Abstract
Mammalian immunoregulatory families of genes encoding activating and inhibitory Ig-like receptor pairs have been located on distinct chromosomes. In chicken, a single Ig-like receptor family with many members had been described so far. By looking at sequence similarity and synteny conservations in the chicken genome, the signal-regulatory protein (SIRP), triggering receptor expressed on myeloid cells (TREM), and CMRF35/CD300L Ig-like gene families were identified on chromosomes 20, 26, and 3, respectively. Further analysis of the three corresponding genomic regions and partial bacterial artificial chromosome sequencing were used to identify more members and to realign several contigs. All putative genomic sequences were monitored by investigating existing expressed sequence tag and cloning cDNA. This approach yielded a single pair of activating and inhibitory SIRP, two inhibitory, and one activating TREM as well as one inhibitory CMRF35/CD300L with a potentially soluble variant and an additional member lacking categorizing motifs. The CMRF35/CD300L and TREM receptors were composed of one or two V-set Ig domains, whereas in SIRP, either a single Ig V domain was present or a combination of a V and C1 domains. Like in many Ig superfamily members, separate exons encode individual Ig domains. However, in two CMRF35/CD300L genes, the signal peptide and the distal Ig domain were encoded by a single exon. In conclusion, the mammalian diversity of immunoregulatory molecules is present the chicken suggesting an important role for TREM, SIRP, and CMRF35/CD300L in a functionally conserved network.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The dogma of the antigen receptors as master switches for lymphocyte activation has been challenged by the discovery of multiple different immunoregulatory receptor families (Long 1999; Vivier and Malissen 2005). Binding of the antigen receptors to their cognate antigen results in activation signal; however, the additional inhibitory and costimulating signals of immunoregulatory receptors may modulate this antigen-mediated signal. The lymphocytes integrate all different signals obtained from a vast array of these immunoregulatory receptors and only after a cell and maturation specific threshold is reached, the lymphocytes become fully activated.
Initially, these receptors were mainly described as costimulatory molecules, such as CD28 acting as a “second signal” for T cell activation (Lenschow et al. 1996). The analyses of natural killer (NK) cell receptors revealed that there is a second mode of immunoregulation caused by inhibitory receptors (Ljunggren and Karre 1990). Different families of immunoregulatory genes exist, that encode receptors with activating and inhibitory function. These receptor families have several common features. They contain multiple members clustered on a specific chromosomal region. The activating and inhibitory receptor pairs within a given receptor family frequently share a high degree of similarity in their extracellular regions. In contrast, the transmembrane and cytoplasmic domains are decisive for the classification into prototypic activating and inhibitory family members. Activating receptors contain a short cytoplasmic tail and a positively charged transmembrane residue that mediates the association to adaptor molecules such as DAP-10, DAP-12/KARAP, and FcɛRIγ (Borrego et al. 2002; Brown et al. 2004; Moretta and Moretta 2004). These adaptor molecules display cytoplasmic motifs capable of triggering an intracytoplasmic activation cascade. In contrast, inhibitory members are characterized by their uncharged transmembrane region and a long cytoplasmic domain that harbors one or more immunoreceptor tyrosine-based inhibitory motifs (ITIM) (Ravetch and Lanier 2000). Following receptor ligation, these ITIM are phosphorylated and associate with cellular SH2 domain containing protein tyrosine phosphatases that interrupt cellular activation by dephosphorylation of downstream target molecules (Campbell and Colonna 2001). Biochemically, the immunoregulatory families are divided into type II transmembrane C-type lectins and type I transmembrane Ig superfamily receptors. The first receptor families with these features such as KIR and Ly49 were described as NK cell specific receptor families (Barten et al. 2001); however, by now, several immunoregulatory receptor families have been described that are expressed on a wide variety of hemopoietic cell types.
The different immunoregulatory Ig-like receptors are currently grouped into the following families [nomenclature according to Human Genome Organization (HUGO) nomenclature]: KIR (killer cell Ig-like receptors), LILR (leukocyte Ig-like receptors), FCR (Fc receptors), SIRP (signal-regulatory protein), TREM (triggering receptor expressed on myeloid cells), CMRF35/CD300L (CD300 antigen like family member), and PILR (paired Ig-like type 2 receptor) (Colonna 2003; Van den Berg et al. 2004).
We and others have previously characterized a unique chicken immunoregulatory receptor family designated “chicken Ig-like receptors” (CHIR) (Dennis et al. 2000; Viertlboeck et al. 2004). CHIR display all features of a classical immunoregulatory receptor family and were identified as orthologous to the mammalian leukocyte receptor complex by their location on microchromosome 31, which show conserved synteny to human chromosome 19q13.4. The CHIR gene family has some hallmarks that are distinct from any mammalian immunoregulatory family. Most importantly, it contains more than 60 functional receptor genes that display extensive haplotypic and allelic variations. In addition to the prototypic inhibitory and activating receptor types, novel receptor types combining inhibitory and activating features have been described (Viertlboeck et al. 2005). The expression pattern varies between individual CHIR family members, so that unique combinations of individual CHIR are found on virtual every leukocyte subpopulation. This enormous variability in gene number, allelic differences, and expression patterns in a single immunoregulatory receptor gene family that is not represented in mammals provokes to question if CHIR are the only immunoregulatory gene family in chickens that has been vastly expanded to compensate for the lack of various mammalian receptor families or, alternatively, if other chicken immunoregulatory gene families exist on different chromosomes like in mammals and what level of complexity they show? Although the identification of chicken homologues to mammalian gene families would have been a daunting task 2 years ago, the recent publication of the first draft of the chicken genome (Hillier et al. 2004) allowed addressing this question in detail, demonstrating the power of whole genome sequencing.
