Abstract
Polymorphic variability in immune response genes, such as IL12B, encoding the IL-12p40 subunit is associated with susceptibility to severe malaria in African populations. Since the role of genetic variation in conditioning severe malaria in Thai adults is largely unexplored, the functional association between IL12B polymorphisms [i.e. IL12Bpro (rs17860508) and IL12B 3′ UTR T/G (rs3212227)], severe malaria and cytokine production was examined in patients with Plasmodium falciparum infections (n = 355) recruited from malaria endemic areas along the Thai–Myanmar border in northwest Thailand. Circulating IL-12p40 (p = 0.049) and IFN-γ (p = 0.051) were elevated in patients with severe malaria, while only IL-12p40 was significantly higher in severe malaria patients with hyperparasitaemia (p = 0.046). Carriage of the IL12Bpro1.1 genotype was associated with enhanced severity of malaria (OR, 2.34; 95% CI, 0.94–5.81; p = 0.066) and hyperparasitaemia (OR, 3.42; 95% CI, 1.17–9.87; p = 0.025) relative to the IL12Bpro2.2 genotype (wild type). Individuals with the IL12Bpro1.1 genotype also had the lowest IL-12p40 (p = 0.002) and the highest IFN-γ (p = 0.004) levels. Construction of haplotypes revealed that carriage of the IL12Bpro-2/3′ UTR-T haplotype was associated with protection against severe malaria (OR, 0.51; 95% CI, 0.29–0.90; p = 0.020) and reduced circulating IFN-γ (p = 0.06). Thus, genotypic and haplotypic variation at IL12Bpro and IL12B 3′ UTR in this population influences susceptibility to severe malaria and functional changes in circulating IL-12p40 and IFN-γ levels. Results presented here suggest that protection against severe malaria in Thai adults is associated with genotypic variants that condition enhanced IL-12p40 and reduced IFN-γ levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infection with Plasmodium falciparum displays a wide spectrum of disease manifestations ranging from asymptomatic presentation to severe disease. The host immune response to malaria is coordinated by the release of pro- and anti-inflammatory cytokines (Kurtzhals et al. 1999; Luty et al. 2000; Othoro et al. 1999; Perkins et al. 2000; Prakash et al. 2006). Depending on the temporal expression and magnitude of production, pro-inflammatory (type-1) cytokines, such as interleukin (IL)-12 and interferon (IFN)-γ, promote both protective and pathogenic responses in malaria (McDevitt et al. 2004). IL-12 is produced primarily by antigen presenting cells (Ing et al. 2006) and promotes IFN-γ release from cells of the innate immune system, including natural killer (NK) cells (Artavanis-Tsakonas and Riley 2002; Hansen et al. 2007), NK-T cells (Schmieg et al. 2003) and γδ T cells (D’Ombrain et al. 2007). IL-12 is also important in adaptive immunity through its ability to augment IFN-γ production from CD4+ T cells (Stephens and Langhorne 2006) and CD8+ T cells (Schmidt et al. 2009). As such, IL-12-promoted release of IFN-γ is a primary mechanism for limiting intracellular pathogen growth (Chehimi and Trinchieri 1994).
IL-12 plays an important protective role against malaria in murine (Mohan and Stevenson 1998b; Sam and Stevenson 1999) and human systems (Boutlis et al. 2003; Keller et al. 2006; Perkins et al. 2000; Prakash et al. 2006; Wroczynska et al. 2005). Reduced circulating levels of IL-12 are associated with enhanced malaria pathogenesis in African children (Luty et al. 2000; Perkins et al. 2000) and non-immune adults (Wroczynska et al. 2005), while elevated plasma concentrations are associated with increased severity of malaria in Asian adults (Gosi et al. 1999; Prakash et al. 2006).
In addition, IFN-γ is an essential mediator of protective immunity against erythrocytic malaria (Favre et al. 1997; Stevenson et al. 1995). Early release of IL-12, IFN-γ and TNF-α in murine models of malaria promotes resistance to infection (De Souza et al. 1997; Favre et al. 1997; Mitchell et al. 2005; Shear et al. 1989; Stevenson et al. 1995). Results from studies in humans also show that enhanced cellular IFN-γ responses are associated with protective immunity against clinical malaria (Iriemenam et al. 2009; Luty et al. 1999; Migot-Nabias et al. 1999; Robinson et al. 2009). In contrast, other investigations have found that elevated circulating levels of IFN-γ are associated with increased pathophysiology of falciparum malaria (Day et al. 1999; Prakash et al. 2006).
Susceptibility to malaria infections and the clinical course of disease once an individual becomes infected are influenced by host genetic variation (Verra et al. 2008, 2009; Weatherall 2008). Association studies in malaria have shown that variation in both innate and adaptive immune pathways condition disease susceptibility and outcomes (Kwiatkowski 2005). Since IL-12 is important for mediating malaria disease outcomes, the current study investigated the role of genetic variation in IL12B in conditioning susceptibility to severe malaria in Thai adults infected with P. falciparum. IL-12 is a heterodimer composed of IL-12p35 and IL-12p40 subunits, encoded by IL12A and IL12B genes located on chromosomes 3p12-q13.2 and 5q31-33, respectively (Sieburth et al. 1992). A number of single nucleotide polymorphisms have been identified in the IL12B gene, including an IL12B promoter polymorphism (IL12Bpro, a bi-allelic promoter polymorphism located at −2,703 bp from the transcription initiation site, rs17860508) and a TaqI polymorphism (a T to G transition at 1188) in the IL12B 3′ untranslated region (referred to as IL12B 3′ UTR from hence forth, rs321227) (Huang et al. 2000). These variants have been shown to be important for conditioning susceptibility to a number of infectious (Marquet et al. 2008; Morahan et al. 2002; Mueller et al. 2004; Tso et al. 2004) and inflammatory diseases (Cargill et al. 2007; Zwiers et al. 2004). Although the relationship between genetic variability in IL12B and susceptibility to severe malaria has shown mixed results (Barbier et al. 2008; Marquet et al. 2008; Morahan et al. 2002), homozygosity for the CTCTAA (IL12Bpro1) allele of the IL12B promoter variant is associated with increased mortality in Tanzanian children with cerebral malaria, but not Kenyan children with severe malaria (Morahan et al. 2002). Variability in IL12B was further associated with P. falciparum parasitaemia in Burkina Faso (Flori et al. 2003; Rihet et al. 1999); however, subsequent familial-based studies failed to show a significant correlation between IL12Bpro and a polymorphism in the 3′ untranslated region of IL12B (IL12B 3′ UTR) and hyperparasitaemia (Barbier et al. 2008).
