Abstract
The white appearance of skim milk is due to strong light scattering by colloidal particles called casein micelles. Bovine casein micelles comprise expressed proteins from four casein genes together with significant fractions of the total calcium, inorganic phosphate, magnesium and citrate ions in the milk. Thus, the milk salts are partitioned between the casein micelles, where they are mostly in the form of nanoclusters of an amorphous calcium phosphate sequestered by caseins through their phosphorylated residues, with the remainder in the continuous phase. Previously, a salt partition calculation was made assuming that the nanoclusters are sequestered only by short, highly phosphorylated casein sequences, sometimes called phosphate centres. Three of the four caseins have a proportion of their phosphorylated residues in either one or two phosphate centres and these were proposed to react with the nanoclusters equally and independently. An improved model of the partition of caseins and salts in milk is described in which all the phosphorylated residues in competent caseins act together to bind to and sequester the nanoclusters. The new model has been applied to results from a recent study of variation in salt and casein composition in the milk of individual cows. Compared to the previous model, it provides better agreement with experiment of the partition of caseins between free and bound states and equally good results for the partition of milk salts. In addition, new calculations are presented for the charge on individual caseins in their bound and free states.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caseins are present in bovine milk as a polydisperse distribution of more-or-less spherical, highly solvated, particles containing nanoclusters of calcium phosphate. The particles are known as casein micelles but it is important to recognise that their structure bears no resemblance to that of detergent micelles. Many models of casein micelle structure have been proposed (Dalgleish 2011; de Kruif and Holt 2003; Horne 1998; Huppertz et al. 2017; McMahon and Oommen 2012; Rollema 1992; Schmidt 1982; Waugh 1971). The calcium phosphate nanocluster model developed by (de Kruif and Holt 2003; Holt et al. 2003) is derived from independent experiments on simplified model systems demonstrating that amorphous calcium phosphate can be dispersed by competent phosphopeptides to form equilibrium core-shell nanoclusters (Clegg and Holt 2009; Holt et al. 1996, 1998, 2009; Lenton et al. 2015b, 2016; Little and Holt 2004). The classical nucleation model for the growth of a crystalline phase from a solution has been largely replaced in recent years by the two-stage model in which an initial amorphous, metastable, liquid-like precursor forms within the spinodal boundary and subsequently matures into the thermodynamically stable crystal phase (Gebauer and Coelfen 2011; Gower 2008; Pouget et al. 2009; Vekilov 2010). The free energy of sequestration by a competent phosphoprotein can convert metastable precursors of either calcium carbonate or calcium phosphate into a thermodynamically stable nanocluster complex (Holt et al. 2009; Ibsen et al. 2016). The equilibrium size of the calcium phosphate core of a nanocluster can be expressed in terms of the free energy of forming the core and the free energy of sequestering the core in a shell of competent phosphorylated proteins or peptides (Holt 2013; Holt et al. 2009, 2014). The same, or very similar, nanoclusters explain the source and scale of the substructure of native bovine casein micelles (de Kruif 2014; Holt et al. 2003; Ingham et al. 2015; Marchin et al. 2007; McMahon and McManus 1998). Thus, according to the calcium phosphate nanocluster model a typical bovine casein micelle of 100 nm radius contains about 800 calcium phosphate nanoclusters, each of 60 kDa mass and radius 2.4 nm with an average spacing between nearest neighbours of 18 nm. Each nanocluster is surrounded by a sequestering shell of the phosphorylated regions of the caseins. Most phosphorylated residues are close together in the casein sequences forming what are known as phosphate centres. The term sequestered calcium phosphate is preferred to the older term colloidal calcium phosphate because the nanocluster is a thermodynamically stable complex of calcium phosphate with the sequestering casein phosphopeptides. Caseins are intrinsically disordered proteins (IDPs) with the ability to act as molecular chaperones (Bhattacharyya and Das 1999; Yong and Foegeding 2010) and three of the four bovine caseins can form amyloid fibrils (Farrell et al. 2003; Thorn et al. 2005, 2008; Treweek et al. 2011). These important discoveries require further development of our ideas of casein micelle structure and function (Thorn et al. 2015).
