV, there have been no reports of this in the non-human primate models of HIV. Here, we sought to determine the frequency of MHC-I peptide promiscuity in the simple, well-defined system of SIV-infected rhesus macaques, focusing on the defined peptide-binding repertoires of the common and important MHC-I molecules A1*001:01 and −B*017:01 (formerly A*01 and B*17, respectively).
Materials and methods
ELISPOT
ELISPOT assays were performed as previously described (Wilson et al. 2009). Briefly, 1 × 105 fresh or frozen PBMC, isolated from EDTA-anticoagulated blood using Ficoll-Paque PLUS (GE Healthcare Systems, Uppsala, Sweden) and density centrifugation were added to each well on precoated ELISpotPLUS plates (Mabtech USA, Mariemont, OH, USA) and the assays were run according to the manufacturer's instructions. Tests were performed in duplicate or triplicate using individual peptides at 10 uM or serial dilutions thereof. The positive control, Con A (Sigma-Aldrich, St. Louis, MO, USA), was used at a final concentration of 5 μg/ml. Negative control wells lacked stimulation. The plates were incubated for 12–18 h at 37°C in 5% CO2.
Wells were imaged and spot forming cells were counted with an AID EliSpot reader version 4.0 (AID, Strassberg, Germany) and analyzed as described (Wilson et al. 2009). Responses were considered positive if the mean of the number of SFC was more than 50 spots per million cells, and significance was determined using a one-tailed t test where alpha = 0.05, where the null hypothesis (Ho): background level ≥ treatment level.
Construction of MHC class I cDNA libraries
Total RNA was isolated from ~3 × 107 cells from B-lymphoblastoid cell lines (BLCL) using the RNeasy Protect Mini Kit (QIAGEN, Valencia, CA). For each animal, ~3 μg mRNA was isolated from 150 μg total RNA using the Oligotex Midi Kit (QIAGEN). One microgram of mRNA from each animal served as the template for first-strand cDNA synthesis, using the SuperScript plasmid system for cDNA synthesis and cloning (Invitrogen, Carlsbad, CA) by following the manufacturer's instructions. Size-fractionated cDNA containing SalI and NotI restriction endonuclease cohesive ends was ligated into the multiple cloning site of pCMV.SPORT6 and used to transform DH5α chemically competent Escherichia coli (Invitrogen). Recombinant plasmids containing cDNA were isolated from ~5 × 105 ampicillin resistant colonies and purified using the HiSpeed Plasmid Midi kit (QIAGEN). Five micrograms of plasmid DNA from each macaque's library served as the target DNA for hybridization to a 40-mer biotinylated oligonucleotide, 5′-[BIOTEG]GAGRCCAYCYTGAGRTGCTGGGCYC TGGGCTTCTACCCTG-3′. The sequence of the capture oligonucleotide was derived from a highly conserved region of the MHC class I alpha-3 domain. Of this probe, 0.5 μg was incubated with 4 μg of purified RecA protein prior to the addition of the cDNA plasmid library according to a procedure describing RecA-mediated affinity capture (Zhumabayeva et al. 1999). More than 150 MHC class I clones were captured and sequenced from each library. Sequencing was performed on an ABI 3730 DNA Analyzer (Applied Biosystems, Foster City, CA). Full-length sequences of MHC class I cDNAs were obtained by using four forward and four reverse primers. The forward primers were: SP6 (5′-GGCCTATTTAGGTGACACTATAG-3′), C/1+ (5′-GCAGATACCTGGAGAACGGG-3′), IV (5′-GGAACCTTCCAGAAGTGGG-3′), and 3′UTR (5′-CAGGGCTCTGATGTGTCTCTCACG-3′). The reverse primers were: T7 (5′-TAATACGACTCACTATAGGG-3′), E2 (5′-CYCCACCTCCTCACATKATGC-3′), F1 (5′-CCAGGTCAGTGTGATCTCCG-3′), and G1 (5′-ATGTAATCCTTGCCGTCGTA-3′). Sequences were analyzed using CodonCode Aligner version 1.6.3 (CodonCode, Deadham, MA). MHC class I alleles were considered part of the cDNA library after at least two copies were verified by sequencing
MHC-I transfectants and restriction mapping
Transient expression of cloned MHC class I cDNA was achieved by electroporation of plasmid DNA into the MHC class I deficient human B cell line 721.221 (DeMars et al. 1985). Briefly, 5 μg of plasmid DNA was added to 5 × 106 721.221 cells in 100 μl Nucleofector™ Solution C and electroporated using program G-16 on a Nucleofector I device (Amaxa, Köln, Germany). Transfectants were used 48–72 h post-electroporation.
MHC-I surface expression on transient MHC-I transfectants was measured by W6/32 antibody surface staining. For restriction mapping, peptides were pulsed on autologous, immortalized macaque B cell lines (positive controls), 721.221 cells (negative controls), or transiently transfected 721.221 cells. After 90 min, peptides were removed with three washes, and these presenting cells were mixed with equal numbers of antigen-specific CTL. Recognition was measured either with IFN-γ ELISPOT or with intracellular cytokine staining (ICS) measuring IFN-γ and TNF-α, 24 h later. ICS assays were acquired on a BD LSR II (BD Biosciences) and analyzed using FlowJo version 9.1 (Treestar, Ashland, OR, USA).
Viral sequencing
Viral RNA was extracted from plasma using the QIAGEN MinElute kit (Qiagen, Valencia, CA, USA) or by a guanidine thiocyanate extraction (Friedrich et al. 2007). We then used the QIAGEN One Step RT-PCR kit to amplify regions encoding the targeted epitopes. The RT-PCR conditions for all amplicons were as follows: 50°C for 30 min; 95°C for 15 min; 45 cycles of 94°C for 30 s, 53°C for 1 min, and 72°C for 150 s; and 68°C for 20 min. Cycling ramp rates were 2°C per second. Amplicons were purified for sequencing using ExoSAP-IT® (USB Corporation).
