Abstract
Certain combinations of the killer immunoglobulin-like receptors (KIR) and major histocompatibility complex class I ligands in humans predispose carriers to a variety of diseases, requiring sophisticated genotyping of the highly polymorphic and diverse KIR and HLA genes. Particularly, KIR genotyping is challenging due to polymorphisms (allelic substitutions), genomic diversity (presence/absence of genes), and frequent duplications. Rhesus macaques are often used as important animal models of human diseases such as, e.g. AIDS. However, typing of rhesus macaque KIR genes has not been described so far. In this study, we report the identification of additional novel rhesus macaque KIR cDNA sequences and a sequence-specific KIR genotyping assay. From a cohort of four rhesus macaque families with a total of 70 individuals, we identified 25 distinct KIR genotypes. Segregation analyses of KIR genes and of two polymorphic microsatellite markers allowed the identification of 21 distinct KIR haplotypes in these families, with five to 11 segregating KIR genes per haplotype. Our analyses confirmed and extended knowledge on differential gene KIR gene content in macaques and indicate that rhesus macaque and human KIR haplotypes show a comparable level of diversity and complexity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Killer cell immunoglobulin-like receptors (KIRs) are cell surface receptors of the immunoglobulin (Ig) superfamily expressed on the cell surface of natural killer (NK) cells and subsets of T lymphocytes (Gardiner 2008; Lanier 2008; Parham 2005). Mapping to the leukocyte-receptor complex in a head to tail fashion, KIR genes encode type I transmembrane glycoproteins with either two or three extracellular Ig-like domains as well as stem, transmembrane, and cytoplasmic regions (Kelley et al. 2005). Inhibitory KIRs have long cytoplasmic tails with immunoreceptor tyrosine-based inhibitory motifs (ITIM), whereas activating KIRs possess short tails lacking ITIMs and instead contain a positively charged amino acid in the transmembrane region that mediates interaction with immunoreceptor tyrosine-based activating motif-containing adaptor molecules (Feng et al. 2005; Lanier 1998; Long 1999). The nomenclature of KIR genes is based on these structural and functional features (Marsh et al. 2003).
A hallmark of KIR genes is their diversity and polymorphism as seen in substantial differences in gene content and numerous alleles of certain genes, respectively (Uhrberg et al. 1997; Valiante et al. 1997). Sixteen KIR genes are currently known in humans and numerous KIR genotypes and haplotypes have been defined (Gardiner 2008; Robinson and Marsh 2007; Vilches and Parham 2002). All human KIR haplotypes contain the KIR3DL3 and KIR3DL2 genes at the centromeric and telomeric end of the KIR locus, respectively, in addition to KIR3DP1, KIR2DL4, KIR2DL2/3, and KIR3DL1/KIR3DS1, which are present on almost all haplotypes. According to their gene content, KIR haplotypes have been classified into either group A or B, with A haplotypes lacking activating KIR genes except KIR2DS4, and all other haplotypes grouped as B (Hsu et al. 2002; Uhrberg et al. 1997). Differential gene contents are usually created by non-reciprocal recombination (Martin et al. 2003; Norman et al. 2009; Uhrberg 2005; Wilson et al. 2000) and the extent is typically determined by polymerase chain reaction (PCR) involving sequence-specific primers (Houtchens et al. 2007; Martin et al. 2008; Uhrberg et al. 1997; Vilches et al. 2007). Remarkably, certain combinations of the highly variable KIRs and their HLA class I ligands predispose carriers to various infectious and autoimmune diseases and influence reproduction (Hiby et al. 2008; Johansson et al. 2006; Khakoo et al. 2004; Martin et al. 2007; Nelson et al. 2004; Parham 2005).
Despite the importance as non-human primate model of human infectious diseases (Bontrop and Watkins 2005), detailed knowledge of KIR haplotypic gene content and diversity is rather limited in rhesus macaques. Previously published reports point to substantial diversity of rhesus macaque KIR genes and haplotypes (Hershberger et al. 2001; Sambrook et al. 2005). In particular, rhesus macaques show extensive expansions of KIR3D genes, which are likely the result of co-evolution with Mamu-A and Mamu-B class I genes that are substantially expanded in rhesus macaques (Otting et al. 2007; Otting et al. 2008). Notably, the impact of KIR diversity on the outcome of infectious/autoimmune diseases in rhesus macaque disease models is not known. Furthermore, rhesus macaques possess different types of activating KIR genes compared to human and great apes (Blokhuis et al. 2009a; Hershberger et al. 2001).