Materials and methods
Milk samples
Predictions of the model are compared with experiment using the same individual milk sample compositions as were used in the Model 1 calculations (Holt 2004). These were derived from the work of White and Davies (White and Davies 1958). Citrate concentrations were corrected for an average systematic error (White and Davies 1963). These data do not include the composition of the casein, so average values derived from the composition of bulk milks were applied to each sample (Davies and Law 1977, 1980). Further comparisons were made using compositional data from milk samples from 18 individual cows, whose casein composition and salt partition were reported by (Bijl et al. 2014). The concentration of milk salts in the continuous phase was estimated by analysis of either an ultrafiltrate (Davies and White 1960; White and Davies 1958) or the supernatant formed by ultracentrifugation.
Average relative molecular masses of caseins
In the development of a thermodynamic description of the casein micelle, it is desirable to use units that reflect the number of molecules present in the structure rather the weight of components. This was implemented some time ago for the milk salts (Holt 1985) which are now commonly reported in molar units but for the proteins there are some difficulties that relate to genetic variants and the variable glycosylation and phosphorylation of individual caseins. The procedure for calculating the molar concentrations of individual caseins from their experimental weight concentrations is described in Online Resource Section 1.
Partition and compositional mole fractions
Casein mole fraction is used in two different ways, namely the partition mole fraction and the composition mole fraction. The partition mole fraction is the proportion of an individual casein that is either bound or free of any linkage to the nanoclusters of calcium phosphate. The composition mole fraction is the mole fraction of an individual casein in whole casein. These two senses are combined when the composition mole fractions are calculated for the free and bound states.
Theory of salt partitioning
In bovine milk, about two thirds of the total calcium, half the inorganic phosphate (Pi), one third of the magnesium and smaller proportions of citrate and the other small ions are bound to the casein micelles with the balance in the continuous phase (Davies and White 1960). An abundance of evidence indicates that there is a thermodynamic equilibrium between the salts in the micelle and those in the continuous phase [reviewed by (de Kruif and Holt 2003; Holt 1997)]. Various attempts have been made to calculate the partition of bovine milk salts without taking account of the intimate association of the non-diffusible fraction with the caseins (Holt et al. 1981; Lyster 1981; Mekmene et al. 2009). A difficulty with these calculations is the discrepancy between the chemical formula of calcium phosphate needed to explain the continuous phase composition and the chemical formula of the non-diffusible fraction. Thermodynamic equilibrium between the ions in the continuous phase and those in the casein micelle requires that the same chemical formula should apply to both.
Equilibrium model of salt partitioning in bovine milk (Model 1)
It was shown that the partitioning of milk salts can be precisely calculated from first principles, without any arbitrary assumptions or fitted parameters using equilibrium thermodynamics (Holt 2004; Lam et al. 2017; Little and Holt 2004). The assumption is that the micellar calcium phosphate of milk is essentially identical in composition and solubility to calcium phosphate nanoclusters prepared in the laboratory using short casein phosphopeptides (Clegg and Holt 2009; Cross et al. 2016; Holt 2004; Holt et al. 1996, 1998; Little and Holt 2004). In calcium phosphate nanoclusters, the casein phosphorylated residues are bound to the calcium phosphate and are part of its chemical formula. The same chemical formula for the inorganic constituents could then be applied to the micelle and continuous phase. This method of calculating the partition of milk salts is referred to here as Model 1 and the revisions and improvements described herein comprise Model 2.
In Model 1, phosphate centres are assumed to react equally and independently with the calcium phosphate nanoclusters. A casein molecule with several phosphate centres, thereby, forms a distribution of unbound, partially bound and fully bound states (Holt 2004; Little and Holt 2004). For example, a casein molecule with two phosphate centres can be free of any linkage to the calcium phosphate or can be bound through one or other of its phosphate centres or through both. The calculated salt partition from Model 1 provides precise agreement with experiment. Although no direct comparison with experimental data on casein partition was made, it was clear that the predicted concentrations of unbound caseins were smaller than typical estimates obtained by a urea solubilisation method (Aoki et al. 1986, 1987). Also, a small number of the milk samples, mainly from cows in late lactation or with mastitis, were predicted to be thermodynamically unstable, thus violating the starting assumption.