The aim of the present study was to determine the role of IL12Bpro and IL12B 3′ UTR variants in conditioning susceptibility to severe malaria and functional changes in IL-12 and IFN-γ levels in Thai adults with falciparum malaria. The primary hypothesis of the study is that haplotypes of IL12B form functional blocks that mediate susceptibility to severe malaria by altering the levels of two critical cytokines that regulate innate and adaptive immune responses: IL-12 and IFN-γ. Determining the role of genetic variability in conditioning susceptibility to severe malaria in Thai adults, particularly in terms of haplotypic blocks, is significant, since the genes and gene pathways that mediate disease outcomes are largely unexplored in this population. Results presented here demonstrate that genotypes/haplotypes of IL12Bpro and IL12B 3′ UTR are associated with differing susceptibilities to severe malaria and altered circulating levels of IL-12 and IFN-γ.
Methods and materials
Study subjects
Patients with falciparum malaria (n = 355; age 18 to 67 years, mean ± SD = 28.3 ± 10.5) admitted to the Hospital for Tropical Diseases, Faculty of Tropical Medicine, Mahidol University, Bangkok were enrolled in an unmatched case–control study. The patients included those with severe malaria (n = 103, cases) and those with uncomplicated malaria (n = 252, controls) who had been living along the Thai–Myanmar border in the northwest of Thailand where malaria is endemic. These areas are considered to be of low malaria endemicity with two peak seasonal transmissions in May–July and November–January (Luxemburger et al. 1997; Nacher et al. 2001). Incidence rates of malaria were two to six cases per 1,000 population in 2001 (Socheat et al. 2003). In this region, adults are most at risk for the complications of severe malaria including hyperparasitaemia, jaundice, renal dysfunction and cerebral malaria, with severe malarial anaemia occurring only rarely. All patients with non-falciparum malaria infections, mixed Plasmodium infections, and the signs and symptoms of AIDS defining illness and/or meningitis were excluded from the study.
All patients were positive for P. falciparum infection by microscopic examination of thin and thick blood smears stained with Giemsa. Severe and uncomplicated malaria were defined according to World Health Organization criteria (WHO 2000). Cerebral malaria was defined as an unarousable coma with positive asexual forms of P. falciparum in peripheral blood. Severe malaria, in the absence of cerebral malaria, was defined as individuals with one or more of the following signs: hyperparasitaemia (>250,000 parasites/μL), hypoglycaemia (glucose <2.2 mmol/L), severe anaemia (haematocrit <20% or haemoglobin <7.0 g/dL) or increased serum levels of creatinine >3.0 mg/dL. Study participants with positive P. falciparum blood films who lacked these signs of severe malaria were categorised as uncomplicated malaria (controls). Patients with severe malaria were further stratified into hyperparasitaemia (≥250,000 parasites/µL) and non-hyperparasitaemia (<250,000 parasites/µL). The study was approved by the Ethical Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, with informed written consent obtained from all study participants.
Laboratory measures
Venipuncture blood (3–5 mL) was collected aseptically in EDTA-containing tubes prior to any treatment interventions. Plasma and packed cells were separated by centrifugation and stored at −20°C until use. Laboratory measures included parasite density determination, complete blood count and clinical biochemistry tests. Parasite densities were determined, and the number of parasites per 1,000 erythrocytes (in thin blood films) or parasites per 200 leukocytes (in thick films) were calculated and expressed as P. falciparum parasites per microlitre of blood.
DNA extraction and whole genome amplification
Genomic DNA was extracted using a FlexiGene DNA extraction kit (QIAGEN, Valencia, CA, USA). Prior to genotyping, whole genome amplification was performed by isothermal strand displacement using GenomiPhi V2 DNA Amplification kit (GE Healthcare, Piscataway, NJ, USA).
Genotyping
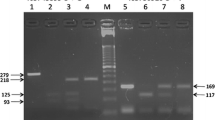
IL12B 3′ UTR (rs3212227) polymorphisms were genotyped using TaqMan® 5′ Allelic Discrimination Pre-Designed Assays (Applied Biosystems, USA). IL12Bpro (rs17860508) genotyping was performed by polymerase chain reaction (PCR)–restriction fragment length polymorphism as described previously (Khoo et al. 2004) with some minor modifications. Briefly, the primers were 5′-TACAGCCTGTCTCCGAG AGAA-3′ and 5′-GAGGAAGTGGTTCTCGTACTTTAGC-3′. The PCR reaction was performed in a 25-μL reaction mixture containing 100 ng DNA, 2.5 mmol/L MgCl2, 200 mmol/L dNTPs, 12.5 ng and 1 unit of Taq DNA polymerase (Promega, Medison, WI, USA) in buffer (10 mmol/L Tris–HCl, pH 9.0 and 50 mmol/L KCl). Underlined bases (indicted in the forward and reverse primers) were inserted to create an AluI site in allele 1 (CTCTAA) or in allele 2 (GC). PCR conditions consisted of an initial 3-min denaturation at 95°C, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 62°C for 30 s, and extension at 72°C for 45 s with a final extension at 72°C for 7 min. PCR products (15 μL) were further digested in a 20-μL reaction volume containing 1.5 units of AluI (New England Biolabs, USA) at 37°C for 14 h. Digested products were separated on 4% high resolution agarose gel (Agarose SFR, Amresco, Solon, OH, USA) containing ethidium bromide and visualised under UV illumination.