Here, we established a rhesus macaque KIR genotyping based on 13 novel full-length and previously published KIR sequences. Typing of 70 animals from four families allowed to define 25 genotypes and 21 haplotypes. The number of distinguishable KIR genes in the tested families varies from five to 11 per haplotype.
Materials and methods
Animals
All rhesus macaques are housed in the facilities of the German Primate Center. Blood samples were obtained during regular veterinary inspections. All experiments were carried out in accordance with the German Animal Welfare Law, guidelines of the German Research Foundation, and the European Communities Council Directive (86/609/EEC). Families consist of single dominant males with multiple females and their offspring.
RNA extraction, cDNA library construction, and isolation of KIR-containing clones
Blood from 15 to 30 unrelated rhesus macaques was obtained and pooled into two large samples, respectively. Peripheral blood mononuclear cells were obtained by centrifugation using Ficoll-Hypaque 1.077 (Sigma) for 40 min at 600×g. The cell pellet was washed twice with 1× PBS followed by enrichment of CD16-positive cells using CD16 microbeads for isolation of NK cells from non-human primates (Miltenyi Biotec) according to the supplier’s recommendations. Total RNA was isolated from enriched CD16-positive cells with the RNeasy Plus Mini Kit (Qiagen) and cDNA libraries were constructed with the Creator SMART cDNA Library Kit (Clontech) according to the supplier's recommendations. KIR-containing cDNA clones were isolated from the library by hybridization with a P32-labelled rhesus macaque KIR PCR fragment. All KIR-containing clones were completely sequenced. KIR amino acid sequences were aligned using ClustalX (Thompson et al. 1997).
Genomic DNA extraction
The cellular fraction of blood samples (5-15 ml) was incubated in lysis buffer (155 mM NH4CL, 10 mM KHCO3, 0.1 mM EDTA, pH 8.0) for 20 min to lyse erythrocytes and centrifuged for 10 min at 7 °C and 200×g. The pellet was washed with 10 ml lysis buffer and incubated over night at 37 C in 5 ml SE buffer (75 mM NaCl, 25 mM EDTA), 250 µl 20% SDS and 20 µl Pronase E. After adding 2 ml 5 M NaCl, the reaction was centrifuged for 10 min at 1,250×g. The DNA was precipitated with 14 ml 100% EtOH, washed with 5 ml 70% EtOH, and resolved in H2O.
KIR-specific PCR-SSP typing
KIR genotyping was performed using sequence-specific primers (Table 1) and a ‘hot start’ PCR. Aliquots of 30 µl were set up by using 1 U Taq DNA polymerase (Biotherm), 3 µl 10× buffer, 5 mM dNTPs, and 50 ng DNA. KIR primers and internal control primers (actin, Table 1) were used in 0.16 pmol/µl and 0.06 pmol/µl concentration, respectively. PCR conditions are the same for all primers, except for the annealing temperature (Table 1): initial denaturation at 94 °C for 3 min, followed by 30 cycles of 94 °C for 30 s, annealing for 30 s, and elongation for 45 s at 72 °C, followed by a final elongation at 72 °C for 5 min. All PCR products were analysed by agarose gel electrophoresis.
Microsatellite analysis
Short tandem repeats were identified in the sequenced KIR haplotype (Sambrook et al. 2005) by manual inspection. Flanking primers (Table 1) were designed and used in a PCR consisting of an initial denaturation step at 94 °C for 3 min, followed by 30 cycles of 94 °C for 30 s, 59 °C for 30 s, and 45 s at 72 °C, and a final extension step for 5 min at 72 °C. 6-FAM and HEX-labelled PCR products were analysed in an ABI3130xl sequencer (Applied Biosystems) along with the Genescan 400 HD ROX size standard (Applied Biosystems). Allele sizes were calculated with Gene Mapper v4.0 (Applied Biosystems).
Database accession numbers
Rhesus macaque KIR cDNA sequences reported here have been assigned database accession numbers FN424249-FN424261.