Improved equilibrium model of salt partition (Model 2)
In the improved calculation described here (Model 2), all single and grouped phosphorylated residues on a given casein molecule react with a calcium phosphate nanocluster after an initial binding event, so that individual casein molecules are either fully bound through all their phosphorylated residues or not bound at all to the calcium phosphate nanocluster. Thus, individual phosphate centres within a casein molecule react together, co-operatively, rather than in the independent way envisaged in Model 1. Because there are no intermediate bound states, a higher proportion of casein is free compared with Model 1, and very few milk samples are found to be thermodynamically unstable.
A further assumption of model 2 is that competent sequestering caseins must contain at least one strong divalent cation binding site occupied by a divalent cation which is essential for the initial binding event. Strong cation binding sites are formed by phosphate centres. In Model 1, all phosphate centres were assumed to be equal. The equivalent assumption in Model 2 is that the strong binding sites are equally reactive. Caseins that contain no strong binding site, such as bovine κ-casein, or caseins having all their strong binding sites occupied by hydronium ions (H+), cannot bind to or sequester the calcium phosphate. This property of both Models 1 and 2 is in agreement with experiments suggesting that none of the κ-casein is bound to the micellar calcium phosphate (Aoki et al. 1986, 1987).
Ion binding by the caseins in Model 2 is integrated more fully into the calculation of the ion equilibria among the salts than in Model 1. It is assumed in the model that the most important equilibria involving the caseins are the binding of divalent cations and the hydronium ion. Binding of monovalent ions to the acidic and basic residues is likely to be weak. Also neglected, because of a lack of literature data, are salt bridges between casein sequences which could be strong even for IDPs in an amorphous structure such as the casein micelle (Borgia et al. 2018). For each individual casein, the proportion bound to the calcium phosphate nanoclusters, its charge in the bound state, the proportion free of such a linkage and its charge in the free state are all calculated.
The final improvement of Model 2 over Model 1 is that the other modifications make it possible to calculate the stability of milk as a function of pH to identify, among other things, whether there is a pH above which there is insufficient casein to ensure thermodynamic stability.
Binding of divalent cations to caseins in Model 2
Ion binding by globular proteins of known structure can be calculated from the electrical field at the binding site or from a mean field solution to the Poisson–Boltzmann distribution (Froloff et al. 1997). In globular proteins, the fold generates the binding site, usually from several residues, some of which may be from remote positions in the sequence. However, for intrinsically disordered proteins like caseins, having an ensemble of interchangeable conformations, in an electrolyte solution with a Debye–Hückel shielding length of less than 1 nm, only nearest neighbour interactions need to be considered. Except for the most highly phosphorylated sequences, we treat the ion binding properties of caseins as a sum of the binding properties of individual residues (Mekmene and Gaucheron 2011). In previous work (Little and Holt 2004), the calcium ion binding isotherm of the tryptic phosphopeptide of β-casein, residues 1–25 of the mature sequence including a cluster of 4 phosphorylated residues, was measured at room temperature as a function of pH in the range 5–8. No physico-chemical model of binding was developed; instead polynomial representations of the isotherms were used to interpolate the binding at any given pH and free calcium ion concentration. Here, an explicit physico-chemical model of the competitive binding of divalent cations and the hydronium ion to casein sequences is developed. There is insufficient information on the binding of magnesium ions to caseins to enable a detailed model to be built comparable to the one for calcium ions. Instead it is assumed that the binding constants for magnesium ions are the same as for calcium ions. The binding of hydronium ions to Lys, Arg, His and the N-terminus was also included in the model to allow the net charge to be calculated over the pH range 2–8. Arg and Lys are fully charged in this pH range, the pKH of His was put equal to 6.1 and the pKH of the N-terminus was put equal to 8.0. The model does not include complexes formed by caseins with monovalent ions as these are likely to be small. Nor does the model include any other types of interaction by charged residues such as bridging between side chains. Such interactions among IDPs can be very strong with dissociation constants in the pM range (Borgia et al. 2018), but no comparable strength of association has been reported for caseins.