Determination of circulating IL-12p40 and IFN-γ levels
Plasma IL-12p40 levels were determined by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (BD Biosciences Pharmingen, San Diago, CA, USA). Circulating levels of IFN-γ were determined by ELISA (R&D systems, Minneapolis, MN, USA) as previously described (Tangteerawatana et al. 2007). Optical densities were measured at 405-nm wavelength, and cytokine concentrations were calculated from the standard curve using recombinant human IL-12 p40 and IFN-γ, respectively. Lower limits of detection for IL-12p40 and IFN-γ were 10 and 25 pg/mL, respectively.
Statistical analyses
Statistical analyses were performed using Minitab® 15 Statistical Software (Minitab Inc., State College, PA, USA) and SPSS (Version 15.0, SPSS Inc., Chicago, IL, USA). Deviation from Hardy–Weinberg equilibrium was determined using web-based calculations (http://www.kursus.kvl.dk/shares/vetgen_Popgen/genetic/appleys/kitest.htm). Chi-square (χ 2) analyses were used to compare proportions. Students’s t test was used to compare demographic and clinical characteristics in uncomplicated vs. severe malaria. Across-group comparisons were determined by Kruskal–Wallis tests, and where significant, Mann–Whitney U tests were used for post hoc comparisons. IL12B haplotypes (IL12Bpro and IL12B 3′ UTR T/G) were constructed using HPlus software (Version 2.5). The association between individual polymorphisms (and haplotypes) and malaria clinical outcomes were determined by multivariate logistic regression analysis, controlling for the confounding effects of age and gender. Statistical significance was defined as p ≤ 0.05.
Results
Demographic clinical and laboratory characteristics of the study subjects
The demographic, clinical and laboratory characteristics of the study subjects are shown in Table 1. No significant differences were observed in the distribution of age (p = 0.082) between the severe (SM) and the uncomplicated malaria (UM) groups. Gender differed significantly between the two groups (p = 0.002). Peripheral parasitaemia was also significantly different between the groups (p < 0.001) with the highest parasitaemia present in the severe malaria group. Although haemoglobin (Hb) concentrations were not significantly lower in the SM group (p = 0.091), the number of red blood cells was significantly reduced (p = 0.007) in these patients. The white blood cell count was elevated in the SM group (p < 0.001), while lymphocyte counts and platelets were significantly lower (p < 0.001 and p < 0.001, respectively). Total bilirubin and creatinine concentrations were significantly higher in patients with severe malaria (p < 0.001 and p = 0.029, respectively).
Circulating levels of IL-12p40 and IFN-γ in malaria-infected patients
Prior to determining the influence of IL12B genotypes/haplotypes on susceptibility to severe malaria and functional changes in cytokine production, circulating levels of IL-12p40 and IFN-γ were determined in the UM and SM groups and in patients with severe malaria with and without hyperparasitaemia. As shown in Table 2, patients with severe malaria had significantly higher circulating IL-12p40 levels [median (IQR) 95.1 (27.6–133.1)] than those with uncomplicated malaria [median (IQR) 27.0 (10.0–98.8), p = 0.049]. In addition, circulating IFN-γ levels were also higher in patients with severe malaria [median (IQR) 387.0 (144.2–632.0)] compared to the uncomplicated malaria group [median (IQR) 283.5 (118.5–473.5), p = 0.051, Table 2].
Analysis of IL-12 and IFN-γ in patients with severe malaria, stratified according to hyperparasitaemia, demonstrated that individuals with hyperparasitaemia had significantly higher circulating IL-12p40 levels [median (IQR) 121.9 (43.3–163.3)] than those with non-hyperparasitaemia [median (IQR) 35.1 (12.5–103.9)], p = 0.046 (Table 2). However, circulating IFN-γ levels were not significantly different in severe malaria patients with and without hyperparasitaemia (Table 2).
Distribution of IL12Bpro and IL12B 3′ UTR genotypic frequencies
The distribution of genotypes for IL12Bpro and IL12B 3′ UTR are presented in Table 3. The distribution of the IL12Bpro1.1 and IL12B 3′ UTR-GG genotypes was significantly different between the UM and the SM groups (p = 0.017 and p = 0.021, respectively). None of the other genotypes showed any significant differences in frequency between the UM and SM groups.
Further investigation of the IL12Bpro polymorphism revealed significant departure from Hardy–Weinberg equilibrium (HWE) in the UM (χ 2 = 63.28, p < 0.001) and SM (χ 2 = 16.41, p < 0.001) groups, respectively. However, the IL12B 3′ UTR polymorphism failed to display significant departure from HWE in both the UM (χ 2 = 1.28; p > 0.05) and SM (χ 2 = 2.68; p > 0.05) groups, respectively.
Association between IL12B polymorphisms and susceptibility to severe malaria and hyperparasitaemia
Multivariate logistic regression analyses were used to determine the association between variability in each of the two IL12B polymorphisms and severe malaria, controlling for the confounding effects of age and gender. In addition, since hyperparasitaemia is a prominent feature of severe malaria in Thailand, multivariate modelling (controlling for identical co-factors) was used to examine the relationship between variation in IL12B genes and hyperparasitaemia in patients with severe malaria. Presence of the IL12Bpro1.1 genotype was associated with increased susceptibility to severe malaria (OR, 2.34; 95% CI, 0.94–5.81, p = 0.066) and hyperparasitaemia (OR, 3.42; 95% CI, 1.17–9.97, p = 0.025, Table 4).
In addition, heterozygous (TG) individuals at the IL12B 3′ UTR locus were 39% less likely to develop severe malaria (OR, 0.61; 95% CI, 0.33–1.00, p = 0.096), while homozygous polymorphic individuals (GG) were 30% less likely to develop severe malaria (OR, 0.60; 95% CI, 0.36–1.39, p = 0.311, Table 4). Variation at IL12B 3′ UTR did not show any prominent relationships with hyperparasitaemia.