KIR gene nomenclature
At the KIR Polymorphism Workshop in Berkeley in 2009, it became obvious that due to substantially increasing numbers of sequences, a robust and sustainable nomenclature system for macaque KIR genes and alleles must urgently be introduced to avoid further confusion. As information of KIR gene content was available only for a single haplotype at that time, a nomenclature of macaque KIR genes was worked out by a committee (see Immuno Polymorphism Database (IPD) database at http://www.ebi.ac.uk/ipd/kir/). In order to install a nomenclature even in the absence of genotype and haplotype data, sequences of KIR genes/alleles were assigned based on clustering in phylogenetic trees. In those cases where an assignment of a certain KIR sequence as either an allele of a known gene or a distinct gene was not obvious, a "W" (workshop) was introduced in the gene symbol to account for its preliminary name. The same rules of the human KIR gene nomenclature (Marsh et al. 2003) were applied to designate macaque KIR genes. A report of the committee on KIR gene nomenclature in macaques will be published soon and will soon be available on the IPD website. Therefore, the new nomenclature is already applied in this study to avoid repeated and short-term renaming.
Results
Identification of novel rhesus macaque KIR cDNA sequences
Two groups of unrelated animals of Indian origin were chosen to establish two cDNA libraries from enriched NK cells. Altogether, 13 full-length KIR cDNA clones were isolated from both libraries and completely sequenced. All isolated KIR cDNA clones show nucleotide substitutions compared to already known rhesus macaque KIR sequences (not shown), thus representing novel sequences. The sequences were sent to the IPD and received official designations (Table 2). These cDNA sequences code for seven inhibitory and six activating KIR3D molecules (Fig. 1). Characteristic features of primate KIR3D molecules were found: the inhibitory KIRs show two ITIMs in their cytoplasmic region, whereas the activating KIRs lack these motifs and display a charged amino acid in the transmembrane region, which is in all cases an arginine residue (Fig. 1). Thus, our data confirm and extend previous findings by others that macaque activating KIRs have arginine instead of a lysine residue (Bimber et al. 2008; Blokhuis et al. 2009a; Hershberger et al. 2001; Sambrook et al. 2005). The deduced amino acid sequences of some KIRs are nearly identical, e.g. clones 6 and 12 differ by a single amino acid and clones 21 and 26 differ at two positions (Fig. 1), indicating that these cDNA sequences represent alleles of two distinct KIR genes. According to assignment of the Rhesus Macaque KIR Gene Nomenclature Committee (see “Materials and methods” section), clones 6 and 12 belong to the KIR3DLW03 gene (alleles *004 and *005) and clones 21 and 26 are derived from the KIR3DL11 gene (alleles *003 and *004). cDNA clone 2 is also assigned to KIR3DL11 (allele *002), but shows 12 and 14 amino acid substitutions to 3DL11*003 and 3DL11*004, respectively, and obviously is a more distantly related allele. Clones 9 and 10 represent a further pair of allelic sequences, which differ by six amino acid residues and were assigned the names KIR3DSW08*006 and KIR3DSW08*007.
Comparison of deduced amino acid sequences of the newly identified Indian rhesus macaque KIR3D cDNA sequences. Identical amino acid residues are indicated by a dot, dashes denote introduced gaps to maximise homology. Immunoreceptor tyrosine-based inhibitory motifs (ITIM) are shown in bold, and the positively charged arginine residues in the transmembrane region of activating KIRs are marked in black. KIR clone 3 (see also Table 2) represents an alternatively spliced transcript of KIR3DL11 and has the DDBJ/EMBL/GenBank database accession number FN424251
KIR cDNA clone 3 carries a 150 bp deletion in the exon encoding the D2 domain and most likely represents a product derived from usage of cryptic splice sites of the KIR3DL011 gene and is similar to transcript variant 4 of a KIR3DL gene described by Hershberger et al. 2001.