The effect of binding at one residue on the affinity of nearest neighbours was modelled only for the case of three consecutive phosphorylated seryl residues (-SerP-SerP-SerP-). The need for an interactive model was demonstrated some years ago by the measured calcium binding isotherms of β- or αS1-caseins, which showed that the first calcium ion was bound more strongly than any subsequent one (Dalgleish and Parker 1980; Parker and Dalgleish 1981). Likewise, when the pH titration of the phosphate cluster in a cyanogen bromide fragment of αS1-casein was measured by 31P-nmr spectroscopy, it exhibited the collective pKH of 6.65 whereas the single isolated phosphoseryl residue titrated at pKH = 5.95 (Sleigh et al. 1983b). To model the cluster of 3 SerP residues in a typical bovine casein phosphate centre, it was assumed that the cluster exhibits higher affinity for the first hydronium or metal ion but that a second or subsequent ion was bound with the same affinity as an isolated SerP residue. Likewise, two adjacent phosphorylated residues and single phosphorylated residues adjacent to Glu or Asp were treated as isolated binding sites. These assumptions reduced the number of fitting parameters to a practical level so that a unique fit could be obtained. Equilibrium constants were obtained by fitting this model to the experimental binding isotherms shown in (Fig. 1a).
a Divalent metal ion binding to β-casein (4P) 1–25 at ionic strength I = 0.08 in the pH range 5–8 fitted to an ion pair model in which a high affinity binding site in the phosphate cluster can bind either H+ or M2+. Other sites of lower affinity are derived from the remaining SerP residues, 7 Glu residues and the C-terminus. b Contours of the net charge on whole casein as a function of the free divalent cation concentration and pH
In general, the phosphorylated residues dominate the sites of binding to αS1-, αS2- and β-caseins above pH 5 but the reverse is true at pH 5 or below and for κ-casein the carboxyl group binding is dominant at all pH values. The most important ion species, the acid dissociation constants and the divalent metal ion association constants are given in Table 1.
The equilibrium constants in Table 1 are practical constants applicable to the conditions in the solution, most notably its temperature and ionic strength. Following convention, KH is an acid dissociation constant whereas KM is an association constant for a 1:1 complex of the divalent metal ion with a ligand.
In the following, a subscript italicised lower-case s denotes a strong binding site and italicised lower-case w denotes a weaker binding site. Let NSerP, s be the total number of moles of strong binding sites per mole of casein. In the ion equilibria model, the total concentration is expressed as a sum of the important species.
Terms in square brackets are molar concentrations. Similar balance equations can be written for each of the other types of binding sites formed by charged residues in casein.
Application of the model to casein requires an enumeration of the strong and weak phosphorylated binding sites. The number of strong sites is zero in κ-casein, one in β-casein, two in αS1-casein and two in the 10- and 11-P phosphoforms of αS2-casein. Although it is in a potential kinase recognition sequence, Thr-130 in αS2-casein is not even partially phosphorylated (Fang et al. 2017). If it were phosphorylated, then another strong binding site would be created. In the more highly phosphorylated forms, it is therefore assumed that Thr-130 is not phosphorylated so that no additional strong binding site is created in αS2-casein. For the purpose of the calculation, the molar proportions of the phosphoforms of the αS-caseins were taken from Table 1 of (Fang et al. 2017) for Montbéliarde cattle. The effect of competitive binding of hydronium and divalent metal ions on the net charge of a casein mixture is shown in Fig. 1b. Contour lines in the figure correspond to net charge per mole of whole casein. The individual caseins in the mixture were in the molar proportions κ-:β-:αS1-:αS2-casein of 0.156:0.372:0.370:0.103 and correspond to the standard bulk milk composition used throughout this work (Davies and Law 1977, 1980). The model correctly predicts the isoelectric point of whole casein to be at about pH 4.6, depending weakly on the free divalent cation concentration.