Association between IL12B haplotypes and susceptibility to severe malaria and hyperparasitaemia
Following analyses of the individual variants, haplotypes were constructed using HPlus software. The following haplotypic distributions were generated: 68.9% (184/267) IL12Bpro-2/3′ UTR-T; 26.2% (70/267) IL12Bpro-2/3′ UTR-G; 12.7% (34/267) IL12Bpro-1/3′ UTR-T; and 68.9% (184/267) IL12Bpro-1/3′ UTR-G. Multivariate logistic regression analyses, controlling for the confounding effects of age and gender, were used to determine the association of IL12B haplotypes with severe malaria and hyperparasitaemia. The model was constructed such that those with vs. those without presence of the haplotype were compared. The IL12Bpro-2/3′ UTR-T haplotype was associated with significant protection against severe malaria (OR, 0.51; 95% CI, 0.29–0.90, p = 0.020) and non-significant protection against hyperparasitaemia (OR, 0.57; 95% CI, 0.28–1.18, p = 0.132, Table 5). Additional analyses failed to show any significant haplotypic associations with susceptibility to either severe malaria or hyperparasitaemia for IL12Bpro-1/3′ UTR-G, IL12Bpro-2/3′ UTR-G and IL12Bpro-1/3′ UTR-T (Table 5).
Functional relationship between the IL12B polymorphisms and circulating levels of IL-12p40 and IFN-γ
Since variation in IL12B may influence IL-12p40 and IFN-γ production, circulating levels of IL-12p40 and IFN-γ were compared across the UM and SM groups stratified according to genotype for their respective polymorphisms (Table 6). There was a significance difference in IL-12p40 levels across the UM group (p = 0.002), but not in individuals with SM (p = 0.919). Post hoc testing of the UM group revealed that carriage of the IL12Bpro1.2 genotype was associated with significantly higher IL-12p40 levels [median (IQR) 75.4 (13.5–112.5)] compared to the IL12Bpro1.1 genotype [median (IQR) 10.0 (10.0–10.0), p = 0.002] and the IL12Bpro2.2 genotype [median (IQR) 20.0 (10.0–69.3), p = 0.040]. In addition, patients with the IL12Bpro2.2 genotype also had higher IL-12p40 levels than those with IL12Bpro1.1 genotype (p = 0.002).
Stratification according to IL12Bpro genotypes showed that IFN-γ levels were also significantly different across the UM group (p = 0.004), but not across the SM group (p = 0.508). Post hoc analyses of the UM group revealed that carriage of the IL12Bpro1.1 genotype [median (IQR) 438.5 (227.7–585.2)] was associated with significantly higher IFN-γ levels compared to the IL12Bpro2.2 genotype [median (IQR) 140.0 (43.0–314.0), p = 0.002]. In addition, individuals with the IL12Bpro1.2 genotype [median (IQR) 338.5 (125.5–522.2)] also had significantly higher IFN-γ levels than those with the IL12Bpro2.2 genotype (p = 0.004).
Comparison of IL-12p40 and IFN-γ levels across the UM and SM groups stratified according to the IL12B 3′ UTR polymorphisms did not reveal any significant differences.
Functional relationship between IL12B haplotypes and circulating IL-12 and IFN-γ levels
To determine if haplotypes were associated with functional changes in IL-12 and IFN-γ production, circulating concentrations of these mediators were compared across the haplotypic groups. Although differences in IFN-γ levels approached significance between individuals with the IL12Bpro-2/3′ UTR-T haplotype [median (IQR); 267.5 (117.5–527.0)] relative to those with the non-IL12Bpro-2/3′ UTR-T haplotype [median (IQR); 387.0 (164.0–597.0), p = 0.06], none of the other haplotypes showed any functional association with circulating IL-12 and IFN-γ levels (Table 6).
Discussion
Although the literature is replete with studies investigating genetic susceptibility to severe malaria in African populations, much less information is available about how variation in immune response genes condition severe disease outcomes in Southeast Asia. As such, we utilised a candidate gene approach to focus on the role of IL-12 in shaping severe malaria outcomes in Thai adults with falciparum malaria. IL-12 was selected for investigation, since this type I cytokine plays a critical role in protection against malaria in both animal models (Mohan and Stevenson 1998a; Sam and Stevenson 1999) and in humans (Boutlis et al. 2003; Gosi et al. 1999; Keller et al. 2006; Luty et al. 2000; Perkins et al. 2000; Wroczynska et al. 2005). We hypothesised that exploration of variation in two previously identified polymorphisms in IL12B (i.e. IL12Bpro and IL12B 3′UTR) may provide insight into the role of IL-12 in mediating susceptibility to severe malaria and another primary feature of severe falciparum malaria in Thai adults: hyperparasitaemia. The cross-sectional study design utilised for the current investigation included only patients with P. falciparum parasitaemia, so that we could explore the role of IL-12 in mediating severe disease outcomes once an individual acquires malaria.