Determination of KIR genotypes
Based on multiple alignment of our new (Fig. 1) and of already known KIR sequences, we identified sequence-specific substitutions that were exploited to establish specific primers (Table 1). For KIR3DL11 alleles *003 and *004, it was not possible to establish specific primers that would allow unambiguous priming. Therefore, these two alleles were not tested in our analysis. The various primer pairs allow for discrimination of rhesus macaque KIR sequences at different levels: some primers allow detection of alleles (e.g. primer pairs 9 and 10), while others detect distinct genes (e.g. primer pair 8). Altogether, a set of 31 primer pairs were established (Table 1) and used to type a panel of four families with a total number of 70 animals. We identified 25 KIR genotypes, revealing ten to 16 specific PCR products out of 31 reactions (Table 3). Whereas KIR genes 2DL4, 3DL11, 3DL20 (2DL5), and 3DSW08 were found in all studied rhesus macaque individuals, we did not detect any monkey carrying 3DL06, 3DL07, 3DS06, or 3DSW07. It should be noted that KIR2DL5 likely represents an alternatively spliced product of the KIR3DL20 gene, which shares closer relationship with human KIR2DL5 only in one Ig domain-encoding exon (Bimber et al. 2008; Rajalingam et al. 2004; Sambrook et al. 2005).
We used the primer set only for analysis of presence/absence polymorphisms of KIR genes. However, this set can also be exploited for transcription studies of individual KIR genes because all primer pairs were located in exons, except primer pairs 2 and 11 that are specific for KIR3DL01 and KIR3DL08, respectively, and are located over exon-intron boundaries.
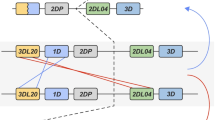
Determination of KIR haplotypes
Having established KIR genotypes, we analysed four rhesus macaque families and followed the segregation of KIR sequences in the offspring to determine KIR haplotypes. An example of a pedigreed family is shown in Fig. 2. A total of 21 different haplotypes were identified, with numbers of segregating KIR genes varying between five and 11 (Table 4). In accord with the previously noted diversity of rhesus macaque KIR genes (Hershberger et al. 2001; Sambrook et al. 2005), only KIR haplotypes 15 and 16 were found in more than one family (not shown). Nevertheless, it was possible to identify common features: genes KIR2DL4, KIR3DL11, KIR3DL20 (KIR2DL5), and KIR3DSW08 were found in all haplotypes studied here, suggesting that these could represent framework KIR genes. Three of these are three-domain-KIRs, further emphasising the diverse nature of lineage II KIR genes in rhesus macaques.
Pedigree of one of the analysed rhesus macaque families. Animal identification numbers are indicated. G and H denote KIR genotype and KIR haplotype, respectively. DNA of rhesus macaque individuals 1561 (died in 2002) and 1636 (delivered to another institution in 1997) is no longer available and their KIR haplotypes were partially inferred from offspring
In several cases, we noticed a specific PCR product, but could not observe segregation in the offspring (indicated by question mark (?) in Table 4). Thus, the studied families are not informative in these cases and more families need to be typed. It should be noted, however, that rhesus macaque families consist of a single dominant male breeding with several females and, therefore, only few offspring of a distinct pair are available for segregation studies. Interestingly, KIR haplotypes 2 (3DL10), 7 (3DL01, 3DSW08), 8 (3DL11), 10 (3DL10), and 11a (3DL10) showed presence of two 'allelic' sequences on a single haplotype, indicating duplication of the corresponding KIR gene. However, another explanation with similar probability is that these sequences do not represent alleles, but belong to different KIR genes that co-segregate in the offspring. If this would be the case, the nomenclature of genes 3DL01, 3DL10, 3DL11, and 3DSW08 demands respective revision. In any case, further haplotype analyses with additional rhesus macaque families are needed to clarify this point.
We noticed nine microsatellite markers in the completely sequenced KIR haplotype (Sambrook et al. 2005). Two of them turned out to be polymorphic in the studied animals and were used to confirm the segregation and haplotype analyses. One microsatellite marker is located 6 kb 5′ of KIR2DL4, the other maps outside the KIR region approximately 30 kb 3′ of the FCAR gene and is located in the NLRP7 gene. Both microsatellite markers were not used in the analysis of cynomolgus macaques published by Bimber et al. 2008. Especially, the latter microsatellite shows a remarkable degree of polymorphism (Table 4). We used the two polymorphic microsatellite markers as additional tools to follow the segregation of maternal and paternal KIR haplotypes in the offspring. Notably, subgroups of haplotypes 11, 15, and 16 were identified. Within these haplotypes, differences in KIR genes were not obvious, but the members of a subgroup differ in their microsatellite markers. It remains to be shown whether differences between these haplotypes are only seen in those microsatellites, or can be found in KIR genes, too.
