Abstract
By determining the nucleotide sequences of more than 700 cDNA clones isolated from 16 cynomolgus monkeys, we identified 26 Mafa-B alleles. In addition, nine sequences with similarity to Mamu-I alleles were identified. Since multiple Mafa-B alleles were found in each individual, it was strongly suggested that the cynomolgus MHC class I B locus might be duplicated and that the Mafa-I locus was derived from the B locus by gene duplication, as in the case of the Mamu-I locus of rhesus monkeys.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well established that CD8+ T-cell activation is triggered through recognition of the MHC class I molecule loaded with an antigenic peptide by an antigen-specific T-cell receptor. The MHC molecules of the mammals including primates are known to influence the outcome of many diseases such as infectious diseases, cancer, and metabolic disorders. HLA class I genes are divided into three different categories, classical (HLA-A, -B, and -C), non-classical (HLA-E, -F, and -G), and pseudogene (HLA-H, -J, -K, and -L), according to their degree of polymorphism and cell surface expression, and the presence of orthologues of the human HLA-A, -B, -E, -F, and -G genes were identified in several species of the Old World monkeys (Alvarez et al. 1997; Boyson et al. 1996a, b; Evans et al. 2000; Lafont et al. 2004; Otting and Bontrop 1993; Prilliman et al. 1996; Sidebottom et al. 2001; Uda et al. 2004). Cynomolgus monkeys as well as rhesus monkeys are preferentially used for biomedical research; however, cynomolgus MHC class I was not extensively studied compared with those in rhesus monkeys. We have previously reported the nucleotide sequences of cynomolgus MHC class I A locus and have shown that at least 14 Mafa-A alleles were present in cynomolgus monkeys (Uda et al. 2004). Although the MHC class I B locus is the most polymorphic MHC locus in primates, little information is available concerning the MHC class I B locus of cynomolgus monkeys. In this study, therefore, we have expanded our analysis on cynomolgus MHC class I genes and identified 26 B locus alleles by analyzing 16 monkeys. We have also found the presence of a novel locus that is very similar to MHC class I I locus recently identified in rhesus monkeys.
Materials and methods
Animals
All cynomolgus monkeys were raised and reared in the Tsukuba Primate Center for Medical Science, the National Institute of Infectious Diseases (NIID). Both genders were involved, and the cynomolgus monkeys were between 5 and 24 years old. This study was conducted in accordance with the Guides for Animal Experiments Performed at the NIID.
RT-PCR and nucleotide sequencing
Preparation of mRNA from peripheral blood mononuclear cells (PBMC) and RT-PCR were performed as described before (Uda et al. 2004). Primers used in this study are listed in Table 1. 5′ MBS and 3′ MBS primers designed to amplify the gene products of the rhesus MHC class I B locus by Boyson et al. (1996b) were also used to amplify the cynomolgus MHC class I B locus. PCR amplification was performed at least twice for each animal. PCR products were cloned into pCR4Blunt-TOPO plasmids (Invitrogen, Carlsbad, Calif., USA) and 48 clones were sequenced by 310 Capillary DNA Sequencer (Applied Biosystems, Foster City, Calif., USA) or 3100-Avant Capillary DNA Sequencer (Applied Biosystems). The Mafa-B nucleotide sequences were assembled with the Contig Manager of the DNASIS pro (Hitachi Software, Yokohama, Japan). The Clustal W algorithm provided in DNASIS PRO was used to align sequences.