Both strands of each amplicon were sequenced on a 3730 DNA Analyzer (Applied Biosystems, Carlsbad, CA, USA) using DYEnamic ET Terminators (GE Healthcare, Uppsala, Sweden) and their respective RT-PCR primers. The sequencing cycling conditions for all amplicons were as follows: 30 cycles of 95°C for 20 s, 50°C for 15 s, and 60°C for 1 min. Sequences were assembled using CodonCode Aligner version 3.7.1 (CodonCode Corporation, Deadham, MA, USA). DNA sequences were conceptually translated and aligned to wild-type SIVmac239 in MacVector 11.1.1 trial version (MacVector,Inc, Cary, NC, USA).
Results
We showed previously that the rhesus macaque MHC-I molecules A1*001:01 and B*017:01 each bind multiple epitopes derived from SIV (Allen et al. 1998, 2001; Mothe et al. 2002). Further, we and others have shown that expression of these molecules is correlated with vaccine-induced [A1*001:01 (Casimiro et al. 2005; McDermott et al. 2005)] and spontaneous [B*017:01 (Maness et al. 2008; Yant et al. 2006)] control of SIVmac239. It is possible that understanding the mechanisms of control of viral replication will involve understanding the nature of the interactions between these MHC-I molecules and the epitopes they bind. In this study, we sought to identify rhesus macaque MHC-I molecules that present SIV-derived peptides identical to those associated with these two MHC-I molecules.
To identify promiscuous peptides derived from SIV, we screened 48 A1*001:01-negative, SIVmac239-infected rhesus macaques for responses to 14 SIV-derived peptides meeting two criteria. First, the peptides were known to bind the A1*001:01 molecule. Second, responses against the peptide must have been detected in A1*001:01-positive, SIVmac239-infected animals in previous studies (Allen et al. 2001, 1998). These animals were predominantly of Indian origin, but a small number were either of Chinese or of Indian/Chinese mixed origin. Only two A1*001:01-negative animals made detectable responses to these peptides (Table 1). However, these results were intriguing because responses against both of these epitopes are potentially important. Specifically, responses against the immunodominant Gag CM9 (CTPYDINQM) epitope have been associated with vaccine-induced control of subsequent SIV challenge (Barouch et al. 2002). This control could be due to fitness costs associated with viral escape from this epitope (Friedrich et al. 2004a, b). Further, CTL directed against the Gag QI9 epitope (QNIPIVGNI), normally subdominant relative to other A1*001:01-bound epitope-directed responses, were recently shown to exhibit distinct kinetics of recognition of infected cells, perhaps due to unique processing requirements of this peptide (Sacha et al. 2008).
Next, we screened 55 SIVmac239-infected, B*017:01-negative animals for responses to 13 peptides that met the same criteria for this molecule (known to bind the B*017:01 molecule and positive responses detected in animals positive for the allele (Maness et al. 2007; Mothe et al. 2002)). Surprisingly, we detected positive responses against 5 of the 13 peptides, with some peptides eliciting responses in multiple animals and some animals responding to multiple peptides (Table 2). One of the animals, r95003, responded to two of these five peptides (Vif HW8, which binds B*017:01 with an IC50 of 2.9 nM (Maness et al. 2008) and the alternate reading frame-derived cRW9, which binds with an IC50 of 32nM (Maness et al. 2007)). cRW9 is derived from an alternate reading frame product translated from the Env-encoding mRNA (Maness et al. 2009). This product of translation encodes several CTL epitopes (Maness et al. 2010). We showed previously that responses against Vif HW8 and cRW9 were among the five most immunodominant B*017:01-restricted responses during SIVmac239 infection (Maness et al. 2008). B*017:013-positive macaques, however, targeted the other three epitopes (Vif CY9, Env LF11 and Env LY10) much less frequently.
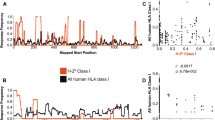
True epitope promiscuity addresses the binding of identical peptides by distinct MHC-I proteins. Hence, we tested PBMC from responding animals using serial dilutions of peptides representing the previously mapped epitope and slight derivations of it, including or excluding one or two amino acids on the C or N terminus. The optimal peptide was found to be identical to the previously mapped peptide in all cases but one (data not shown and Fig. 1). The strong positive response detected against the Gag CM9 peptide in a A1*001:01-negative macaque was found to be against a peptide 11 amino acids long, incorporating the CM9 peptide in addition to two amino acids on the C terminus (Fig. 1a), while the dilutions demonstrated that A1*001:01-positive animals respond strongest to the CM9 peptide (Fig. 1b, c). Although this result is intriguing, and understanding the effects of such a strong response against a region of the viral Gag protein known to be under functional constraints will be important, it indicates that this is not a case of MHC-I promiscuity and was excluded from further analysis in this study. However, the Gag QI9 peptide, bound by A1*001:01 and another unknown molecule and all five of the B*017:01-bound peptides were subjected to further analysis because they met the criteria of promiscuity.