Discussion
Rhesus macaques serve as important non-human primate models of human infectious and autoimmune diseases, and in transplantation studies. Combinations of KIR and (major histocompatibility complex) MHC class I genotypes are known to influence these human diseases. However, knowledge of rhesus macaque KIR genotypes and methods to determine those were not available until now. Here, we report the establishment of a PCR-based sequence-specific KIR genotyping in the rhesus macaque and its usage for determination of KIR haplotypes in family studies. The typing resulted in identification of 25 genotypes and 21 haplotypes among 70 rhesus macaques from four families, emphasising the considerable diversity of KIR genes in rhesus macaques. Detailed knowledge of rhesus macaque KIR genotypes and haplotypes will be important for evaluation of non-human primate animal model studies of those human diseases where contributions of individual KIRs and their specific MHC class I ligands play important roles in determination of disease susceptibility and resistance. However, data on specific KIR and MHC class I interactions are still not available for the rhesus macaque.
Based on 25 genotypes in segregating families, we were able to identify 21 KIR haplotypes (Table 4). The number of segregating KIR genes per haplotype can vary between five and 11 in the analysed cohort. Differential KIR gene content and duplication of KIR2DL4 were previously observed in rhesus macaques (Blokhuis et al. 2009b; Sambrook et al. 2005) and duplications were also found in cynomolgus macaques (Bimber et al. 2008). Our study extends these data of differential gene content and suggests that inhibitory and activating KIR3D genes might be duplicated on some haplotypes. In an accompanying paper in this issue of Immunogenetics, (Blokhuis and colleagues 2010) also obtained evidence for KIR gene duplications as they found three cDNA sequences derived from the same KIR gene in a single animal. Such duplications, in particular recent ones, can result in complicated genetic situations as for example the same KIR gene sequence can be either derived from an allele or from a distinct gene, making sequence-specific genotyping technically demanding. Future studies involving complete sequencing of rhesus macaque KIR haplotypes will significantly contribute to identification of recombinant KIR genes and recent duplications, but also of KIR gene fusions that can result from deletions (Abi-Rached et al. 2010).
The previously described rhesus macaque KIR haplotype carries only five KIR genes: 3DL20, 1D, 2DL4, 3DL10, and 3DL01 (Sambrook et al. 2005). Interestingly, this sequenced haplotype does not contain typical activating KIR genes, which are present in all haplotypes identified in this study. Furthermore, it contains only two (KIR3DL20 and KIR2DL4) of four framework genes identified here. Thus, the sequenced KIR haplotype is obviously rather uncommon. In humans, KIR haplotypes are assigned to either group A or group B, which differ considerably in both number and type of KIR genes (Hsu et al. 2002; Uhrberg et al. 1997). Although also rhesus macaque KIR haplotypes strikingly vary in gene content of both inhibitory and activating KIR genes, we could not detect clear differences in our cohort that would allow for clear-cut discriminations similar to the human group A and B haplotypes.
Our study describes the first sequence-specific typing approach of KIR genes in a macaque species. The advantage of this method is its speed and cost-effectiveness, making it ideal for high-throughput screening of large cohorts. Due to the short sizes of PCR products, also samples obtained by non-invasive methods (e.g. faeces) may be typed, which would allow sampling from free-living macaques. The disadvantages are that no novel alleles or genes are detected. In addition, recombinant genes might lead to misinterpretation of the obtained genotyping data. However, as with all complex genotyping, the method will constantly be improved upon knowledge of further rhesus macaque KIR sequences. The newly developed genomic sequencing technologies (second and third generation sequencing) are suitable to sequence entire rhesus macaque KIR haplotypes, resulting in substantially improved knowledge of KIR genes and allotypes and improved sequence-specific KIR genotyping. All these efforts will contribute to make association studies of KIR and MHC genotypes possible in rhesus macaque disease models.