Phylogenetic analysis
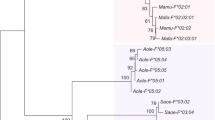
The full-length nucleotide sequences of Mafa-B, Mafa-I, Mafa-A, Mamu-A, Mamu-B, Mamu-I, HLA-A, and HLA-B were aligned using Clustal W provided online by the DNA Data Bank of Japan [(DDBJ) http://www.ddbj.nig.ac.jp]. A phylogenetic tree of these nucleotide sequences was constructed by the neighbor-joining method of the Molecular Evolution Genetics Analysis, version 2.1 (MEGA 2.1). Genetic distances were estimated using the method of Jules-Canter. At the sites in which alignment indicated a gap, nucleotides at this position in all the sequences were deleted. The reliability of the tree topology was tested by the bootstrap method. Thousand relationships and 64,238 random seeds were used for determining bootstrap values (Fig. 2a, b). Since the bootstrap values of less than 50% were unreliable, the values of less than 50% were not shown in Fig. 2a, b.
GenBank accession numbers
The Mafa-B and Mafa-I sequences described in this manuscript had been deposited in the DDBJ and were assigned accession numbers AB195431 to AB195465. We previously deposited Mafa-A alleles in the DDBJ, and these alleles were assigned accession numbers AB154760 to AB154773. The GenBank accession numbers for other sequences used in this study are as follows: HLA-A*0201, U07161; HLA-B*2702, L38504; HLA-B*5701, AJ458991; Mafa-E*01, U02976; Mamu-A*01, U50836; Mamu-A*02, U50837; Mamu-A*03, U41379; Mamu-A*04, U41380; Mamu-B*02, U41833; Mamu-B*03, U41825; Mamu-B*04, U41826; Mamu-B*05, U41827; Mamu-B*06, U41828; Mamu-B*07, U41829; Mamu-B*08, U41830; Mamu-B*36, AJ556886; Mamu-I*01011, AF161865; Mamu-I*02012, AF161869, Mamu-I*04, AF4161874; Mamu-I*07, AF161875; Mamu-I*08, AF161876; Mamu-I*09, AF161877; Mamu-I*10, AF161878; and Mamu-I*11, AF161879.
Results
Detection of 26 MHC class I B locus alleles and nine I locus alleles in cynomolgus monkeys
To amplify cynomolgus MHC class I B locus genes, PCR was carried out using primers that were successfully used for amplification of rhesus MHC class I B locus genes along with newly designed ones (Table 1; Boyson et al. 1996b). We obtained 48 clones from each animal. The nucleotide sequences that were found in just one clone were excluded from the subsequent analyses to avoid incorporation of artificial sequences generated by PCR error or during the cloning procedure into public databases. Ambiguous sequences were also excluded. When the nucleotide sequence was shared by more than two clones, regardless of whether they were derived from one animal or multiple animals, the sequences were regarded as a consensus sequences representing a particular alleles of each animal. Eventually, 43 candidate alleles were obtained, and 34 of 43 were found to have substantial homology with Mamu-B alleles. Amino acid sequences deduced from the nucleotide sequences of these 34 candidate B alleles were further subjected to phylogenetic analysis using the neighbor-joining method (Saitou and Nei 1987; data not shown). When the predicted amino acid sequence variation between two candidates was negligible (d<0.025), the amino acid sequence shared by a majority of the clones was regarded as representing a particular allele. The other sequence shared by a minority of clones was excluded from the subsequent analyses. As the result of the analysis, 26 Mafa-B alleles were identified. It was found that the remaining nine candidate alleles were closely related to those of Mamu-I locus reported by Urvater et al. (2000b). Since Urvater et al. also identified two Mafa-I alleles (Mafa-I*01011 and Mafa-I*01012), we named tentatively alleles identified here Mafa-I*01013 through Mafa-I*09. The Mafa-I*01013 allele was identical in amino acid sequence with Mafa-I*01011 and Mafa-I*01012, but there were several synonymous nucleotide changes scattered around the sequence. We therefore considered that this alleles was a variant of Mafa-I*01, although reported sequences of Mafa-I*01011 and Mafa-I*01012 were incomplete. The