Materials and methods
Animals, cell preparation, and cell lines
Chicken line M11 (B2/B2) and commercial LSL birds (Lohmann, Cuxhaven) were hatched at the institute and the animals were used for experiments in the age of 3 to 10 weeks. Leukocytes from bursa, thymus, spleen, bone marrow, and blood were prepared using standard procedures. ConA-activated T cells were generated by stimulating splenocytes with 10 μg/ml ConA for 24 h and harvesting the cells after 72 h. Intestinal intraepithelial lymphocytes were prepared as described before (Göbel 2000) and CD3–CD8+ were FACS sorted from splenocytes and, in vitro, expanded with recombinant chicken IL-2 (Kaufman et al. 1999).
Macrophage preparation from peripheral blood leukocytes (PBL) were performed as described (Peck et al. 1982). A 100-mg piece of cerebrum was taken after opening the skull and used directly for RNA preparation.
The chicken cell lines HD11 (Beug et al. 1979) and OU2 (Ogura and Fujiwara 1987) were maintained in RPMI 1640 medium with Glutamax (Invitrogen, Germany) supplemented with 10% FCS in a CO2 incubator at 40°C.
Database searches
Following databases were used to identify Ig-like receptor family members in different species: Gene database at http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene (Maglott et al. 2005), HomoloGene database at http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?DB=homologene and the chicken genome assembly at http://www.ensembl.org/Gallus_gallus/index.html. Putative protein sequences were further analyzed using SMART (http://smart.embl-heidelberg.de/) (Letunic et al. 2004; Schultz et al. 1998) and corresponding chicken expressed sequence tag (EST) clones were identified using the BLAST program (Altschul et al. 1997) limited to the National Center for Biotechnology Information (NCBI) “gallus gallus” EST database. This database contains more than 550.000 sequences, which are a summary of all published chicken EST databases. The original EST databases are provided by the BBSRC (http://www.chick.umist.ac.uk/), by the Delaware Biotechnology Institute (http://www.chickest.udel.edu/), and by the Bursal Transcript Database (http://pheasant.gsf.de/DEPARTMENT/DT40/dt40Transcript.html) and are generated from many different embryonic and adult tissues (B-cells, T-cells, macrophages, spleen, PBL, bone marrow, liver, fat, reproductive tract, muscle, pancreas, small intestine, cerebrum, cerebellum, pituitary gland, hypothalamus, pineal gland, kidney, adrenal, chondrocytes, embryo heads and limbs, and whole embryos from different stages).
BAC preparation
Bacterial artificial chromosome (BAC) clones CH261-174G2 and CH261-44K10, specific for TREM and CMRF35/CD300L genes, respectively, according to the chicken genome assembly, were obtained from BACPAC Resources of the Children’s Hospital Oakland (http://bacpac.chori.org/order.php). They were amplified in LB medium containing 12.5 μg/ml chloramphenicol. BACs were purified using NucleoBond PC 100 (Macherey-Nagel, Düren, Germany) and partially sequenced (GATC, Konstanz).
Cloning procedures
Total RNA was prepared using Trizol (Invitrogen, Karlsruhe, Germany) and cDNA synthesis was performed with the Revert H Minus first strand cDNA synthesis kit (MBI Fermentas, St. Leon-Rot, Germany). Herculase enhanced DNA polymerase (Stratagene, Amsterdam, Netherlands) was used for polymerase chain reaction (PCR) at 2 min of denaturation at 95°C, 35 cycles of 10 s at 95°C, 30 s at primer specific temperature, 2 min at 72°C and a final extension time of 10 min at 72°C. Primer sequences and their specific temperatures are summarized in Table 1. Each primer pair was tested on the different cDNAs. The PCR product with the best result was used for cloning. PCR products were cloned into a pcDNA3.1/V5-His TOPO Vector (Invitrogen, Karlsruhe, Germany), colonies were screened by PCR, and plasmids of positive colonies were isolated using the NucleoSpin Plasmid Kit (Macherey-Nagel, Düren, Germany) and sequenced (GATC, Konstanz, Germany). Deduced amino acid sequences were further analyzed using PSORT II (http://psort.nibb.ac.jp/form2.html) and Jpred http://www.compbio.dundee.ac.uk/∼www-jpred/) for secondary structure prediction. Protein sequences were assembled using ClustalW (http://www.ebi.ac.uk/clustalw/index.html) and phylogenic trees were performed using MEGA 3.1 (Chenna et al. 2003; Kumar et al. 2004).