References
Abi-Rached L, Kuhl H, Roos C, ten Hallers B, Zhu B, Carbone L, de Jong PJ, Mootnick AR, Knaust F, Reinhardt R, Parham P, Walter L (2010) A small, variable and irregular killer cell immunoglobulin-like receptor (KIR) locus accompanies the absence of MHC-C and MHC-G in gibbons. J Immunol (in press)
Bimber BN, Moreland AJ, Wiseman RW, Hughes AL, O'Connor DH (2008) Complete characterization of killer Ig-like receptor (KIR) haplotypes in Mauritian cynomolgus macaques: novel insights into nonhuman primate KIR gene content and organization. J Immunol 181:6301–6308
Blokhuis JH, Doxiadis GG, Bontrop RE (2009a) A splice site mutation converts an inhibitory killer cell Ig-like receptor into an activating one. Mol Immunol 46:640–648
Blokhuis JH, van der Wiel MK, Doxiadis GG, Bontrop RE (2009b) Evidence for balancing selection acting on KIR2DL4 genotypes in rhesus macaques of Indian origin. Immunogenetics 61:503–512
Blokhuis JH, van der Wiel MK, Doxiadis GGM, Bontrop RE (2010) The mosaic of KIR haplotypes in rhesus macaques. Immunogenetics. doi:10.1007/s00251-010-0434-3
Bontrop RE, Watkins DI (2005) MHC polymorphism: AIDS susceptibility in non-human primates. Trends Immunol 26:227–233
Feng J, Garrity D, Call ME, Moffett H, Wucherpfennig KW (2005) Convergence on a distinctive assembly mechanism by unrelated families of activating immune receptors. Immunity 22:427–438
Gardiner CM (2008) Killer cell immunoglobulin-like receptors on NK cells: the how, where and why. Int J Immunogenet 35:1–8
Hershberger KL, Shyam R, Miura A, Letvin NL (2001) Diversity of the killer cell Ig-like receptors of rhesus monkeys. J Immunol 166:4380–4390
Hiby SE, Regan L, Lo W, Farrell L, Carrington M, Moffett A (2008) Association of maternal killer-cell immunoglobulin-like receptors and parental HLA-C genotypes with recurrent miscarriage. Hum Reprod 23:972–976
Houtchens KA, Nichols RJ, Ladner MB, Boal HE, Sollars C, Geraghty DE, Davis LM, Parham P, Trachtenberg EA (2007) High-throughput killer cell immunoglobulin-like receptor genotyping by MALDI-TOF mass spectrometry with discovery of novel alleles. Immunogenetics 59:525–537
Hsu KC, Chida S, Geraghty DE, Dupont B (2002) The killer cell immunoglobulin-like receptor (KIR) genomic region: gene-order, haplotypes and allelic polymorphism. Immunol Rev 190:40–52
Johansson S, Hall H, Berg L, Hoglund P (2006) NK cells in autoimmune disease. Curr Top Microbiol Immunol 298:259–277
Kelley J, Walter L, Trowsdale J (2005) Comparative genomics of natural killer cell receptor gene clusters. PLoS Genet 1:129–139
Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, Cox S, Little AM, Alexander GJ, Cramp ME, O'Brien SJ, Rosenberg WM, Thomas DL, Carrington M (2004) HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305:872–874
Lanier LL (1998) NK cell receptors. Annu Rev Immunol 16:359–393
Lanier LL (2008) Evolutionary struggles between NK cells and viruses. Nat Rev Immunol 8:259–268
Long EO (1999) Regulation of immune responses through inhibitory receptors. Annu Rev Immunol 17:875–904
Marsh SG, Parham P, Dupont B, Geraghty DE, Trowsdale J, Middleton D, Vilches C, Carrington M, Witt C, Guethlein LA, Shilling H, Garcia CA, Hsu KC, Wain H (2003) Killer-cell immunoglobulin-like receptor (KIR) nomenclature report, 2002. Immunogenetics 55:220–226
Martin MP, Bashirova A, Traherne J, Trowsdale J, Carrington M (2003) Cutting edge: expansion of the KIR locus by unequal crossing over. J Immunol 171:2192–2195
Martin M, Qi Y, Gao X, Yamada E, Martin J, Pereyra F, Colombo S, Brown E, Shupert W, Phair J, Goedert J, Buchbinder S, Kirk G, Telenti A, Connors M, O'brien S, Walker B, Parham P, Deeks S, McVicar D, Carrington M (2007) Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet 39:733–740