Prior to determining the effect of IL12B genotypes and haplotypes on conditioning disease outcomes and functional changes in IL-12p40 and IFN-γ production, we examined these cytokines in parasitized individuals stratified according to uncomplicated and severe malaria and in severe malaria patients with and without hyperparasitaemia. Circulating levels of IL-12p40 and IFN-γ were highest in patients with severe malaria and hyperparasitaemia, respectively. The patterns of cytokine expression observed here are both similar and different from a number of previous studies conducted in populations with differing ages and levels of malaria endemicity. For example, results presented here in the largely less immune adult population to malaria most closely resemble studies in non-immune adults undergoing experimental infection with P. falciparum in which there was an early release of plasma IL-12p40 and IFN-γ (Hermsen et al. 2003). Our results differ somewhat from previous studies in Cameroon in which plasma levels of IL-12p40 and IL-12p70 were not significantly elevated in children with severe malaria, while circulating concentrations of IFN-γ were significantly higher in this group (Hermsen et al. 2003). In addition, results presented here differ from investigations in Gabon and Kenya demonstrating that severe childhood malaria is characterised by suppression of circulating IL-12 p40/p70 levels (Keller et al. 2006; Luty et al. 2000; Perkins et al. 2000). An additional explanation for the heterogeneity in IL-12p40/p70 and IFN-γ responses in the differing populations may be, at least in part, due to host genetic factors (Artavanis-Tsakonas and Riley 2002; D’Ombrain et al. 2007; D’Ombrain et al. 2008; Mueller et al. 2004; Peng et al. 2006; Shimokawa et al. 2009). Genotyping of the overall population in the study revealed frequencies for the IL12Bpro polymorphism at 8.8% (IL12Bpro1.1), 72.8% (IL12Bpro1.2) and 18.4% (IL12Bpro2.2), while frequencies for the IL12B 3′ UTR polymorphism were 26.2% (TT), 44.7% (TG) and 29.1% (GG). Among patients with severe malaria, there were significantly elevated frequencies of IL12Bpro1.1 and IL12B 3′ UTR-GG compared to the uncomplicated malaria group, while none of the other distributions significantly differed. Frequencies of the IL12Bpro polymorphism in the Thai population investigated here differ from those observed in Chinese (Tso et al. 2004) and Spanish (Orozco et al. 2005) populations. Distributions of the IL12B 3′ UTR polymorphism were comparable to those reported previously in Thai (Nair et al. 2000) and Japanese (Yang et al. 2006) populations, but different from observations in Caucasian Americans and African Americans (Ma et al. 2003).
Multivariate analyses, controlling for the confounding effects of age and gender, revealed that carriage of the IL12Bpro1.1genotype was associated with a non-significant increase in the risk of severe malaria and a significant increase in susceptibility to hyperparasitaemia. However, variation at the IL12B 3′ UTR was not associated with susceptibility to either severe malaria or hyperparasitaemia. Since haplotypes can identify important associations with disease outcomes that may not be reflected by analysis of individual loci, we performed haplotypic analyses. Construction of haplotypes for the two IL12B polymorphisms revealed that carriage of the IL12Bpro-2/3′ UTR-T haplotype was associated with a 49% reduction in severe malaria (p = 0.020) and a 43% reduction in hyperparasitaemia (p = 0.132). Additional haplotypic analyses did not reveal any significant associations with severe malaria or hyperparasitaemia. Although not statistically significant, it is important to note that individuals with the IL12Bpro-1/3′ UTR-T haplotype had a 115% increase in susceptibility to hyperparasitaemia relative to those without the haplotype. The lack of statistical significance for this haplotype likely reflects reduced sample size issues in the current study that may deserve closer inspection in a larger cohort.
Recent reports illustrate that the IL12Bpro and IL12B 3′ UTR polymorphisms affect IL-12 gene expression (Shimokawa et al. 2009) and IL-12 production in vitro (Muller-Berghaus et al. 2004; Peng et al. 2006; Seegers et al. 2002). Results presented here demonstrate that carriage of the heterozygous (i.e. IL12Bpro1.2) genotype was associated with significantly higher IL-12p40 levels in patients with uncomplicated malaria. In individuals with severe malaria, carriage of the IL12Bpro2 allele was associated with the highest levels of IL-12p40, suggesting that this allele may functionally influence increased IL-12 production. These results are consistent with the fact that the IL12Bpro2 allele appears to influence IL-12 production by altering Sp1-mediated transcription activity (Shimokawa et al. 2009). These results also parallel the studies in Papua New Guineans and Africans showing that the IL12Bpro1.1 genotype was associated with reduced circulating levels of IL-12p40 and elevated parasitaemia (Boutlis et al. 2003). In contrast to IL-12, IFN-γ levels were highest in individuals with the IL12Bpro1.1 polymorphism in both uncomplicated and severe malaria. Thus, although enhanced IL-12 production is typically associated with elevated IFN-γ levels, the results presented here illustrate that this relationship is not present in Thai adults with malaria and that this relationship is conditioned by variation in the IL12B promoter. Analysis of cytokine production stratified according to haplotypes revealed that the IL12Bpro-2/3′ UTR-T that conditioned decreased susceptibility to severe malaria was also associated with reduced IFN-γ levels. These results are consistent with our finding that individuals with severe malaria have the highest levels of circulating IFN-γ, suggesting that over-expression of IFN-γ in this population may be important for enhancing the development of severe malaria.
In conclusion, results presented here in Thai adults with falciparum malaria illustrate that elevated expression of IL-12 and IFN-γ are associated with severe disease and a primary clinical feature of severe disease (i.e. hyperparasitaemia) in this population. This study further illustrates that haplotypes of the IL12Bpro and 3′ UTR polymorphisms, namely the IL12Bpro-2/3′ UTR-T, is associated with decreased susceptibility to severe malaria and reduced circulating levels of IFN-γ. As such, it appears that one primary effect of variation in the IL12B promoter on conditioning disease severity in this population may be, at least in part, mediated through IFN-γ. Future studies aimed at examining additional variation in IL12B, as well as IFN-γ genes, may provide important insight into the complex genetic pathways that condition susceptibility to severe malaria in Thai adults.