Results
General strategy to identify chicken Ig-like receptor families
The first draft of the chicken genome assembly of March 2004 was searched by the keywords SIRP, TREM, CMRF, FCR, and PILR. For those, which were not identified by keyword search BLAST searches on the chicken genome assembly, were performed with known chicken sequences or in case of their absence with human sequences. In case of SIRP, TREM, and CMRF, single or multiple putative homologues were located on different chromosomes within the chicken genome. To verify that these genes really belong to chicken gene families that represent homologues to their mammalian counterparts, the respective genomic regions were screened for highly conserved, unrelated genes flanking the identified candidate genes that were also found to be present in the human genome at a similar location (Fig. 1). This approach would strongly favor a syntenic relationship of the receptor families found. Moreover, the area between such conserved genes was analyzed for the presence of putative Ig-like receptors to demonstrate the multigene character of the families. The predicted genomic sequences encoding putative Ig-like receptors were then used to screen various chicken EST databases to identify expressed transcripts (Table 2). One result of this analysis was to estimate the quality of the different predicted genomic sequences. The ENSEMBL predictions (e.g., ENSGALT00000009971 for ggSIRP-A1) seemed to be rather accurate in terms of gene number; e.g., one ENSGALT was encoding one gene. In contrast, the NCBI prediction of annotated genomic sequences using GNOMON (e.g., XM_417440) turned out to display very long transcripts, where different genes with identical transcriptional orientation were put together in one prediction (here ggSIRP-B1, ψSIRP, and STK35). None of the programs, however, predicted the correct genes, cloned from cDNA. The alignment and comparison of all matching genomic and EST sequences for each individual gene allowed the design of specific primer pairs. Finally, all genes identified by this strategy were PCR-amplified, cloned, and sequenced. All novel sequences were used for BLAST searches on the chicken genome assembly to identify potential additional members located elsewhere in the chicken genome; however, these BLAST searches did not yield other receptors.
Comparative chromosomal organization of SIRP, TREM, and CMRF35/CD300L families. Predicted gene order and orientation (arrows) of chicken a SIRP, b TREM, and c CMRF35/CD300L gene families and comparison to the corresponding human chromosomal regions. Highly conserved flanking genes are boxed. The contigs containing the chicken genes are displayed below the genes in dotted boxes. Although, gene lengths and the distances between individual genes are not drawn to scale, the figure displays accurate order and occurrence of all genes in that area. In the case of TREM and CMRF35/CD300L genes, our analyses have corrected some of the predictions. The single genes predicted on chicken contig 65.72 on chromosome 26 and contig 137.40–37 on chromosome 3_random turned out to be more than one gene. The entire gene designation according to HUGO nomenclature: FKBP1A FK506 binding protein 1A; FOXP4 forkhead box P4; GPRC5C G protein-coupled receptor, family C, group 5, member C; MDFI MyoD family inhibitor; NFYA nuclear transcription factor Y, alpha; NSFL1C NSFL1 (p97) cofactor (p47); SLC9A3R1 solute carrier family 9, member 3 regulator 1; STK35 serine/threonine kinase 35
To establish a nomenclature for the different immunoregulatory Ig-like receptor families and their members, the name of the human homologues according to HUGO was combined with the prefix “gg (gallus gallus)” and a letter based on classification by sequence features such as activating (-A), inhibitory (-B), soluble (-S) and other molecules without classifying motifs (-X) succeeded by an arbitrary number (e.g., ggSIRP-A1).
SIRP, TREM, and CMRF35/CD300L chicken gene families are present on chromosomes 20, 26 and 3
Employing this approach, we could clearly identify chromosomal areas on chromosome 20 and 26 containing chicken SIRP and TREM homologues, respectively (Fig. 1), that were surrounded by highly conserved genes, also flanking the equivalent human receptor families. Chicken homologues for the CMRF35/CD300L gene family were located on chromosome 3. Because the assembly of this particular chromosome has not been finished in the first draft (assigned “chromosome 3_random”), a flanking conserved gene could only be identified on one side of the chicken CMRF35/CD300L genes. In contrast, all attempts to identify chicken FCR or PILR homologues were unsuccessful. Even locus-specific flanking genes could only be identified on unassembled chromosomes.
One activating and inhibitory SIRP receptor pair
Two SIRP-like molecules were predicted on contig 18.104 on chicken chromosome 20 and corresponding EST clones were identified (Fig. 1, Table 2). A 1,181 bp long fragment could be amplified by PCR that encodes a 382 amino acid long protein that was designated ggSIRP-A1 due to a short cytoplasmic region and a lysine residue in the transmembrane region (Fig. 2). The extracellular domain of ggSIRP-A1 was composed of three Ig domains. While two of the Ig domains were identified as C1 type (Williams and Barclay 1988), the third membrane distal Ig domain resembled a V-set domain with a J-like motif present in the g-strand that displayed the consensus sequence GxGTxL/VxV (Du Pasquier 2000), commonly found in mammalian SIRP. The activating human SIRPB1 shared 34% amino acid identity and more importantly displayed identical features such as three Ig domains, a transmembrane lysine and a short cytoplasmic domain. An additional chicken SIRP-like sequence, more than 90% identical to ggSIRP-A1, was found on an adjacent contig. It was designated ψSIRP because we could not amplify a transcript out of a variety of different cDNAs, and no corresponding EST clones specific for signal peptide or transmembrane could be identified either. The inhibitory counterpart designated as ggSIRP-B1 was identified on contig 18.104. The corresponding 830 bp cDNA encodes a 262 amino acid protein with a single V-set Ig domain (Fig. 2) that shares 56% amino acid identity with the membrane distal Ig domain of ggSIRP-A1. GgSIRP-B1 has an uncharged transmembrane region followed by a long cytoplasmic tail with two ITIM matching the consensus sequence of (I/V/L/S)-x-Y-x-x-(L/V) (Burshtyn et al. 1997). Although ggSIRP-B1 displays only a single Ig domain, it is most homologous to the inhibitory human PTPNS1 that has three Ig domains, but otherwise identical features. In conclusion, a pair of activating and inhibitory chicken SIRP was identified on chromosome 20.