a PBMC from animal r00078 (A1*001:01-negative) were tested for reactivity to the CM9 (A1*001:01-restricted) peptide as well as overlapping peptides to determine the minimal optimal peptide recognized by this animal. b, cA1*001:01-positive animals r95061 and r96141 were also tested. Peptide dilutions were used in an IFN-γ ELISPOT assay to determine the minimal optimal epitopes targeted by these animals
We next needed to verify that the peptides we had identified as promiscuous were mapped to the correct MHC-I molecule in previous studies. The Gag QI9 peptide was indeed presented by A1*001:01 in animals expressing this molecule, as evidenced by the binding of MHC-I tetramers folded with this peptide to CTL specific for the epitope (data not shown). In addition, the Vif HW8 and cRW9 were verified as being B*017:01-restricted [(Maness et al. 2008) and data not shown)]. However, the other three putatively B*017:01-restricted peptides recognized in B*017:01-negative animals (Vif CY9, Env LF11 and LY10) were never tested for restriction with MHC-I transferents, and tetramers were never folded with these peptides (Mothe et al. 2002), likely because they are rarely recognized by B*017:01-positive animals (Maness et al. 2008). To test whether these three epitopes are presented by B*017:01 in animals possessing this allele, we expanded T cell lines against the peptides from a B*017:01-positive, SIVmac239 infected macaque. Unfortunately, we could not expand T cells recognizing the Env LY10 peptide. T cells against the other two peptides were successfully expanded in vitro, and we were able to test whether they recognized the peptide bound to the B*017:01 molecule when stably expressed in K562 cells, which do not express MHC-I molecules. We found that neither the Vif CY9 nor the Env LF11 peptides were presented by B*017:01 in this animal, while a T cell line against the verified B*017:01-restricted Nef IW9 peptide was presented by B*017:01 (Fig. 2), indicating that the Vif CY9 and Env LF11 peptides are not presented by B*017:01 in SIV-infected animals. We did not determine the actual restricting MHC-I molecule for these peptides. The possibility exists that the Env LY10 peptide is indeed presented by B*017:01 in infected animals. However, this is unlikely due to the relatively high fraction of B*017:01-negative animals responding to Env LY10 and its poor binding coefficient to the B*017:01 molecule (IC50 = 439 nM), which is just below the threshold to be considered physiologically relevant (Sette et al. 1994).
Putatively promiscuous epitopes in B*017:01-negative animals. We expanded CTL lines against the peptides in vitro and tested for restriction by B*017:01 using K562 cells stably expressing this molecule or another molecule used as a negative control. A line against the Nef IW9 peptide was used as a positive control. Using intracellular cytokine staining measuring IFN-γ and TNF-α production as a measure of recognition of the peptide bound to B*017:01. Y-axis, the percentage of the maximum cytokine production for each CTL line is plotted. Positive sign is with peptide and negative sign is without peptide to establish background for each cell line
After controlling for non-identical peptides and peptides that are not presented by the MHC-I molecule they were originally found to bind, we were left with 3 SIVmac239-derived peptides that met our criteria for promiscuity. We next conducted experiments to determine the MHC-I molecules that actually present these peptides in animals that did not express the original presenting molecule. We first sought to determine the full complement of MHC-I alleles present in these animals using RecA capture of cDNA libraries, using oligos designed to capture all MHC-I alleles extracted from cells from these animals. The products of these experiments are full-length, directionally cloned cDNAs that can be transfected into MHC-I null cells to determine restriction. Both rh2256 and r95003 expressed a multitude of MHC-I alleles (Table 3), typical of the immense complexity of the MHC-I loci in macaques (Otting et al. 2005; Wiseman et al. 2009).
All of the alleles expressed by animal rh2256, which recognized the Gag QI9 peptide, were typical of Chinese ancestry (Karl et al. 2008; Otting et al. 2008). In contrast, animal r95003 expressed rhesus alleles indicative either of ancestral admixture or of purely Chinese ancestry. One Mamu-A allele in this animal, A2*05:26, has been detected in animals of both Chinese and Burmese origin (Doxiadis et al. 2011; Otting et al. 2007). Another A4*14:03:01 has been detected in animals of Burmese, Chinese, and Indian origin macaques (Doxiadis et al. 2011; Karl et al. 2008; Otting et al. 2005). In addition, several Mamu-B alleles expressed in this animal, including B*036:02 and B*037:01, were originally identified in animals of Chinese ancestry (Karl et al. 2008; Otting et al. 2007, 2008), while the alleles B*001:01:01, B*007:02, and B*030:02 are found in animals of either Indian or Chinese ancestry (Karl et al. 2008; Otting et al. 2005). Together, these data suggest a Chinese ancestry for animal rh2256 and either a full or partial Chinese ancestry for animal r95003.
The cDNA clones isolated from the typing experiments were next used to determine restriction of the promiscuous peptides. We tested whether CTL lines specific for each of the peptides could recognize peptide-pulsed 721.221 cells transfected with each of the cDNA clones. Our results clearly show that, in animal rh2256, the Chinese molecule B*056:01 was responsible for presenting the Gag QI9 peptide to T cells (Fig. 3a). Further, we found that the Chinese molecule A2*05:26 presented both the Vif HW8 and the cRW9 peptides to T cells in this animal (Fig. 3b, c).
Restriction determination of verified promiscuous epitopes. We expanded CTL lines in vitro against the promiscuous peptides and tested for restriction using MHC-I-null 721.221 cells transfected with plasmids encoding each of the MHC-I molecules expressed by each animal, as identified by RecA capture of cDNA libraries. IFN-γ ELISPOT was used to determine reactivity. For simplicity, shown are only the mock-transfected (no DNA), non-transfected autologous BLCL, the molecule found to present the peptide and an example of a molecule that did not present the molecule. The Gag QI9 peptide was presented by B*056:01 in animal rh2256 (a); the Vif HW8 (b) and cRW9 (c) peptides were presented by A2*0526 in animal r95003. MHC surface expression was allele-specific and ranged from 5% to 30% in these assays (not shown)
We next aligned the α1 and α2 domains of the MHC-I molecules responsible for presenting identical peptides. We reasoned that, despite substantial sequence variation overall, conserved residues in the B and F pocket residues, responsible for anchoring the bound peptides into the MHC-I groove, might be conserved between the molecules. We identified sequence similarity in key residues in both the B and F pockets between A1*001:01 and B*056:01 (Fig. 4a, blue and red shaded residues). Intriguingly, however, all of the F pocket residues deemed to be key for peptide binding were identical between the molecules. More experiments will be necessary to determine whether this similarity is responsible for overlapping peptide-binding repertoires. Surprisingly, the same relationship was found between B*017:01 and A2*05:26 (variation in the B pocket and identity in the F pocket, Fig. 4b). It is not known whether this convergent evolution is responsible for the promiscuity seen in this study. Together, these alignments show that substantial differences can exist within key residues in the peptide-binding residues of MHC-I molecules and yet the molecules can have overlapping peptide-binding repertoires.