Calculation of the salt partition and binding of phosphorylated residues to the calcium phosphate nanoclusters in Model 2
This aspect of Model 2 follows closely the procedure used in Model 1 by taking the same solubility constant for the calcium phosphate (KS) and the same composition figures for the nanocluster complexes (Holt 2004; Little and Holt 2004) as determined by independent experiments on nanoclusters formed by a single type of short phosphopeptide. An initial value is assumed for the fraction of strong binging sites (α) that have reacted with the calcium phosphate. This is sufficient to calculate the concentration of the nanoclusters and the fractions of casein residues involved in sequestering them. The remaining salts and casein residues then form an equilibrium distribution of free ions and complexes allowing the ion activity product (IAP) for the calcium phosphate to be calculated (Holt et al. 1981). The concentration of the nanoclusters is increased if IAP/KS > 1 or decreased if IAP/KS < 1 and the cycle repeated until equilibrium is reached (IAP/KS = 1). A further condition of equilibrium is that α ≤ 1. Thus, if \([{\text{MSerP}}_{\text{s}} ] < 0\), the calculation is stopped while the milk remains in a metastable state, liable to precipitate calcium phosphate, because all the competent caseins have reacted to form nanocluster complexes.
The salt partition calculation gives the concentrations of ions bound to the caseins and the diffusible ion concentrations in the continuous phase. To compare the continuous phase concentrations with those measured in a centrifugal supernatant, ultrafiltrate or equilibrium diffusate, allowance must be made for differences between the ion activity coefficients arising from the excluded volume of co-solutes and a Donnan correction made arising from the net charge of the non-diffusible components, as described previously (Holt 1997).
Calculation of the net charge on bound and free caseins in Model 2
The net charge on a free casein is readily calculated from the ion equilibrium model without any further assumptions. For the bound casein, it is necessary to specify which residues are incorporated in the nanocluster complexes. The calcium phosphate nanoclusters formed by β-casein (4P) 1–25 have been intensively studied to give their composition, structure and size. NMR spectra, measured composition, charge balance and physical proximity are consistent with the inclusion of the carboxyl groups from Glu residues adjacent to the phosphate centre (Cross et al. 2016; de Kruif and Holt 2003) as part of the surface structure of the nanocluster complex. Calculations of free and bound carboxyl groups were made for the phosphoforms of all the bovine caseins and the glycoforms of the A and B genetic variants of κ-casein. Table 2 reveals that approximately one carboxyl group is involved in sequestration of the nanoclusters for every casein phosphate moiety, e.g. β-CN with 5 phosphate groups contributes 5 out of its total of 24 carboxyl groups to the sequestration reaction (Table 2).
Calculation of the stability diagram of milk in Model 2
The stabilisation of milk against precipitation of calcium phosphate through the sequestration of nanoclusters of amorphous calcium phosphate has been presented as a particular example of a general physiological mechanism for the stabilisation of biofluids (Holt et al. 2014) and the theory and experimental evidence to support this idea has been broadened to include sequestration by other phosphoproteins (Clegg and Holt 2009; Holt et al. 2009; Jahnen-Dechent et al. 2011). Osteopontin is of particular significance in this context because of its very wide distribution among species, tissues and biofluids (Mazzali et al. 2002). In developing the theory of calcium phosphate sequestration by phosphoproteins using Model 1, the idea of a stability diagram was introduced. In its original form, the stability diagram displays the minimum concentration of phosphate centres needed to ensure thermodynamic stability in a biofluid at a given pH. In milk, because its nutritional function requires a high concentration of sequestered calcium phosphate, the minimum concentrations of sequestering caseins are normally in the mM range but in other biofluids such as blood, the concentrations needed are about three orders of magnitude smaller (Holt et al. 2014). The condition of stability in Model 2 is that when IAP/KS = 1 we require [MSerPs] ≥ 0 for thermodynamic stability and the minimum sequestering phosphoprotein concentration is at [MSerPs]min = 0. The stability curve is characterised by a critical pH, pHc, above which the solution requires a finite concentration of competent sequestering agent for it to be stable and it exhibits a limiting plateau concentration of sequestrant, [SerPs]max at high pH.