Martin MP, Single RM, Wilson MJ, Trowsdale J, Carrington M (2008) KIR haplotypes defined by segregation analysis in 59 Centre d'Etude Polymorphisme Humain (CEPH) families. Immunogenetics 60:767–774
Nelson G, Martin M, Gladman D, Wade J, Trowsdale J, Carrington M (2004) Cutting edge: heterozygote advantage in autoimmune disease: hierarchy of protection/susceptibility conferred by HLA and killer Ig-like receptor combinations in psoriatic arthritis. J Immunol 173:4273–4276
Norman PJ, Abi-Rached L, Gendzekhadze K, Hammond JA, Moesta AK, Sharma D, Graef T, McQueen KL, Guethlein LA, Carrington CV, Chandanayingyong D, Chang YH, Crespi C, Saruhan-Direskeneli G, Hameed K, Kamkamidze G, Koram KA, Layrisse Z, Matamoros N, Mila J, Park MH, Pitchappan RM, Ramdath DD, Shiau MY, Stephens HA, Struik S, Tyan D, Verity DH, Vaughan RW, Davis RW, Fraser PA, Riley EM, Ronaghi M, Parham P (2009) Meiotic recombination generates rich diversity in NK cell receptor genes, alleles, and haplotypes. Genome Res 19:757–769
Otting N, de Vos-Rouweler AJ, Heijmans CM, de Groot NG, Doxiadis GG, Bontrop RE (2007) MHC class I A region diversity and polymorphism in macaque species. Immunogenetics 59:367–375
Otting N, Heijmans CM, van der Wiel M, de Groot NG, Doxiadis GG, Bontrop RE (2008) A snapshot of the Mamu-B genes and their allelic repertoire in rhesus macaques of Chinese origin. Immunogenetics 60:507–514
Parham P (2005) MHC class I molecules and KIRs in human history, health and survival. Nat Rev Immunol 5:201–214
Rajalingam R, Parham P, Abi-Rached L (2004) Domain shuffling has been the main mechanism forming new hominoid killer cell Ig-like receptors. J Immunol 172:356–369
Robinson J, Marsh SG (2007) IPD: the Immuno Polymorphism Database. Methods Mol Biol 409:61–74
Sambrook JG, Bashirova A, Palmer S, Sims S, Trowsdale J, Abi-Rached L, Parham P, Carrington M, Beck S (2005) Single haplotype analysis demonstrates rapid evolution of the killer immunoglobulin-like receptor (KIR) loci in primates. Genome Res 15:25–35
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Uhrberg M (2005) The KIR gene family: life in the fast lane of evolution. Eur J Immunol 35:10–15
Uhrberg M, Valiante NM, Shum BP, Shilling HG, Lienert-Weidenbach K, Corliss B, Tyan D, Lanier LL, Parham P (1997) Human diversity in killer cell inhibitory receptor genes. Immunity 7:753–763
Valiante NM, Uhrberg M, Shilling HG, Lienert-Weidenbach K, Arnett KL, D'Andrea A, Phillips JH, Lanier LL, Parham P (1997) Functionally and structurally distinct NK cell receptor repertoires in the peripheral blood of two human donors. Immunity 7:739–751
Vilches C, Parham P (2002) KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol 20:217–251
Vilches C, Castaño J, Gómez-Lozano N, Estefanía E (2007) Facilitation of KIR genotyping by a PCR-SSP method that amplifies short DNA fragments. Tissue Antigens 70:415–422
Wilson MJ, Torkar M, Haude A, Milne S, Jones T, Sheer D, Beck S, Trowsdale J (2000) Plasticity in the organization and sequences of human KIR/ILT gene families. Proc Natl Acad Sci USA 97:4778–4783
Acknowledgement
The authors gratefully acknowledge the expert technical assistance of Nicole Otto. PK is a member of the Göttingen Graduate School of Neurosciences and Molecular Biology (GGNB) "Molecular Biology of Microbial, Animal and Plant Cells". This study was supported by European Union grant "EUPRIM-Net" (FP6 026155) to LW.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kruse, P.H., Rosner, C. & Walter, L. Characterization of rhesus macaque KIR genotypes and haplotypes. Immunogenetics 62, 281–293 (2010). https://doi.org/10.1007/s00251-010-0433-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-010-0433-4