Alignments of the peptide binding regions (a1 and a2 domains) of molecules that present promiscuous peptides. A1*001:01 aligned to B*056:01, which both present the Gag QI9 peptide. a B*017:01 aligned to A2*05:26, which both present the Vif HW8 and cRW9 peptides. Open blue boxes, B pocket residues. Shaded blue boxes, B pocket key residues. Open red boxes, F pocket residues. Shaded red boxes, F pocket key residues
Finally, we were interested in comparing the in vivo dynamics of the CTL responses directed against the epitopes as presented by their newly identified presenting molecules. Acute samples were not available for animal r95003, so this analysis was restricted to animal rh2256, which presented the Gag QI9 peptide via the B*056:01 molecule. We tested samples from several time points throughout infection, ranging from day 0, the day of viral challenge, to past week 20. This response was immunodominant at week 2 and was maintained at a high level before waning by week 20 post-challenge (Fig. 5, orange line). In contrast, the dynamics of the CTL response directed against the Gag QI9 peptide show a much more fleeting pattern in 6 A1*001:01-positive animals used as vaccine-naïve controls in a recent vaccine experiment (Wilson et al. 2006) (Fig. 5, blue lines). The Gag QI9 response in these animals was of variable magnitude but tended to be strong in very early infection and rapidly dropped below the limit of ELISPOT detection. However, the limited sample size in this study precludes drawing generalized conclusions about the immunodominance of CTL against Gag QI9 in B*056:01-positive animals.
In vivo kinetics of the Gag QI9 response in animal rh2256 and six A1*001:01-positive animals used as vaccine-naïve animals in a recent vaccine study (Wilson et al. 2006). IFN-γ ELISPOT was used to measure the magnitude of the response at timepoints ranging from day of infection (week 0) to more than 40 weeks post-infection. Responses were considered positive if they were more than 50 spots per million cells and were determined using a one-tailed t test and alpha = 0.05, where the null hypothesis (Ho): background level ≥ treatment level
We also wished to determine the patterns of viral evolution in the epitopes targeted and to compare them with the well-studied patterns in animals expressing A1*001:01 and B*017:01. We sequenced the portion of the SIV gag gene that encodes QI9 from viral RNA from several timepoints throughout infection in animal rh2256. We did not observe any variation in this epitope throughout infection (data not shown). Likewise, we have never seen variation in the QI9 epitope in more than 20 A1*001:01-positive animals, implicating strong functional constraints on this region of the Gag protein. We also sequenced the regions of the SIV genome ecoding the A1*001:01-restricted Gag CM9 and Tat SL8 epitopes to determine whether this animal had targeted them, resulting in viral escape. No sequence variation was observed in either epitope. Likewise, neither of these peptides were recognized by this animal in our ELISPOT assays. Together, these data imply this animal did not target these epitopes. These data indicate that although A1*001:01 and B*056:01 have overlapping peptide-binding repertoires, they are not identical and likely do not share immunodominance patterns.
We also sequenced the regions of the viral genome that encode the Vif HW8 and cRW9 epitopes in animal r95003. In this case, viral variation was observed in both epitopes. Surprisingly, the variation we found was identical to the typical patterns of viral escape from these responses in B*017:01-postive animals. Specifically, we found a position 1 change from H to Y in the Vif HW8 epitope and a position 9 change from W to R in cRW9 (Fig. 6). These changes were reported previously when the cRW9 epitope was initially discovered (Maness et al. 2007). Although the Vif HW8 and cRW9 epitopes are among the five most commonly targeted in B*017:01-postive animals (Maness et al. 2008), they are generally less dominant than the other three, Nef IW9, Nef MW9, and Env FW9, in which sequence variation is observed in essentially every SIVmac239-infected, B*017:01-postive animal. Animal r95003 did not target any of these epitopes in our initial screen. However, to determine if this animal might have targeted without detection, we sequenced the regions of Nef and Env encoding these epitopes. No variation was observed. Again, these data indicate that while B*017:01 and A2*05:26 have overlapping peptide-binding repertoires, they are not identical, and do not share the same immunodominance hierarchies. Taken together, our data indicate that MHC-I epitope promiscuity is a real phenomenon but is not commonly observed in SIVmac239 infected rhesus macaques.
Viral sequence alignments of wild-type SIVmac239 (the inoculating sequence) and that sequenced from r95003 at time of euthanasia. Top line is the region of the Vif protein containing the HW8 peptide, in shaded box. Bottom line is the ARF encoding the cRW9 peptide, in shaded box. Dots represent sequence identity
Discussion
Among the most striking features of MHC-I biology is diversity, both within and between individuals. The MHC-I genes are among the most polymorphic known and the differences between MHC-I molecules map largely to the regions known to interact with their peptide antigens (Hughes and Nei 1988; Hughes et al. 1990). This implies strong historical selection for the ability to recognize a broad repertoire of pathogens or of epitopes within particular pathogens or both. Understanding how specific MHC-I molecules interact with pathogen-derived epitopes, such as those derived from HIV and its simian counterpart, SIV, is important in the search for vaccines that elicit CTL responses against these viruses.