A simple analytical expression to describe the stability diagrams is desirable. The stability diagram of milk and some other biofluids (Holt et al. 2014) is similar in shape to a binding isotherm and can be fitted nicely by a type of Hill equation (Hill 1910) using a non-linear least squares method. The equation can be written as:
where the dependent variable on the ordinate is \([ {\text{SerP}}_{\text{s}} ]_{\rm{min} }\) and the independent variable on the abscissa is pH. The fitted parameter pH1/2 is the pH value at which \([ {\text{SerP}}_{\text{s}} ]_{\rm{min} }\) = \([ {\text{SerP}}_{\text{s}} ]_{\rm{max} } /2\) and n is a fitted shape parameter. In the original Hill equation, n described the co-operativity of binding oxygen molecules to molecules of haemoglobin but it does not have a simple physical significance here. An analogous expression to Eq. (2) can be written for the minimum casein concentration, [Cas]min, since
In Model 1, an expression was derived for the thermodynamic stability of milk in terms of the concentration of phosphate centres (Holt 2004). In model 2, we define a stability ratio in terms of the actual milk concentration of casein, [Cas], or strong binding sites, [SerPs],
such that milk is stable over the complete pH range if \(R_{\text{stab}} \ge 1\).
Stability diagrams for a reference bulk milk are shown in Fig. 2. To the right of the thermodynamic stability curve, in the region identified as metastable or unstable, lies the spinodal boundary. This marks the practical limit of metastability for a single-phase system. Experiments to determine the position of the spinodal in a milk-like casein-calcium phosphate system have not been reported, but the approximate position of the spinodal in an artificial blood system using an osteopontin phosphopeptide mixture as the sequestrant has been described (Holt et al. 2014).
Stability diagram of the standard reference milk showing the zones of stability and meta- or instability with respect to calcium phosphate and the parameters used to fit Eq. (2). The minimum concentration of strong divalent cation binding sites required to achieve thermodynamic stability with respect to the precipitation of calcium phosphate is the right-hand axis and the left-hand axis gives the corresponding minimum concentration of casein. Also shown is the position of the natural milk
Results
Comparisons of Model 1 and Model 2 with experiment for a reference bulk skim milk
The milk sample chosen for this illustrative calculation is a combination of the salt analysis on the bulk milk sample of (White and Davies 1958) combined with the average casein composition of the bulk milk samples of (Davies and Law 1977, 1980). Results of the calculation using Models 1 and 2 are compared with experimental ultrafiltrate concentrations of the principal salts in Table 3.
The Model 2 calculation for this milk is that 72% of the competent caseins are bound to the calcium phosphate nanoclusters, whereas Model 1 required 83% of the casein and 91% of the phosphate centres to be bound (Holt 2004).
The results for the casein composition of the free and bound states are shown in the columns of Table 4 for Models 1 and 2. For example, the bound fraction according to Model 2 has 44% β-, 44% αS1- and 12% αS1-casein but contains no κ-casein. In Model 2, the free fraction is enriched, as expected, in κ-casein but all the other caseins are represented until α reaches 1 where only κ-casein remains free. The main difference between Model 1 and Model 2 is the higher concentrations in the latter of free αS1- and αS2-caseins.
The net charge on the individual caseins when either sequestering calcium phosphate or free is also shown in Table 4. While most caseins retain a net negative charge in the milk, the effect of calcium phosphate sequestration by αS2-casein is to give the casein a large net positive charge, mainly because of its high content of Lys residues. Whereas the net charge of the bound fraction is small and positive, the free fraction caries a large negative charge.
Figure 2 shows the stability diagram for the reference bulk milk, calculated according to Model 2. For the standard reference bulk milk, the fitted values and standard errors according to Eq. (2) are [Casein]max = 26.65 g L−1 (± 0.92, P < 0.0004), [MSerPs]max = 1.73 mM (± 0.06, P < 0.0004), pHc = 5.65 (± 0.01, P < 0.0001), n = 1.28 (± 0.11, P < 0.0013) and pH1/2 = 6.19 (± 0.03, P < 0.0004). The maximum concentrations are below the actual concentrations, so this milk is stable to pH adjustment at ambient temperature through the entire pH range.