Amino acid alignment of chicken and human SIRP. The amino acid sequences of cloned chicken SIRP-A1, SIRP-B1, human SIRPB1 (acc. no. Y10376), and human PTPNS1 (acc. no. BC038510) were aligned using ClustalW. The accession numbers of all cloned chicken genes are summarized in Table 2. Conserved residues are boxed. The signal peptide (SP), Ig domains (VJ and C1), transmembrane regions (TM), and cytoplasmic domains (CY) are aligned in separate blocks. The predicted position of the transmembrane domain is indicated by a bar above the sequence. The positions of the β strands in the Ig domains are indicated above with arrows and the conserved cysteines forming an intrachain disulfide bond are indicated by an asterisk. The J-like consensus motif is indicated underneath the sequence. The positively charged transmembrane residue is boxed and the ITIM are indicated by a bar above the sequence
The chicken TREM family contains an activating and two inhibitory genes
The genome scan identified a single TREM-like molecule on chicken chromosome 26 (Fig. 1). Further analysis and comparison to EST clones revealed that this genomic region encoded two separate chicken TREM molecules closely linked on the chromosome in the same transcriptional orientation (Table 2, Fig. 3a). GgTREM-A1, an activating receptor with a single V-set Ig domain and a transmembrane lysine residue, is encoded by a 713-bp transcript (Fig. 3b). Human TREM2 displays exactly the same structural features. The chicken and human Ig domains show 44% amino acid identity, which is higher than the amino acid identity between different human TREM Ig domains. GgTREM-B1, a 411 amino acid long molecule, was identified adjacent to ggTREM-A1 and had two V-set Ig domains (Fig. 3b). It is interesting to note that in the membrane proximal Ig domain, the conserved cysteine located in the f strand, that normally forms an intramolecular disulfide bond was absent. In the cytoplasmic tail, two ITIM and an additional motif, designated immunoreceptor tyrosine-based switch motif, which is also known to mediate cellular inhibition, was found in ggTREM-B1.
Chromosomal organization and sequence alignment of the TREM gene family. a Schematic representation of the identified chicken TREM genes on chromosome 26. The ggTREM-A and ggTREM-B1 genes, located on contig 65.72, were originally predicted as a single gene in the ENSEMBL database. The TREM-B2 was only identified following sequencing the gap between contig 65.70 and contig 65.71 with the primers shown as arrows. The two Ig domains essential to identify Ig-like receptors are indicated. b Amino acid alignment of the cloned chicken TREM genes with the most related human TREM (huTREM2, acc. no. AF213457, and huTREML1, acc. no. AF534822). The decoration is identical to Fig. 2, however, both Ig domains of TREM-B1 and TREM-B2 (V-1 as membrane distal Ig domain, V-2 as membrane proximal Ig domain) were aligned together with the single Ig domains found in TREM-A1 and the human TREMs
The chromosomal region containing chicken TREM genes has been built using 16 nonoverlapping contigs between the flanking genes FOXP4 and NFYA; therefore, there are still gaps of unknown size in between these contigs. The BAC clone CH261-174G2 spans the entire region and was used to perform PCR with primers specific for the ends of each individual contig to define the gap lengths between contigs, and one gap larger than 1,000 bp was successively sequenced (Fig. 3a). By this strategy, all exons encoding a third chicken TREM gene (ggTREM-B2) were identified, two of which encoding the diagnostic Ig domains located in the gap between the two contigs 65.70 and 65.71 (Fig. 3a).
It is interesting to note that PCR amplification of this chicken TREM revealed two distinct transcripts of 1,014 bp and 1,341 bp. The long transcript (ggTREM-B2-v1) encoded two V-set Ig domains, an uncharged transmembrane region and a cytoplasmic domain containing two ITIM. The shorter form (ggTREM-B2-v2) was identical; however, the membrane proximal Ig domain was missing, most likely as a result of alternative splicing (Table 2, Fig. 5). Because there is no mammalian TREM with two Ig domains, a true homologue was difficult to assign; therefore, the inhibitory human TREM-L1 was used for the alignment (Fig. 3b). The identities between human and chicken inhibitory TREM V-set Ig domains ranged between 10 and 30%. In summary, using genome database searching in combination with EST database comparison and sequencing of gaps between contigs, a total of three chicken TREMs were identified, an activating ggTREM-A1 and the inhibitory ggTREM-B1, and ggTREM-B2. Alternative splicing of ggTREM-B2 further diversifies this receptor family.
The CD300L family on chromosome 3 contains one inhibitory, one soluble member, and a third member without categorizing motifs
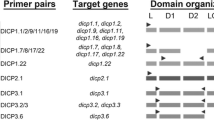
An initial database search for chicken CMRF35/CD300L homologues identified two potential genes on chromosome 3_random, one two-Ig domains encoding gene on contigs 137.40 to 37 and one single-Ig domain molecule on contig 137.36 (Fig. 1). This latter gene potentially encoded an activating receptor; however, we could not detect a transcript by PCR with different primer pairs on the various cDNAs. Moreover, no corresponding EST clone could be identified, indicating that this gene may represent a pseudogene.