Abbreviations
- SNP:
-
Single nucleotide polymorphism
- IL-12:
-
Interleukin-12
- IL-12p40:
-
40-kDa subunit of IL-12
- IFN-γ:
-
Interferon gamma
- IL12B :
-
Gene encoding 40-kDa subunit of IL-12, IL12B 3′ UTR:A → C SNP located in the 3′ untranslated region (UTR) at position 1188 of the IL12B gene
- IL12Bpro:
-
A compound polymorphism involving a GC/TT transition combined with an AGAG micro-insertion within the promoter region of the IL12Bpro
References
Artavanis-Tsakonas K, Riley EM (2002) Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol 169:2956–2963
Barbier M, Atkinson A, Fumoux F, Rihet P (2008) IL12B polymorphisms are linked but not associated with Plasmodium falciparum parasitaemia: a familial study in Burkina Faso. Genes Immun 9:405–411
Boutlis CS, Lagog M, Chaisavaneeyakorn S, Misukonis MA, Bockarie MJ, Mgone CS, Wang Z, Morahan G, Weinberg JB, Udhayakumar V, Anstey NM (2003) Plasma interleukin-12 in malaria-tolerant Papua New Guineans: inverse correlation with Plasmodium falciparum parasitaemia and peripheral blood mononuclear cell nitric oxide synthase activity. Infect Immun 71:6354–6357
Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, Matsunami N, Ardlie KG, Civello D, Catanese JJ, Leong DU, Panko JM, McAllister LB, Hansen CB, Papenfuss J, Prescott SM, White TJ, Leppert MF, Krueger GG, Begovich AB (2007) A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet 80:273–290
Chehimi J, Trinchieri G (1994) Interleukin-12: a bridge between innate resistance and adaptive immunity with a role in infection and acquired immunodeficiency. J Clin Immunol 14:149–161
D’Ombrain MC, Hansen DS, Simpson KM, Schofield L (2007) Gammadelta-T cells expressing NK receptors predominate over NK cells and conventional T cells in the innate IFN-gamma response to Plasmodium falciparum malaria. Eur J Immunol 37:1864–1873
D’Ombrain MC, Robinson LJ, Stanisic DI, Taraika J, Bernard N, Michon P, Mueller I, Schofield L (2008) Association of early interferon-gamma production with immunity to clinical malaria: a longitudinal study among Papua New Guinean children. Clin Infect Dis 47:1380–1387
Day NP, Hien TT, Schollaardt T, Loc PP, Chuong LV, Chau TT, Mai NT, Phu NH, Sinh DX, White NJ, Ho M (1999) The prognostic and pathophysiologic role of pro-and antiinflammatory cytokines in severe malaria. J Infect Dis 180:1288–1297
De Souza JB, Williamson KH, Otani T, Playfair JH (1997) Early gamma interferon responses in lethal and nonlethal murine blood-stage malaria. Infect Immun 65:1593–1598
Favre N, Ryffel B, Bordmann G, Rudin W (1997) The course of Plasmodium chabaudi chabaudi infections in interferon-gamma receptor deficient mice. Parasite Immunol 19:375–383
Flori L, Kumulungui B, Aucan C, Esnault C, Traore AS, Fumoux F, Rihet P (2003) Linkage and association between Plasmodium falciparum blood infection levels and chromosome 5q31–q33. Genes Immun 4:265–268
Gosi P, Khusmith S, Looareesuwan S, Sitachamroom U, Glanarongran R, Buchachart K, Walsh DS (1999) Complicated malaria is associated with differential elevations in serum levels of interleukins 10, 12, and 15. Southeast Asian J Trop Med Public Health 30:412–417
Hansen DS, Bernard NJ, Nie CQ, Schofield L (2007) NK cells stimulate recruitment of CXCR3+ T cells to the brain during Plasmodium berghei-mediated cerebral malaria. J Immunol 178:5779–5788
Hermsen CC, Konijnenberg Y, Mulder L, Loe C, van Deuren M, van der Meer JW, van Mierlo GJ, Eling WM, Hack CE, Sauerwein RW (2003) Circulating concentrations of soluble granzyme A and B increase during natural and experimental Plasmodium falciparum infections. Clin Exp Immunol 132:467–472
Huang D, Cancilla MR, Morahan G (2000) Complete primary structure, chromosomal localisation, and definition of polymorphisms of the gene encoding the human interleukin-12 p40 subunit. Genes Immun 1:515–520
Ing R, Segura M, Thawani N, Tam M, Stevenson MM (2006) Interaction of mouse dendritic cells and malaria-infected erythrocytes: uptake, maturation, and antigen presentation. J Immunol 176:441–450
Iriemenam NC, Okafor CM, Balogun HA, Ayede I, Omosun Y, Persson JO, Hagstedt M, Anumudu CI, Nwuba RI, Troye-Blomberg M, Berzins K (2009) Cytokine profiles and antibody responses to Plasmodium falciparum malaria infection in individuals living in Ibadan, southwest Nigeria. Afr Health Sci 9:66–74
Keller CC, Yamo O, Ouma C, Ong’echa JM, Ounah D, Hittner JB, Vulule JM, Perkins DJ (2006) Acquisition of haemozoin by monocytes down-regulates interleukin-12 p40 (IL-12p40) transcripts and circulating IL-12p70 through an IL-10-dependent mechanism: in vivo and in vitro findings in severe malarial anaemia. Infect Immun 74:5249–5260
Khoo SK, Hayden CM, Roberts M, Horak E, de Klerk N, Zhang G, Robertson CF, Goldblatt J, Le Souef P (2004) Associations of the IL12B promoter polymorphism in longitudinal data from asthmatic patients 7 to 42 years of age. J Allergy Clin Immunol 113:475–481
Kurtzhals JA, Akanmori BD, Goka BQ, Adabayeri V, Nkrumah FK, Behr C, Hviid L (1999) The cytokine balance in severe malarial anaemia. J Infect Dis 180:1753–1755
Kwiatkowski DP (2005) How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet 77:171–192
Luty AJ, Lell B, Schmidt-Ott R, Lehman LG, Luckner D, Greve B, Matousek P, Herbich K, Schmid D, Migot-Nabias F, Deloron P, Nussenzweig RS, Kremsner PG (1999) Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis 179:980–988