deduced amino acid sequences of Mafa-B and Mafa-I alleles were shown in Fig. 1 along with those of alleles reported for other primates. The total numbers of clones obtained and the numbers of animals having the allele were shown in the figure. The putative glycosylation site was located at residue 86, and the conserved cysteine residues occurred at positions 101 and 164 in α2 and at positions 203 and 259 in α3. To evaluate whether the nucleotide sequences of Mafa-B and Mafa-I alleles established in this study were gene products of class I B and I loci, respectively, Mafa-B and Mafa-I alleles were phylogenetically analyzed (Fig. 2a). The full-length nucleotide sequences of Mafa-B, Mafa-I, Mafa-A, Mamu-A, Mau-B, Mamu-I, HLA-A, and HAL-B were aligned by Clustal W. A phylogenetic tree was constructed by the neighbor-joining method of MEGA2.1 software. The reliability of the tree topology was tested by the bootstrap method, and the bootstrap values are shown in Fig. 2a. Since the bootstrap values of less than 50% were unreliable, the bootstrap values of greater than 50% are shown in Fig. 2a. Several Mafa-B alleles (Mafa-B*21, 22, 24, 25, and 26) appeared to cluster with Mamu-I or Mafa-I allele rather than B locus alleles. Since amino acid difference between alleles of I and B loci were more apparent at the carboxy half of the protein, we reconstructed the phylogenetic tree using the amino acid sequences of the exons 5 to 8. The result clearly showed that these nine alleles clustered with Mamu-I alleles (Fig. 2b). These results strongly suggested that these cDNA clones were derived from distinct alleles on MHC class I B and I loci of cynomolgus monkeys.
Deduced amino acid sequences of Mafa-B and Mafa-I alleles. Amino acid sequences of HLA-A, HLA-B, Mamu-A, Mamu-B, Mamu-I, Mafa-A, and Mafa-E alleles were also included. Amino acids identical to those of HLA-B*2702 are indicated by dots. The deletions of amino acids are indicated by hyphens. The total numbers of clones obtained and the numbers of animals having the allele were indicated after the allele name
Inheritance of Mafa-B and Mafa-I in a family of cynomolgus monkeys
A family consisting of three parents (one sire and two dams) and four offspring was subjected to genetic analysis to study inheritance of Mafa-B and Mafa-I alleles. By nucleotide sequence analysis, ten Mafa-B alleles and four Mafa-I alleles were detected in this family as shown in Table 2. Since certain alleles appeared to be inherited in this family as a complex, we considered those gene complexes as haplotypes and assigned letters A through F to those combinations of alleles (Table 2). Haplotype A (Mafa-B*03 and Mafa-I*09) was detected in 3028, 1113, and 0068, whereas haplotype B (Mafa-B*24 and Mafa-B*25) was carried by 3028, 0079, and 7071 (Fig. 3). Haplotype C (Mafa-B*17, Mafa-B*20, and Mafa-I*06) was found in 1159 and 7071, haplotype D (Mafa-B*01 and Mafa-B*04) in 1159 and 0068, haplotype E (Mafa-B*16, Mafa-I*03) in 3032, 0079, and 1113, and haplotype F (Mafa-B*06, Mafa-B*23, and Mafa-I*02) in 3032 (Fig. 3). We could not detect Mafa-I alleles in monkeys bearing haplotypes B and D. It was evident that Mafa-B alleles were inherited in a Mendelian fashion. Moreover, cynomolgus monkeys in this family were shown to have two to four Mafa-B alleles. The presence of multiple Mafa-B alleles was confirmed by nucleotide sequences analysis of two additional cynomolgus monkeys unrelated to this family. Table 3 showed that 2010 had four Mafa-B alleles and 5076 had three Mafa-B alleles. These results indicated that MHC class I B locus of cynomolgus monkeys was duplicated as in the case of rhesus monkeys (Boyson et al. 1996b).
Discussion
Although cynomolgus monkeys are widely used as animal models in a variety of biomedical researches, there are no nucleotide sequence data on cynomolgus MHC class I B locus. In this study, we tried to identify the alleles of cynomolgus MHC class I B locus, using PBMC cDNA from 16 cynomolgus monkeys.