Similar to the situation found in the chicken TREM locus, the gene potentially encoding two Ig domains turned out to encode two separate CD300L molecules upon analysis with corresponding EST clones (Fig. 4a, Table 2). One of them with a corresponding cDNA sequence of 559 bp, encoded a single V-set Ig domain without further transmembrane or cytoplasmic domains and was thus designated ggCD300L-S1 referring to a putative soluble CD300L family member (Fig. 4b). The second gene was predicted incompletely, due to the presence of a gap between two contigs. To identify the 3′ part after the Ig domain, we employed the same strategy as for the TREM genes and sequenced the BAC clone CH261-44K10 with a primer binding on the 5′end of contig 137.39 to fill the gap between two contigs. Surprisingly, the alignment of the contigs with the newly obtained sequence reversed the order of two contigs, thus changing the previous assembly. We now predicted a complete CD300L gene in this area and, in addition, a third gene, previously split within two contigs (Fig. 4a). Both of them could be amplified with specific primers. GgCD300L-B1 was cloned as a 1,032 bp transcript with an open reading frame of 933 bp displaying a single V-set Ig domain, an uncharged transmembrane region followed by a long cytoplasmic tail containing four potential tyrosine phosphorylation sites, two of them embedded in an ITIM (Fig. 4b). The second gene was tentatively designated ggCD300L-X1 because the 746 bp transcript encoded a single V-set Ig domain, an uncharged transmembrane region followed by a short cytoplasmic tail without any known signalling motif (Fig. 4b). Both CD300L-B1 and CD300L-X1 seem to have human counterparts with identical features, human CD300A, and human CD300C, respectively (Fig. 4b). In summary, three distinct chicken CD300L gene members were defined, one of which being a classical inhibitory molecule, whereas the others either lacked cytoplasmic signalling motifs or seemed to encode a soluble CD300L version.
Chromosomal organization and sequence alignment of the CMRF35/CD300L gene family. a Schematic representation of the identified CMRF35/CD300L genes on chromosome 3. Sequencing reversed the order of two contigs thus resulting in the identification of two additional CMRF35/CD300L genes. b Amino acid alignment of the cloned chicken CMRF35/CD300L genes with the human CD300A (acc. no. BC032352) and human CD300C (acc. no. BC022279). Decorations see Fig. 2
Genomic organization of the different chicken Ig-like receptor families
The exon/intron structure of the Ig-like receptor genes could be concluded by the comparison of the cloned Ig-like receptor family members with the corresponding genomic DNA, obtained from the ENSEMBL database and from BAC sequencing. The cDNA and genomic sequences did not always match exactly, most likely due to the different origins. The genome sequence was obtained from red jungle fowl, a wild type ancestor of chickens, whereas the cDNA were cloned from domesticated breeds (Table 2). Nevertheless, the exon/intron boundaries could be clearly identified. All characterized Ig-like receptors conformed to the gt-ag rule and all exons for signal peptides and Ig-like domains were in phase 1, whereas exons for the stalk and transmembrane region were in phase 1 or 2, and cytoplasmic exons were in phase 0. The chicken SIRP genes displayed relatively short introns resulting in quite short total gene lengths, whereas the average size of the chicken TREM genes was more than twice (Fig. 5). The intron length, number, and distribution of the different chicken TREM members varied extremely, similar to the situation of TREM family members in humans (Allcock et al. 2003). The exon/intron structure of ggCD300L-B1 and ggCD300L-X1 displayed a unique feature, compared to all known mammalian Ig-like receptors, lacking the intron between the signal peptide and the Ig domain, however this was not the case for ggCD300L-S1. In addition, ggCD300L-X1 had an extended second exon that encoded the stalk and transmembrane region. In conclusion, each Ig-like receptor family showed unique features of genomic organization and average length.
Exon/intron organization of the different chicken Ig receptors. The lengths of the exons and introns are all drawn to scale and are indicated with numbers. The exons encoding signal peptides (SP), Ig domains (IG), stalk regions (S), transmembrane domains (TM), cytoplasmic domains (CY), and cytoplasmic exons containing ITIM (ITIM) are all displayed by differently hatched boxes in order to simplify comparisons. Positively charged transmembrane residues are indicated by single letter amino acid code. The introns are represented by lines between the boxes
Phylogenetic relation of different chicken Ig-like receptor families
To compare the newly identified chicken immunoregulatory families with the previously identified CHIR and corresponding mammalian families, a phylogenetic tree was constructed utilizing the individual chicken and corresponding human Ig domains for the analysis (Fig. 6). The Ig domains of the putative pseudogenes, ψSIRP and ψCD300L-A1, were also included. Only low bootstrap values were obtained by this analysis, most likely because of the phylogenetic distance between chickens and mammals and due to the fast evolution of IgSF members. Nevertheless, the newly identified chicken genes within a given family cluster together with their predicted human homologues. In contrast to the other gene families, the TREM gene family seems to contain a rather diverse set of individual receptors where the chicken and human genes were more closely related to each other than the various human TREMs. The previously identified CHIR genes form a separate group due their C2-set Ig domains.