Luty AJ, Perkins DJ, Lell B, Schmidt-Ott R, Lehman LG, Luckner D, Greve B, Matousek P, Herbich K, Schmid D, Weinberg JB, Kremsner PG (2000) Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect Immun 68:3909–3915
Luxemburger C, Ricci F, Nosten F, Raimond D, Bathet S, White NJ (1997) The epidemiology of severe malaria in an area of low transmission in Thailand. Trans R Soc Trop Med Hyg 91:256–262
Ma X, Reich RA, Gonzalez O, Pan X, Fothergill AK, Starke JR, Teeter LD, Musser JM, Graviss EA (2003) No evidence for association between the polymorphism in the 3′ untranslated region of interleukin-12B and human susceptibility to tuberculosis. J Infect Dis 188:1116–1118
Marquet S, Doumbo O, Cabantous S, Poudiougou B, Argiro L, Safeukui I, Konate S, Sissoko S, Chevereau E, Traore A, Keita MM, Chevillard C, Abel L, Dessein AJ (2008) A functional promoter variant in IL12B predisposes to cerebral malaria. Hum Mol Genet 17:2190–2195
McDevitt MA, Xie J, Gordeuk V, Bucala R (2004) The anaemia of malaria infection: role of inflammatory cytokines. Curr Hematol Rep 3:97–106
Migot-Nabias F, Luty AJ, Ringwald P, Vaillant M, Dubois B, Renaut A, Mayombo RJ, Minh TN, Fievet N, Mbessi JR, Millet P, Deloron P (1999) Immune responses against Plasmodium falciparum asexual blood-stage antigens and disease susceptibility in Gabonese and Cameroonian children. Am J Trop Med Hyg 61:488–494
Mitchell AJ, Hansen AM, Hee L, Ball HJ, Potter SM, Walker JC, Hunt NH (2005) Early cytokine production is associated with protection from murine cerebral malaria. Infect Immun 73:5645–5653
Mohan K, Stevenson MM (1998a) Dyserythropoiesis and severe anaemia associated with malaria correlate with deficient interleukin-12 production. Br J Haematol 103:942–949
Mohan K, Stevenson MM (1998b) Interleukin-12 corrects severe anaemia during blood-stage Plasmodium chabaudi AS in susceptible A/J mice. Exp Hematol 26:45–52
Morahan G, Boutlis CS, Huang D, Pain A, Saunders JR, Hobbs MR, Granger DL, Weinberg JB, Peshu N, Mwaikambo ED, Marsh K, Roberts DJ, Anstey NM (2002) A promoter polymorphism in the gene encoding interleukin-12 p40 (IL12B) is associated with mortality from cerebral malaria and with reduced nitric oxide production. Genes Immun 3:414–418
Mueller T, Mas-Marques A, Sarrazin C, Wiese M, Halangk J, Witt H, Ahlenstiel G, Spengler U, Goebel U, Wiedenmann B, Schreier E, Berg T (2004) Influence of interleukin 12B (IL12B) polymorphisms on spontaneous and treatment-induced recovery from hepatitis C virus infection. J Hepatol 41:652–658
Muller-Berghaus J, Kern K, Paschen A, Nguyen XD, Kluter H, Morahan G, Schadendorf D (2004) Deficient IL-12p70 secretion by dendritic cells based on IL12B promoter genotype. Genes Immun 5:431–434
Nacher M, Treeprasertsuk S, Singhasivanon P, Silachamroon U, Vannaphan S, Gay F, Looareesuwan S, Wilairatana P (2001) Association of hepatomegaly and jaundice with acute renal failure but not with cerebral malaria in severe falciparum malaria in Thailand. Am J Trop Med Hyg 65:828–833
Nair RP, Stuart P, Henseler T, Jenisch S, Chia NV, Westphal E, Schork NJ, Kim J, Lim HW, Christophers E, Voorhees JJ, Elder JT (2000) Localization of psoriasis-susceptibility locus PSORS1 to a 60-kb interval telomeric to HLA-C. Am J Hum Genet 66:1833–1844
Orozco G, Gonzalez-Gay MA, Paco L, Lopez-Nevot MA, Guzman M, Pascual-Salcedo D, Balsa A, Martin J (2005) Interleukin 12 (IL12B) and interleukin 12 receptor (IL12RB1) gene polymorphisms in rheumatoid arthritis. Hum Immunol 66:710–715
Othoro C, Lal AA, Nahlen B, Koech D, Orago AS, Udhayakumar V (1999) A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anaemia in children residing in a holoendemic malaria region in western Kenya. J Infect Dis 179:279–282
Peng JC, Abu Bakar S, Richardson MM, Jonsson JJ, Frazer IH, Nielsen LK, Morahan G, Thomas R (2006) IL10 and IL12B polymorphisms each influence IL-12p70 secretion by dendritic cells in response to LPS. Immunol Cell Biol 84:227–232
Perkins DJ, Weinberg JB, Kremsner PG (2000) Reduced interleukin-12 and transforming growth factor-beta1 in severe childhood malaria: relationship of cytokine balance with disease severity. J Infect Dis 182:988–992
Prakash D, Fesel C, Jain R, Cazenave PA, Mishra GC, Pied S (2006) Clusters of cytokines determine malaria severity in Plasmodium falciparum-infected patients from endemic areas of Central India. J Infect Dis 194:198–207
Rihet P, Traore Y, Aucan C, Traore-Leroux T, Kumulungui B, Traore AS, Abel L, Fumoux F (1999) Genetic dissection of Plasmodium falciparum blood infection levels and other complex traits related to human malaria infection. Parassitologia 41:83–87
Robinson LJ, D’Ombrain MC, Stanisic DI, Taraika J, Bernard N, Richards JS, Beeson JG, Tavul L, Michon P, Mueller I, Schofield L (2009) Cellular tumor necrosis factor, gamma interferon, and interleukin-6 responses as correlates of immunity and risk of clinical Plasmodium falciparum malaria in children from Papua New Guinea. Infect Immun 77:3033–3043
Sam H, Stevenson MM (1999) Early IL-12 p70, but not p40, production by splenic macrophages correlates with host resistance to blood-stage Plasmodium chabaudi AS malaria. Clin Exp Immunol 117:343–349
Schmidt NW, Butler NS, Harty JT (2009) CD8 T cell immunity to Plasmodium permits generation of protective antibodies after repeated sporozoite challenge. Vaccine 27:6103–6106
Schmieg J, Gonzalez-Aseguinolaza G, Tsuji M (2003) The role of natural killer T cells and other T cell subsets against infection by the pre-erythrocytic stages of malaria parasites. Microbes Infect 5:499–506