Nucleotide sequence analyses and following phylogenetic analysis identified 26 Mafa-B alleles (Figs. 1, 2a). We also found nine clones with the nucleotide sequences showing high homology with those of Mamu-I alleles. Phylogenetic analysis showed that these clones were derived from nine Mafa-I alleles. It was reported that novel MHC class I I locus in rhesus monkeys, Mamu-I, could be amplified with B locus-specific primers, and that the I locus was recently evolved from a classical MHC class I B locus by duplication (Urvater et al. 2000b).
The haplotypes of rhesus MHC class I composed of at least one A locus and at least two B loci (Boyson et al. 1996b). In cynomolgus monkeys, we previously reported that the A locus had been duplicated, because one to four Mafa-A alleles were found in an animal (Uda et al. 2004). The presence of up to six Mamu-B alleles in a rhesus monkey (Urvater et al. 2000a) indicates that rhesus monkeys have three class I B loci. In this study, we also showed that two to four Mafa-B alleles were present in each individual, strongly suggesting that cynomolgus monkeys have multiple MHC class I B loci. Regarding the I locus, it seemed possible that at least one locus was present in each animal, although some individual appeared not to have the locus. The apparent lack of the I locus in some individual was probably due to low efficiency of amplification of the I locus because of the presence of the multiple B loci.
Information on MHC class I molecule is particularly important in better understanding of pathogenesis of various infectious diseases including HIV infection. So far the nucleotide sequence data are available for the alleles of Mafa-A (Uda et al. 2004), Mafa-E (Alvarez et al. 1997; Boyson et al. 1995), Mafa-G (Arnaiz-Villena et al. 1997; Castro et al. 1996), Mafa-I (Urvater et al. 2000b), Mafa-DRB (Gaur et al. 1997; Kriener et al. 2000; Leuchte et al. 2004), Mafa-DQA (Kenter et al. 1992), and Mafa-DQB (Otting et al. 2002) in cynomolgus monkeys. The identification of Mafa-B alleles would, therefore, greatly help understand the pathogenesis of various pathogens that naturally or experimentally infect cynomolgus monkeys.
References
Alvarez M, Martinez-Laso J, Varela P, Diaz-Campos N, Gomez-Casado E, Vargas-Alarcon G, Garcia-Torre C, Arnaiz-Villena A (1997) High polymorphism of Mhc-E locus in non-human primates: alleles with identical exon 2 and 3 are found in two different species. Tissue Antigens 49:160–167
Arnaiz-Villena A, Martinez-Laso J, Alvarez M, Castro MJ, Varela P, Gomez-Casado E, Suarez B, Recio MJ, Vargas-Alarcon G, Morales P (1997) Primate Mhc-E and -G alleles. Immunogenetics 46:251–266
Boyson JE, McAdam SN, Gallimore A, Golos TG, Liu X, Gotch FM, Hughes AL, Watkins DI (1995) The MHC E locus in macaques is polymorphic and is conserved between macaques and humans. Immunogenetics 41:59–68
Boyson JE, Iwanaga KK, Golos TG, Watkins DI (1996a) Identification of the rhesus monkey HLA-G ortholog. Mamu-G is a pseudogene. J Immunol 157:5428–5437
Boyson JE, Shufflebotham C, Cadavid LF, Urvater JA, Knapp LA, Hughes AL, Watkins DI (1996b) The MHC class I genes of the rhesus monkey. Different evolutionary histories of MHC class I and II genes in primates. J Immunol 156:4656–4665
Castro MJ, Morales P, Fernandez-Soria V, Suarez B, Recio MJ, Alvarez M, Martin-Villa M, Arnaiz-Villena A (1996) Allelic diversity at the primate Mhc-G locus: exon 3 bears stop codons in all Cercopithecinae sequences. Immunogenetics 43:327–336