Phylogenetic comparison of chicken immunoregulatory families. Individual Ig domains were aligned by the Clustal W algorithm. The neighbor-joining tree with 1,000 bootstrap replicates and pairwise gap deletions was built using MEGA 3.1. A maximum parsimony tree was also constructed, but because it was essentially the same as the neighbor-joining trees with respect to the major branching patterns, it will not be presented here. Accession numbers of the individual SIRP, TREM, and CMRF35/CD300L molecules according to Figs. 2, 3, and 4. The Ig domains of CHIR-A2 (acc. no. AJ745093), CHIR-B2 (acc. no. AJ639837), and human LILRB1 (acc. no. AF009220) were included in the analysis
Discussion
These studies were initiated to answer specific questions regarding additional immunoregulatory Ig-like families in the chicken because our previous analyses have concentrated on a single family that differs from its human counterpart by both gene number and diversity (Viertlboeck et al. 2005). Precisely, we wanted to clarify, if other immunoregulatory families exist in the chicken, to determine their location in the chicken genome and to estimate their individual gene number. The genes were initially detected by a homology-based approach, but due to the low overall homology between chicken and mammalian genes, several additional criteria were employed to unequivocally define the different families as true chicken homologues to the human SIRP, TREM, and CMRF35/CD300L families.
Firstly, each locus was carefully analyzed for flanking genes that were unrelated to the immunoregulatory family and that were highly conserved to human genes. We could identify conserved flanking genes in each case, suggesting conserved synteny between the respective chicken and human chromosomal areas. In the case of the chicken CD300L family, only a single conserved flanking gene was detected; however, the situation on chromosome 3 is complicated by the fact that it has not been correctly assembled. In a second test, the individual receptor genes between the flanking regions were carefully analyzed. Immunoregulatory family is always characterized by multiple, closely linked genes that may contain various numbers of Ig domains and that are further distinguished by activating and inhibitory cytoplasmic features. These criteria were also met for the three chicken families described. Finally, the individual families are characterized by distinctive structural features in their Ig domains as discussed below.
The chicken SIRP family is characterized by a membrane distal V-set Ig domain that contains a J-like element encoded by one exon and that may be linked to membrane proximal C1-set Ig domains. The fish novel immune-type receptor also contains this J-like element which are associated with an I-set Ig domain; therefore, they may represent ancestral SIRP genes (Van den Berg et al. 2004). In contrast, the chicken receptors in this family can be unequivocally defined as SIRP homologues that have individual corresponding human members. The ggSIRP-B1 seems to be an inhibitory form like human PTPNS1 that was shown to associate with SHP-1 and SHP-2 after CD47 binding (Kharitonenkov et al. 1997; Liu et al. 2002). Vice versa, human SIRPB1 as an activating receptor that associates with DAP12 and induces serotonine release in RBL cells (Tomasello et al. 2000) may be the functional homologue of ggSIRP-A1.
The human TREM gene cluster contains a rather diverse set of individual members, including the natural cytotoxicity triggering receptor 2 (NCR2) gene (Allcock et al. 2003). For example in their Ig domain, the human TREM2 shares only 30% with human TREML1, whereas the human TREM2 shares 44% with ggTREM-A1, a rather unusual observation. Apart from their common chromosomal location, human TREM share two additional features, a single V-set Ig domain and in some cases an extra pair of cysteines in the c- and c′-strand, which are thought to stabilize the β hairpin of the Ig fold. This additional disulfide bond represents a specific structural signature defining a novel Ig subfamily (Cantoni et al. 2003).
These TREM features are all represented in the chicken homologues. Although ggTREM-B1 and ggTREM-B2 both contain two V-set Ig domains, they were also assigned to be chicken TREM family members, because the number of Ig domains is highly variable and provides no evidence for assignment to a specific family.
The additional cysteines in the Ig domains are also found in human and chicken CMRF35/CD300L family. Human CD300A and CD300C (also known as CMRF35-H und -A) are 91. 2% identical in their V-set Ig domain and are therefore believed to have evolved by gene duplication (Clark et al. 2001). Whereas CD300A has a long cytoplasmic tail with three to four potential ITIM, CD300C has only a short cytoplasmic tail and a glutamic acid in the transmembrane region similar to CD3 δ?, CD3 γ, and CD147 (Clevers et al. 1988; Kasinrerk et al. 1992). A similar molecule with a transmembrane glutamic acid is also found in the mouse CMRF35-like molecule 5 (Chung et al. 2003).
The situation in the chicken is comparable with ggCD300L-B1 and ggCD300L-X1 sharing 93.9% amino acid identity in their V-set Ig domain. GgCD300L-B1 has two ITIM and ggCD300L-X1 displays a glutamine instead of the glutamic acid in the transmembrane region. Because both of amino acids represent polar residues that are rarely found in transmembrane domains, they may serve important functions such as mediating the association to linked molecules. Alternatively, both the soluble ggCD300L-S1 and the ggCD300L-X1 may also serve as decoy receptors without signalling capacity.
In carp, an immunoregulatory family has been described as novel Ig-like transcripts (NILT) with single V-set Ig-domains that also display two additional cysteine residues. Due to the similarity to the human CMRF35/CD300L and TREM families, a definitive assignment was not possible (Stet et al. 2005).
The experiments described herein were initiated to clarify if the extraordinarily complex CHIR family may represent a functional substitute of the various smaller human Ig-like immunoregulatory families. Our analyses demonstrate that this is not the case, but that the other families are present in the chicken with comparable numbers of individual receptors. We cannot exclude that there are few additional receptors in these families simply because the respective regions have not been fully sequenced and assembled, however, this will not increase the overall complexity.