Here, we report two MHC-I molecules that present peptides to CTL typically associated with other, unrelated molecules in the same species, Macaca mulatta. Interestingly, we found that an MHC-I molecule in macaques of Chinese ancestry, A2*05:26, presented peptides presented typically by the important MHC-I molecule B*017:01. B*017:01 is one of two macaque alleles with strong associations with elite control of viral replication, along with B*008:01 (Loffredo et al. 2007b; Yant et al. 2006). The A2*05:26-positive animal in this study, r95003, did not exhibit elite control of SIV replication. However, since the majority of animals possessing elite control-associated alleles do not control virus, it cannot be determined whether targeting of epitopes presented by A2*05:26 can result in low viral loads or elite control of SIV replication. These results are also intriguing because a close relative of B*017:01, B*017:02 is known to exist in macaques of Chinese origin. As Chinese macaques become a more common model organism for the study of HIV and anti-HIV vaccines, it will be interesting to see if either B*017:02 or A2*05:26 have beneficial influences on viral dynamics in these animals.
The presentation of peptides normally associated with the elite controller molecule B*017:01 by A2*05:26 is intriguing because the A2*05 family of alleles is one of the most polymorphic and frequently detected in macaques of related species (Wu et al. 2008). In fact, nearly every animal in our colony, of either Indian or Chinese origin, has at least one A2*05 allele. However, based on our ELISPOT data, it appears that the vast majority of these very closely related molecules do not present peptides normally associated with B*017:01. To test this, we examined whether 721.221 cells expressing the very closely related molecule, A2*05:29, could present either Vif HW8 or cRW9 to CTL expanded from r95003 against these peptides. No reactivity was seen (data not shown). It is not clear if this lack of reactivity is due to changes in residues that interact with the peptides or with the T cell receptors of the CTL. In either case, it is an important observation that very closely related alleles could have such divergent peptide presentation abilities. This result has important implications for the diversity of epitopes targeted by infected individuals.
The presentation of the Gag QI9 peptide by the B*056:01 molecule, also of Chinese origin, is also intriguing. This peptide exhibits unique epitope presentation dynamics relative to other peptides encoded by the gag gene product (Sacha et al. 2008). This peptide was targeted by immunodominant CTL as early as 2 weeks post-viral challenge in both A1*001:01-positive animals and in the B*056:01-positive animal in this study. However, the response was detected for much longer when presented by B*056:01, despite no difference in viral variation. This difference might indicate differences in relative peptide binding or it might indicate a lack of competition for MHC-I binding with more dominant A1*001:01-bound peptides such as Gag CM9 and Tat SL8, which were not targeted by our B*056:01-positive animal.
To our knowledge, our serendipitous discovery of SIV epitopes presented by MHC-I molecules of Chinese origin is the first example of mapped SIV-derived epitopes in these increasingly important animal models of HIV infection. A tetramer with an SIV peptide was recently folded with a Chinese origin MHC molecule (Ouyang et al. 2009), but actual immune responses against this peptide were not identified, though their existence can be inferred. Although peptide-binding motifs have been studied in Chinese MHC-I molecules (Solomon et al. 2010), and several investigators have examined the viral replication kinetics of SIV-infected Chinese origin animals (Joag et al. 1994; Marthas et al. 2001; Trichel et al. 2002; Ling et al. 2002; Burdo et al. 2005), few or no studies have examined, in detail, the immune repertoires and subsequent viral evolution in these animals. We examined 77 animals for the ability to target peptides bound by molecules they did not express. Of these, very few were of Chinese origin, yet the only two cases of true promiscuity were identified in one animal of complete Chinese origin and one that was either a mixture of Indian and Chinese ancestry or of pure Chinese ancestry. These data imply that promiscuity is the result of convergent evolution of the ability to recognize identical peptides in geographically separate populations of the same species, while it was not observed between alleles of the same geographic origin.
The exquisite specificity of MHC-I:peptide interactions has led many to suppose that only very closely related molecules can present identical peptides, despite recent reports of a high frequency of such interactions (Frahm et al. 2007; Axelsson-Robertson et al. 2010). Though our results do not directly contradict such findings, they do indicate that cases of true promiscuity in immunity to SIV are less common than one might suppose. They also indicate that cases of “false positives”, supposed cases of promiscuity that are the result of incorrect mapping either of epitope identity or of restricting MHC-I molecule, are actually more common in immunity to SIV than are true cases. However, cases of true promiscuity were identified and might have important implications for future use of macaques in preclinical HIV studies.