Evans DT, Jing P, Allen TM, O’Connor DH, Horton H, Venham JE, Piekarczyk M, Dzuris J, Dykhuzen M, Mitchen J, Rudersdorf RA, Pauza CD, Sette A, Bontrop RE, DeMars R, Watkins DI (2000) Definition of five new simian immunodeficiency virus cytotoxic T-lymphocyte epitopes and their restricting major histocompatibility complex class I molecules: evidence for an influence on disease progression. J Virol 74:7400–7410
Gaur LK, Nepom GT, Snyder KE, Anderson J, Pandarpurkar M, Yadock W, Heise ER (1997) MHC-DRB allelic sequences incorporate distinct intragenic trans-specific segments. Tissue Antigens 49:342–355
Kenter M, Otting N, Anholts J, Leunissen J, Jonker M, Bontrop RE (1992) Evolutionary relationships among the primate Mhc-DQA1 and DQA2 alleles. Immunogenetics 36:71–78
Kriener K, O’hUigin C, Tichy H, Klein J (2000) Convergent evolution of major histocompatibility complex molecules in humans and New World monkeys. Immunogenetics 51:169–178
Lafont BA, Buckler-White A, Plishka R, Buckler C, Martin MA (2004) Pig-tailed macaques (Macaca nemestrina) possess six MHC-E families that are conserved among macaque species: implication for their binding to natural killer receptor variants. Immunogenetics 56:142–154
Leuchte N, Berry N, Kohler B, Almond N, LeGrand R, Thorstensson R, Titti F, Sauermann U (2004) MhcDRB-sequences from cynomolgus macaques (Macaca fascicularis) of different origin. Tissue Antigens 63:529–537
Otting N, Bontrop RE (1993) Characterization of the rhesus macaque (Macaca mulatta) equivalent of HLA-F. Immunogenetics 38:141–145
Otting N, de Groot NG, Doxiadis GG, Bontrop RE (2002) Extensive Mhc-DQB variation in humans and non-human primate species. Immunogenetics 54: 230–239
Prilliman K, Lawlor D, Ellexson M, McElwee N, Confer D, Cooper DK, Kennedy RC, Hildebrand W (1996) Characterization of baboon class I major histocompatibility molecules. Implications for baboon-to-human xenotransplantation. Transplantation 61:989–996
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sidebottom DA, Kennedy R, Hildebrand WH (2001) Class I MHC expression in the yellow baboon. J Immunol 166:3983–3993
Uda A, Tanabayashi K, Yamada YK, Akari H, Lee YJ, Mukai R, Terao K, Yamada A (2004) Detection of 14 alleles derived from the MHC class I A locus in cynomolgus monkeys. Immunogenetics 56:155–163
Urvater JA, McAdam SN, Loehrke JH, Allen TM, Moran JL, Rowell TJ, Rojo S, Lopez de Castro JA, Taurog JD, Watkins DI (2000a) A high incidence of Shigella-induced arthritis in a primate species: major histocompatibility complex class I molecules associated with resistance and susceptibility, and their relationship to HLA-B27. Immunogenetics 51:314–325
Urvater JA, Otting N, Loehrke JH, Rudersdorf R, Slukvin II, Piekarczyk MS, Golos TG, Hughes AL, Bontrop RE, Watkins DI (2000b) Mamu-I: a novel primate MHC class I B-related locus with unusually low variability. J Immunol 164:1386–1398
Acknowledgements
We thank K. Ono, A. Hiyaoka, M, Maeshima, and other staff in The Corporation of Production and Breeding of Primate for animal care and blood collection. This study was supported by the Program of Fundamental Studies in Health Science of the Organization for Pharmaceutical Safety and Research of Japan, and by the Health Science Research Grants from the Ministry of Health and Welfare of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uda, A., Tanabayashi, K., Fujita, O. et al. Identification of the MHC class I B locus in cynomolgus monkeys. Immunogenetics 57, 189–197 (2005). https://doi.org/10.1007/s00251-005-0782-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-005-0782-6