Our analyses clearly show that various Ig-like immunoregulatory receptor families have been conserved from chicken to man. The different chicken immunoregulatory families are unequivocally identified not only by their chromosomal location, but also by distinctive sequence features that are unique within a given family. In some cases, these conserved sequence motifs even allow the assignment of a particular chicken family member to its human counterpart. We therefore predict that the families that were not identified (FCR, PILR) may still be present in the chicken genome. Moreover, from the conserved sequence features it is anticipated that in many cases, the chicken immunoregulatory Ig-like receptors may bind to similar ligands as in humans and potentially have similar functions. Because in many cases neither the ligand nor the function of individual receptors is currently known, the chicken may serve as an additional model to identify some of these features.
Abbreviations
- CD300L:
-
CD300 antigen like
- CHIR:
-
Chicken Ig-like receptor
- DAP:
-
DNAX activating protein
- ITIM:
-
Immunoreceptor tyrosine-based inhibitory motif
- KARAP:
-
Killer cell-activating receptor-associated protein
- KIR:
-
Killer cell Ig-like receptor
- LILR:
-
Leukocyte Ig-like receptor
- NCR2:
-
Natural cytotoxicity triggering receptor 2
- NILT:
-
Novel Ig-like transcript
- NITR:
-
Novel immune-type receptor
- PILR:
-
Paired Ig-like type 2 receptor
- SHP:
-
Src homology 2 domain containing protein tyrosine phosphatase
- SIRP:
-
Signal-regulatory protein
- TREM:
-
Triggering receptor expressed on myeloid cells
References
Allcock RJ, Barrow AD, Forbes S, Beck S, Trowsdale J (2003) The human TREM gene cluster at 6p21.1 encodes both activating and inhibitory single IgV domain receptors and includes NKp44. Eur J Immunol 33:567–577
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Barten R, Torkar M, Haude A, Trowsdale J, Wilson MJ (2001) Divergent and convergent evolution of NK-cell receptors. Trends Immunol 22:52–57
Beug H, von Kirchbach A, Doderlein G, Conscience JF, Graf T (1979) Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell 18:375–390
Borrego F, Kabat J, Kim DK, Lieto L, Maasho K, Pena J, Solana R, Coligan JE (2002) Structure and function of major histocompatibility complex (MHC) class I specific receptors expressed on human natural killer (NK) cells. Mol Immunol 38:637–660
Brown D, Trowsdale J, Allen R (2004) The LILR family: modulators of innate and adaptive immune pathways in health and disease. Tissue Antigens 64:215–225
Burshtyn DN, Yang W, Yi T, Long EO (1997) A novel phosphotyrosine motif with a critical amino acid at position -2 for the SH2 domain-mediated activation of the tyrosine phosphatase SHP-1. J Biol Chem 272:13066–13072
Campbell KS, Colonna M (2001) Human natural killer cell receptors and signal transduction. Int Rev Immunol 20:333–370
Cantoni C, Ponassi M, Biassoni R, Conte R, Spallarossa A, Moretta A, Moretta L, Bolognesi M, Bordo D (2003) The three-dimensional structure of the human NK cell receptor NKp44, a triggering partner in natural cytotoxicity. Structure (Camb) 11:725–734
Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD (2003) Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31:3497–3500
Chung DH, Humphrey MB, Nakamura MC, Ginzinger DG, Seaman WE, Daws MR (2003) CMRF-35-like molecule-1, a novel mouse myeloid receptor, can inhibit osteoclast formation. J Immunol 171:6541–6548
Clark GJ, Cooper B, Fitzpatrick S, Green BJ, Hart DN (2001) The gene encoding the immunoregulatory signaling molecule CMRF-35A localized to human chromosome 17 in close proximity to other members of the CMRF-35 family. Tissue Antigens 57:415–423
Clevers H, Alarcon B, Wileman T, Terhorst C (1988) The T cell receptor/CD3 complex: a dynamic protein ensemble. Annu Rev Immunol 6:629–662
Colonna M (2003) TREMs in the immune system and beyond. Nat Rev Immunol 3:445–453
Dennis G Jr, Kubagawa H, Cooper MD (2000) Paired Ig-like receptor homologs in birds and mammals share a common ancestor with mammalian Fc receptors. Proc Natl Acad Sci USA 97:13245–13250
Du Pasquier L (2000) The phylogenetic origin of antigen-specific receptors. Curr Top Microbiol Immunol 248:160–185
Göbel TWF (2000) Isolation and analysis of natural killer cells in chickens. Methods Mol Biol 121:337–345