References
Allen TM, Sidney J, del Guercio MF, Glickman RL, Lensmeyer GL, Wiebe DA, DeMars R, Pauza CD, Johnson RP, Sette A, Watkins DI (1998) Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J Immunol 160:6062–6071
Allen TM, Mothe BR, Sidney J, Jing P, Dzuris JL, Liebl ME, Vogel TU, O'Connor DH, Wang X, Wussow MC, Thomson JA, Altman JD, Watkins DI, Sette A (2001) CD8(+) lymphocytes from simian immunodeficiency virus-infected rhesus macaques recognize 14 different epitopes bound by the major histocompatibility complex class I molecule mamu-A*01: implications for vaccine design and testing. J Virol 75:738–749
Axelsson-Robertson R, Weichold F, Sizemore D, Wulf M, Skeiky YA, Sadoff J, Maeurer MJ (2010) Extensive major histocompatibility complex class I binding promiscuity for Mycobacterium tuberculosis TB10.4 peptides and immune dominance of human leucocyte antigen (HLA)-B*0702 and HLA-B*0801 alleles in TB10.4 CD8 T-cell responses. Immunology 129:496–505
Barouch DH, Kunstman J, Kuroda MJ, Schmitz JE, Santra S, Peyerl FW, Krivulka GR, Beaudry K, Lifton MA, Gorgone DA, Montefiori DC, Lewis MG, Wolinsky SM, Letvin NL (2002) Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335–339
Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB (1994) Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol 68:6103–6110
Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, Nelson JA, Gairin JE, Hahn BH, Oldstone MB, Shaw GM (1997) Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med 3:205–211
Burdo TH, Marcondes MC, Lanigan CM, Penedo MC, Fox HS (2005) Susceptibility of Chinese rhesus monkeys to SIV infection. AIDS 19:1704–1706
Carrington M, O'Brien SJ (2003) The influence of HLA genotype on AIDS. Annu Rev Med 54:535–551
Casimiro DR, Wang F, Schleif WA, Liang X, Zhang ZQ, Tobery TW, Davies ME, McDermott AB, O'Connor DH, Fridman A, Bagchi A, Tussey LG, Bett AJ, Finnefrock AC, Fu TM, Tang A, Wilson KA, Chen M, Perry HC, Heidecker GJ, Freed DC, Carella A, Punt KS, Sykes KJ, Huang L, Ausensi VI, Bachinsky M, Sadasivan-Nair U, Watkins DI, Emini EA, Shiver JW (2005) Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with DNA and recombinant adenoviral vaccine vectors expressing Gag. J Virol 79:15547–15555
DeMars R, Rudersdorf R, Chang C, Petersen J, Strandtmann J, Korn N, Sidwell B, Orr HT (1985) Mutations that impair a posttranscriptional step in expression of HLA-A and -B antigens. Proc Natl Acad Sci USA 82:8183–8187
Doxiadis GG, de Groot N, Otting N, Blokhuis JH, Bontrop RE (2011) Genomic plasticity of the MHC class I A region in rhesus macaques: extensive haplotype diversity at the population level as revealed by microsatellites. Immunogenetics 63:73–83
Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee HG (1991) Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 351:290–296
Frahm N, Yusim K, Suscovich TJ, Adams S, Sidney J, Hraber P, Hewitt HS, Linde CH, Kavanagh DG, Woodberry T, Henry LM, Faircloth K, Listgarten J, Kadie C, Jojic N, Sango K, Brown NV, Pae E, Zaman MT, Bihl F, Khatri A, John M, Mallal S, Marincola FM, Walker BD, Sette A, Heckerman D, Korber BT, Brander C (2007) Extensive HLA class I allele promiscuity among viral CTL epitopes. Eur J Immunol 37:2419–2433
Friedrich TC, Dodds EJ, Yant LJ, Vojnov L, Rudersdorf R, Cullen C, Evans DT, Desrosiers RC, Mothe BR, Sidney J, Sette A, Kunstman K, Wolinsky S, Piatak M, Lifson J, Hughes AL, Wilson N, O'Connor DH, Watkins DI (2004a) Reversion of CTL escape-variant immunodeficiency viruses in vivo. Nat Med 10:275–281
Friedrich TC, Frye CA, Yant LJ, O'Connor DH, Kriewaldt NA, Benson M, Vojnov L, Dodds EJ, Cullen C, Rudersdorf R, Hughes AL, Wilson N, Watkins DI (2004b) Extraepitopic compensatory substitutions partially restore fitness to simian immunodeficiency virus variants that escape from an immunodominant cytotoxic-T-lymphocyte response. J Virol 78:2581–2585
Friedrich TC, Valentine LE, Yant LJ, Rakasz EG, Piaskowski SM, Furlott JR, Weisgrau KL, Burwitz B, May GE, Leon EJ, Soma T, Napoe G, Capuano S, Friedrich TC, Valentine LE, Yant LJ, Rakasz EG, Piaskowski SM, Furlott JR, Weisgrau KL, Burwitz B, May GE, Leon EJ, Soma T, Napoe G, Capuano SVr, Wilson NA, Watkins DI (2007) Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J Virol 81:3465–3476
Hughes AL, Nei M (1988) Pattern of nucleotide substitution at major histocompatibility complex class I loci reveals overdominant selection. Nature 335:167–170
Hughes AL, Ota T, Nei M (1990) Positive Darwinian selection promotes charge profile diversity in the antigen-binding cleft of class I major-histocompatibility-complex molecules. Mol Biol Evol 7:515–524
Joag SV, Stephens EB, Adams RJ, Foresman L, Narayan O (1994) Pathogenesis of SIVmac infection in Chinese and Indian rhesus macaques: effects of splenectomy on virus burden. Virology 200:436–446
Karl JA, Wiseman RW, Campbell KJ, Blasky AJ, Hughes AL, Ferguson B, Read DS, O'Connor DH (2008) Identification of MHC class I sequences in Chinese-origin rhesus macaques. Immunogenetics 60:37–46