Seegers D, Zwiers A, Strober W, Pena AS, Bouma G (2002) A TaqI polymorphism in the 3′UTR of the IL-12 p40 gene correlates with increased IL-12 secretion. Genes Immun 3:419–423
Shear HL, Srinivasan R, Nolan T, Ng C (1989) Role of IFN-gamma in lethal and nonlethal malaria in susceptible and resistant murine hosts. J Immunol 143:2038–2044
Shimokawa N, Nishiyama C, Hirota T, Tamari M, Hara M, Ikeda S, Okumura K, Ogawa H (2009) Functional analysis of a polymorphism in the promoter region of the IL-12/23p40 gene. Clin Exp Allergy 39:228–235
Sieburth D, Jabs EW, Warrington JA, Li X, Lasota J, LaForgia S, Kelleher K, Huebner K, Wasmuth JJ, Wolf SF (1992) Assignment of genes encoding a unique cytokine (IL12) composed of two unrelated subunits to chromosomes 3 and 5. Genomics 14:59–62
Socheat D, Denis MB, Fandeur T, Zhang Z, Yang H, Xu J, Zhou X, Phompida S, Phetsouvanh R, Lwin S, Lin K, Win T, Than SW, Htut Y, Prajakwong S, Rojanawatsirivet C, Tipmontree R, Vijaykadga S, Konchom S, Cong le D, Thien NT, Thuan le K, Ringwald P, Schapira A, Christophel E, Palmer K, Arbani PR, Prasittisuk C, Rastogi R, Monti F, Urbani C, Tsuyuoka R, Hoyer S, Otega L, Thimasarn K, Songcharoen S, Meert JP, Gay F, Crissman L, Cho Min N, Chansuda W, Darasri D, Indaratna K, Singhasivanon P, Chuprapawan S, Looareesuwan S, Supavej S, Kidson C, Baimai V, Yimsamran S, Buchachart K (2003) Mekong malaria. II. Update of malaria, multi-drug resistance and economic development in the Mekong region of Southeast Asia. Southeast Asian J Trop Med Public Health 34(Suppl 4):1–102
Stephens R, Langhorne J (2006) Priming of CD4+ T cells and development of CD4+ T cell memory; lessons for malaria. Parasite Immunol 28:25–30
Stevenson MM, Tam MF, Wolf SF, Sher A (1995) IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanism. J Immunol 155:2545–2556
Tangteerawatana P, Pichyangkul S, Hayano M, Kalambaheti T, Looareesuwan S, Troye-Blomberg M, Khusmith S (2007) Relative levels of IL4 and IFN-gamma in complicated malaria: association with IL4 polymorphism and peripheral parasitaemia. Acta Trop 101:258–265
Tso HW, Lau YL, Tam CM, Wong HS, Chiang AK (2004) Associations between IL12B polymorphisms and tuberculosis in the Hong Kong Chinese population. J Infect Dis 190:913–919
Verra F, Avellino P, Bancone G, Mangano V, Modiano D (2008) Genetic epidemiology of susceptibility to malaria: not only academic exercises. Parassitologia 50:147–150
Verra F, Mangano VD, Modiano D (2009) Genetics of susceptibility to Plasmodium falciparum: from classical malaria resistance genes towards genome-wide association studies. Parasite Immunol 31:234–253
Weatherall DJ (2008) Genetic variation and susceptibility to infection: the red cell and malaria. Br J Haematol 141:276–286
WHO (2000) Severe falciparum malaria. Trans R Soc Trop Med Hyg 94:1–90
Wroczynska A, Nahorski W, Bakowska A, Pietkiewicz H (2005) Cytokines and clinical manifestations of malaria in adults with severe and uncomplicated disease. Int Marit Health 56:103–114
Yang JM, Nagasaka S, Yatagai T, Nakamura T, Kusaka I, Ishikawa SE, Saito T, Ishibashi S (2006) Interleukin-12p40 gene (IL-12B) polymorphism and type 1 diabetes mellitus in Japanese: possible role in subjects without having high-risk HLA haplotypes. Diabetes Res Clin Pract 71:164–169
Zwiers A, Seegers D, Heijmans R, Koch A, Hampe J, Nikolaus S, Pena AS, Schreiber S, Bouma G (2004) Definition of polymorphisms and haplotypes in the interleukin-12B gene: association with IL-12 production but not with Crohn’s disease. Genes Immun 5:675–677
Acknowledgements
We wish to thank the late Professor Sornchai Looareesuwan, Department of Clinical Tropical Medicine and Hospital for Tropical Diseases, Mahidol University for providing blood samples and clinical data. We also thank his staff for assistance in blood collection and slide reading. We thank the patients for their kind participation in the study.
Authors’ contributions
SK, DJP and CP designed the study. CP collected blood samples, genotyped the IL12Bpro and IL12B 3′ UTR polymorphisms, participated in data analysis and writing of the manuscript. CO assisted in genotyping of IL12Bpro (rs17860508) polymorphisms, data analysis and writing of the manuscript. PT collected blood samples and determined the circulating IFN-γ levels. JT determined circulating IL-12p40 levels. TW assisted in genotyping of IL12B 3′ UTR polymorphisms and data analysis. DW and YM assisted in data analysis. SK and DJP financed the study, performed data analyses and co-wrote the manuscript. All authors have read and approved the final version of the manuscript.
Financial support
This study was supported by the Royal Golden Jubilee Ph.D. Program (PHD/0197/2549) of the Thailand Research Fund (CP and SK), the Commission on Higher Education of Thailand (CP), the National Institutes of Health [AI51305–06 (DJP) and TW05884–06 (DJP)] and the Faculty of Tropical Medicine, Mahidol University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
There is no conflict of interest for any of the authors of the manuscript due to commercial or other affiliations.
Rights and permissions
About this article
Cite this article
Phawong, C., Ouma, C., Tangteerawatana, P. et al. Haplotypes of IL12B promoter polymorphisms condition susceptibility to severe malaria and functional changes in cytokine levels in Thai adults. Immunogenetics 62, 345–356 (2010). https://doi.org/10.1007/s00251-010-0439-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-010-0439-y