Hillier LW, Miller W, Birney E, Warren W, Hardison RC, Ponting CP, Bork P, Burt DW, Groenen MA, Delany ME, Dodgson JB, Chinwalla AT, Cliften PF, Clifton SW, Delehaunty KD, Fronick C, Fulton RS, Graves TA, Kremitzki C, Layman D, Magrini V, McPherson JD, Miner TL, Minx P, Nash WE, Nhan MN, Nelson JO, Oddy LG, Pohl CS, Randall-Maher J, Smith SM, Wallis JW, Yang SP, Romanov MN, Rondelli CM, Paton B, Smith J, Morrice D, Daniels L, Tempest HG, Robertson L, Masabanda JS, Griffin DK, Vignal A, Fillon V, Jacobbson L, Kerje S, Andersson L, Crooijmans RP, Aerts J, van der Poel JJ, Ellegren H, Caldwell RB, Hubbard SJ, Grafham DV, Kierzek AM, McLaren SR, Overton IM, Arakawa H, Beattie KJ, Bezzubov Y, Boardman PE, Bonfield JK, Croning MD, Davies RM, Francis MD, Humphray SJ, Scott CE, Taylor RG, Tickle C, Brown WR, Rogers J, Buerstedde JM, Wilson SA, Stubbs L, Ovcharenko I, Gordon L, Lucas S, Miller MM, Inoko H, Shiina T, Kaufman J, Salomonsen J, Skjoedt K, Wong GK, Wang J, Liu B, Yu J, Yang H, Nefedov M, Koriabine M, Dejong PJ, Goodstadt L, Webber C, Dickens NJ, Letunic I, Suyama M, Torrents D, von Mering C, Zdobnov EM et al (2004) Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 432:695–716
Kasinrerk W, Fiebiger E, Stefanova I, Baumruker T, Knapp W, Stockinger H (1992) Human leukocyte activation antigen M6, a member of the Ig superfamily, is the species homologue of rat OX-47, mouse basigin, and chicken HT7 molecule. J Immunol 149:847–854
Kaufman J, Milne S, Göbel TW, Walker BA, Jacob JP, Auffray C, Zoorob R, Beck S (1999) The chicken B locus is a minimal essential major histocompatibility complex. Nature 401:923–925
Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A (1997) A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 386:181–186
Kumar S, Tamura K, Nei M (2004) MEGA3: Integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Lenschow DJ, Walunas TL, Bluestone JA (1996) CD28/B7 system of T cell costimulation. Annu Rev Immunol 14:233–258
Letunic I, Copley RR, Schmidt S, Ciccarelli FD, Doerks T, Schultz J, Ponting CP, Bork P (2004) SMART 4.0: towards genomic data integration. Nucleic Acids Res 32:D142–D144 (Database issue)
Liu Y, Buhring HJ, Zen K, Burst SL, Schnell FJ, Williams IR, Parkos CA (2002) Signal regulatory protein (SIRPalpha), a cellular ligand for CD47, regulates neutrophil transmigration. J Biol Chem 277:10028–10036
Ljunggren HG, Karre K (1990) In search of the missing self: MHC molecules and NK cell recognition. Immunol Today 11:237–244
Long EO (1999) Regulation of immune responses through inhibitory receptors. Annu Rev Immunol 17:875–904
Maglott D, Ostell J, Pruitt KD, Tatusova T (2005) Entrez gene: gene-centered information at NCBI. Nucleic Acids Res 33:D54–D58
Moretta L, Moretta A (2004) Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J 23:255–259
Ogura H, Fujiwara T (1987) Establishment and characterization of a virus-free chick cell line. Acta Med Okayama 41:141–143
Peck R, Murthy KK, Vainio O (1982) Expression of B-L (Ia-like) antigens on macrophages from chicken lymphoid organs. J Immunol 129:4–5
Ravetch JV, Lanier LL (2000) Immune inhibitory receptors. Science 290:84–89
Schultz J, Milpetz F, Bork P, Ponting CP (1998) SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci USA 95:5857–5864
Stet RJ, Hermsen T, Westphal AH, Jukes J, Engelsma M, Lidy Verburg-van Kemenade BM, Dortmans J, Aveiro J, Savelkoul HF (2005) Novel immunoglobulin-like transcripts in teleost fish encode polymorphic receptors with cytoplasmic ITAM or ITIM and a new structural Ig domain similar to the natural cytotoxicity receptor NKp44. Immunogenetics 57:77–89
Tomasello E, Blery M, Vely E, Vivier E (2000) Signaling pathways engaged by NK cell receptors: double concerto for activating receptors, inhibitory receptors and NK cells. Semin Immunol 12:139–147
van den Berg TK, Yoder JA, Litman GW (2004) On the origins of adaptive immunity: innate immune receptors join the tale. Trends Immunol 25:11–16
Viertlboeck BC, Crooijmans RP, Groenen MA, Gobel TW (2004) Chicken Ig-like receptor B2, a member of a multigene family, is mainly expressed on B lymphocytes, recruits both Src homology 2 domain containing protein tyrosine phosphatase (SHP)-1 and SHP-2, and inhibits proliferation. J Immunol 173:7385–7393
Viertlboeck BC, Habermann FA, Schmitt R, Groenen MA, Du Pasquier L, Gobel TW (2005) The chicken leukocyte receptor complex: a highly diverse multigene family encoding at least six structurally distinct receptor types. J Immunol 175:385–393
Vivier E, Malissen B (2005) Innate and adaptive immunity: specificities and signaling hierarchies revisited. Nat Immunol 6:17–21
Williams AF, Barclay AN (1988) The immunoglobulin superfamily-domains for cell surface recognition. Annu Rev Immunol 6:381–405
Acknowledgements
We cordially thank Drs. L. du Pasquier and M. Groenen for helpful discussions. The BAC clones were provided by the BACPAC Resources of the Children’s Hospital Oakland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Viertlboeck, B.C., Schmitt, R. & Göbel, T.W. The chicken immunoregulatory receptor families SIRP, TREM, and CMRF35/CD300L. Immunogenetics 58, 180–190 (2006). https://doi.org/10.1007/s00251-006-0091-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-006-0091-8