Leslie A, Price DA, Mkhize P, Bishop K, Rathod A, Day C, Crawford H, Honeyborne I, Asher TE, Luzzi G, Edwards A, Rousseau CM, Mullins JI, Tudor-Williams G, Novelli V, Brander C, Douek DC, Kiepiela P, Walker BD, Goulder PJ (2006) Differential selection pressure exerted on HIV by CTL targeting identical epitopes but restricted by distinct HLA alleles from the same HLA supertype. J Immunol 177:4699–4708
Ling B, Veazey RS, Penedo C, Xu K, Lifson JD, Marx PA (2002) Longitudinal follow up of SIVmac pathogenesis in rhesus macaques of Chinese origin: emergence of B cell lymphoma. J Med Primatol 31:154–163
Loffredo JT, Sidney J, Wojewoda C, Dodds E, Reynolds MR, Napoe G, Mothe BR, O'Connor DH, Wilson NA, Watkins DI, Sette A (2004) Identification of seventeen new simian immunodeficiency virus-derived CD8+ T cell epitopes restricted by the high frequency molecule, Mamu-A*02, and potential escape from CTL recognition. J Immunol 173:5064–5076
Loffredo JT, Friedrich TC, Leon EJ, Stephany JJ, Rodrigues DS, Spencer SP, Bean AT, Beal DR, Burwitz BJ, Rudersdorf RA, Wallace LT, Piaskowski SM, May GE, Sidney J, Gostick E, Wilson NA, Price DA, Kallas EG, Piontkivska H, Hughes AL, Sette A, Watkins DI (2007a) CD8+ T cells from SIV elite controller macaques recognize Mamu-B*08-bound epitopes and select for widespread viral variation. PLoS ONE 2:e1152
Loffredo JT, Maxwell J, Qi Y, Glidden CE, Borchardt GJ, Soma T, Bean AT, Beal DR, Wilson NA, Rehrauer WM, Lifson JD, Carrington M, Watkins DI (2007b) Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J Virol 81:8827–8832
Loffredo JT, Sidney J, Bean AT, Beal DR, Bardet W, Wahl A, Hawkins OE, Piaskowski S, Wilson NA, Hildebrand WH, Watkins DI, Sette A (2009) Two MHC class I molecules associated with elite control of immunodeficiency virus replication, Mamu-B*08 and HLA-B*2705, bind peptides with sequence similarity. J Immunol 182:7763–7775
Madden DR, Gorga JC, Strominger JL, Wiley DC (1992) The three-dimensional structure of HLA-B27 at 2.1 A resolution suggests a general mechanism for tight peptide binding to MHC. Cell 70:1035–1048
Maness NJ, Valentine LE, May GE, Reed J, Piaskowski SM, Soma T, Furlott J, Rakasz EG, Friedrich TC, Price DA, Gostick E, Hughes AL, Sidney J, Sette A, Wilson NA, Watkins DI (2007) AIDS virus specific CD8+ T lymphocytes against an immunodominant cryptic epitope select for viral escape. J Exp Med 204:2505–2512
Maness NJ, Yant LJ, Chung C, Loffredo JT, Friedrich TC, Piaskowski SM, Furlott J, May GE, Soma T, Leon EJ, Wilson NA, Piontkivska H, Hughes AL, Sidney J, Sette A, Watkins DI (2008) Comprehensive immunological evaluation reveals surprisingly few differences between elite controller and progressor Mamu-B*17-positive simian immunodeficiency virus-infected rhesus macaques. J Virol 82:5245–5254
Maness NJ, Sacha JB, Piaskowski SM, Weisgrau KL, Rakasz EG, May GE, Buechler MB, Walsh AD, Wilson NA, Watkins DI (2009) Novel translation products from simian immunodeficiency virus SIVmac239 Env-encoding mRNA contain both Rev and cryptic T-cell epitopes. J Virol 83:10280–10285
Maness NJ, Walsh AD, Piaskowski SM, Furlott J, Kolar HL, Bean AT, Wilson NA, Watkins DI (2010) CD8+ T cell recognition of cryptic epitopes is a ubiquitous feature of AIDS virus infection. J Virol 84:11569–11574
Marthas ML, Lu D, Penedo MC, Hendrickx AG, Miller CJ (2001) Titration of an SIVmac251 stock by vaginal inoculation of Indian and Chinese origin rhesus macaques: transmission efficiency, viral loads, and antibody responses. AIDS Res Hum Retroviruses 17:1455–1466
Matano T, Shibata R, Siemon C, Connors M, Lane HC, Martin MA (1998) Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol 72:164–169
Matsumura M, Fremont DH, Peterson PA, Wilson IA (1992) Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science 257:927–934
McDermott AB, O'Connor DH, Fuenger S, Piaskowski S, Martin S, Loffredo J, Reynolds M, Reed J, Furlott J, Jacoby T, Riek C, Dodds E, Krebs K, Davies ME, Schleif WA, Casimiro DR, Shiver JW, Watkins DI (2005) Cytotoxic T-lymphocyte escape does not always explain the transient control of simian immunodeficiency virus SIVmac239 viremia in adenovirus-boosted and DNA-primed Mamu-A*01-positive rhesus macaques. J Virol 79:15556–15566
Mothe BR, Sidney J, Dzuris JL, Liebl ME, Fuenger S, Watkins DI, Sette A (2002) Characterization of the peptide-binding specificity of Mamu-B*17 and identification of Mamu-B*17-restricted epitopes derived from simian immunodeficiency virus proteins. J Immunol 169:210–219
Otting N, Heijmans CM, Noort RC, de Groot NG, Doxiadis GG, van Rood JJ, Watkins DI, Bontrop RE (2005) Unparalleled complexity of the MHC class I region in rhesus macaques. Proc Natl Acad Sci USA 102:1626–1631
Otting N, de Vos-Rouweler AJ, Heijmans CM, de Groot NG, Doxiadis GG, Bontrop RE (2007) MHC class I A region diversity and polymorphism in macaque species. Immunogenetics 59:367–375
Otting N, Heijmans CM, van der Wiel M, de Groot NG, Doxiadis GG, Bontrop RE (2008) A snapshot of the Mamu-B genes and their allelic repertoire in rhesus macaques of Chinese origin. Immunogenetics 60:507–514
Ouyang D, Wang X, He X, Xu L, Shi H, Gao Q, Guo H (2009) Construction of soluble Mamu-b*1703, a class I major histocompatibility complex of Chinese rhesus macaques, monomer and tetramer loaded with a simian immunodeficiency virus peptide. Cell Mol Immunol 6:117–122
Sacha JB, Reynolds MR, Buechler MB, Chung C, Jonas AK, Wallace LT, Weiler AM, Lee W, Piaskowski SM, Soma T, Friedrich TC, Wilson NA, Watkins DI (2008) Differential antigen presentation kinetics of CD8+ T-cell epitopes derived from the same viral protein. J Virol 82:9293–9298
Sette A, Vitiello A, Reherman B, Fowler P, Nayersina R, Kast WM, Melief CJ, Oseroff C, Yuan L, Ruppert J, Sidney J, del Guercio MF, Southwood S, Kubo RT, Chesnut RW, Grey HM, Chisari FV (1994) The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol 153:5586–5592
Sette A, Sidney J, Bui HH, del Guercio MF, Alexander J, Loffredo J, Watkins DI, Mothe BR (2005) Characterization of the peptide-binding specificity of Mamu-A*11 results in the identification of SIV-derived epitopes and interspecies cross-reactivity. Immunogenetics 57:53–68
Sidney J, Dzuris JL, Newman MJ, Johnson RP, Kaur A, Amitinder K, Walker CM, Appella E, Mothe B, Watkins DI, Sette A (2000) Definition of the Mamu A*01 peptide binding specificity: application to the identification of wild-type and optimized ligands from simian immunodeficiency virus regulatory proteins. J Immunol 165:6387–6399
Solomon C, Southwood S, Hoof I, Rudersdorf R, Peters B, Sidney J, Pinilla C, Marcondes MC, Ling B, Marx P, Sette A, Mothe BR (2010) The most common Chinese rhesus macaque MHC class I molecule shares peptide binding repertoire with the HLA-B7 supertype. Immunogenetics 62(7):451–464
Trichel AM, Rajakumar PA, Murphey-Corb M (2002) Species-specific variation in SIV disease progression between Chinese and Indian subspecies of rhesus macaque. J Med Primatol 31:171–178
Vogel TU, Friedrich TC, O'Connor DH, Rehrauer W, Dodds EJ, Hickman H, Hildebrand W, Sidney J, Sette A, Hughes A, Horton H, Vielhuber K, Rudersdorf R, De Souza IP, Reynolds MR, Allen TM, Wilson N, Watkins DI (2002) Escape in one of two cytotoxic T-lymphocyte epitopes bound by a high-frequency major histocompatibility complex class I molecule, Mamu-A*02: a paradigm for virus evolution and persistence? J Virol 76:11623–11636
Wilson NA, Reed J, Napoe GS, Piaskowski S, Szymanski A, Furlott J, Gonzalez EJ, Yant LJ, Maness NJ, May GE, Soma T, Reynolds MR, Rakasz E, Rudersdorf R, McDermott AB, O'Connor DH, Friedrich TC, Allison DB, Patki A, Picker LJ, Burton DR, Lin J, Huang L, Patel D, Heindecker G, Fan J, Citron M, Horton M, Wang F, Liang X, Shiver JW, Casimiro DR, Watkins DI (2006) Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J Virol 80:5875–5885
Wilson NA, Keele BF, Reed JS, Piaskowski SM, MacNair CE, Bett AJ, Liang X, Wang F, Thoryk E, Heidecker GJ, Citron MP, Huang L, Lin J, Vitelli S, Ahn CD, Kaizu M, Maness NJ, Reynolds MR, Friedrich TC, Loffredo JT, Rakasz EG, Erickson S, Allison DB, Piatak MJ, Lifson JD, Shiver JW, Casimiro DR, Shaw GM, Hahn BH, Watkins DI (2009) Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. J Virol 83:6508–6521
Wiseman RW, Karl JA, Bimber BN, O'Leary CE, Lank SM, Tuscher JJ, Detmer AM, Bouffard P, Levenkova N, Turcotte CL, Szekeres EJ, Wright C, Harkins T, O'Connor DH (2009) Major histocompatibility complex genotyping with massively parallel pyrosequencing. Nat Med 15:1322–1326
Wu J, Bassinger S, Montoya GD, Chavez L, Jones CE, Holder-Lockyer B, Masten B, Williams TM, Prilliman KR (2008) Allelic diversity within the high frequency Mamu-A2*05/Mane-A2*05 (Mane-A*06)/Mafa-A2*05 family of macaque MHC-A loci. Tissue Antigens 72:29–38
Yant LJ, Friedrich TC, Johnson RC, May GE, Maness NJ, Enz AM, Lifson JD, O'Connor DH, Carrington M, Watkins DI (2006) The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol 80:5074–5077
Zhumabayeva B, Chenchik A, Siebert PD (1999) RecA-mediated affinity capture: a method for full-length cDNA cloning. Biotechniques 27:834–836, 838, 840 passim
Acknowledgements
This work was supported by NIH grants R37 AI052056, R01 A1049120, R01 AI076114, R24 RR015371, R24 RR016038, and R21 AI081590 to D.I.W. and grant P51 RR000167 from the National Center for Research Resources, a component of the National Institutes of Health (to the Wisconsin National Primate Research Center, University of Wisconsin-Madison). This research was conducted in part at a facility constructed with support from Research Facilities Improvement grants RR15459-01 and RR020141-01.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maness, N.J., Walsh, A.D., Rudersdorf, R.A. et al. Chinese origin rhesus macaque major histocompatibility complex class I molecules promiscuously present epitopes from SIV associated with molecules of Indian origin; implications for immunodominance and viral escape. Immunogenetics 63, 587–597 (2011). https://doi.org/10.1007/s00251-011-0538-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-011-0538-4