Calculated partition of salts in the milk of individual cows
(White and Davies 1958) also gave the detailed chemical composition of milk from more than 100 individual cows. Although they gave the total casein concentration in each milk sample, the composition of the caseins was unknown. In the calculations of Model 1, the average casein composition of the reference bulk milk sample was used for each sample even though considerable variation in casein composition occurs in individual cows (Bijl et al. 2013; Fang et al. 2017; Heck et al. 2009; Ketto et al. 2017). The agreement between experiment and calculation is generally satisfactory (Fig. 3) but least so for calcium, where a systematic overestimation of ultrafiltrate concentration was found (Table 5). Similar results were previously reported for Model 1(Holt 2004). No attempt has been made to adjust the parameters of Model 2 to the experimental data for calcium because it is not clear what causes the overestimation.
The stability of the individual milks was examined and only two samples were found to be marginally metastable, one in mid-lactation and the other in late lactation, which contrasts with the findings from Model 1 where nearly half the samples of milk from cows in late lactation and mastitic milks were predicted to be unstable. The explanation for this difference is that in Model 2 more of the casein phosphate groups combine with the calcium phosphate than in Model 1 and, therefore, Model 2 predicts more spare capacity for sequestration than Model 1.
The distribution of α values calculated from Model 2 for the individual milk samples from early, middle and late lactation and for cows with sub-clinical mastitis (White and Davies 1958) is shown in Fig. 4a. For the most part, cows in late lactation and with sub-clinical mastitis yield higher α values than cows in early lactation or, to a slightly less extent, middle lactation. This is also the pattern seen in milk pH which might explain the variation in physico-chemical terms. For comparison, the distribution of α values calculated by applying Model 1 to the same samples is shown in Fig. 4b. Model 2 calculates that 98% of the milk samples are in the thermodynamically stable region having 0 ≤ α ≤ 1, with the remaining 2% in the range 1 < α ≤ 1.05. Two thirds of the milk samples were in the range 0.75 ≤ α ≤ 0.9. Model 1 calculations were that nearly 23% of the milk samples were in the region of meta- or instability (α > 1).
Model calculations on the extent of reaction of caseins with calcium phosphate. a Stacked histogram of the frequency of α values from Model 2 found in milks from cows in early, middle and late lactation and of milk from cows with sub-clinical mastitis. b The same milk samples as in (a) but calculated from Model 1
Salt and casein partition calculations on 18 individual milk samples of known casein composition
A comparison has been made between the experimental salt partition in 18 milk samples from individual cows reported by Bijl et al. (2014) and the calculated partition of milk salts from Model 2. After converting the casein concentrations to molar units (Online Resource Section 2), it was confirmed that the important observation of a negative correlation between the average size and the glycosylated κ-casein concentration was preserved. Two of the samples (JL01 and EH01) failed to meet the conditions required for thermodynamic equilibrium. Sample JL01 had higher than normal total concentrations of calcium and Pi whereas samples JL01 and EH01 had lower than average concentrations of strong divalent metal ion binding sites. The results from the remaining 16 samples are summarised in Fig. 5 for calcium, magnesium, Pi and citrate concentrations in the continuous phase. As was found with the comparisons made by either Model 1 or Model 2 using the data of White and Davies (1958) summarised in Fig. 3 and Table 5, the agreement between calculation and experiment is generally satisfactory although calculated calcium concentrations in the continuous phase are again overestimated by about 2 mM.
Comparison of experimental supernatant and calculated ultrafiltrate concentrations of calcium, magnesium, Pi and citrate in 16 milk samples from individual cows (Bijl et al. 2014)
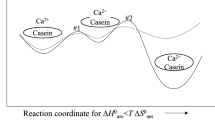
Model 2 allows the partition of individual caseins between bound and free states to be calculated and the net charge in each state to be determined. In Table 6, the results for the 16 samples are summarised. Partition data in Table 6 are in units of μmole casein per litre of defatted milk and the average casein charge is the net number of charged groups per molecule. A negative sign signifies an excess of negative charges and positive numbers can arise because of charge neutralisation of negative groups on the caseins because of the binding of divalent cations (M2+) and the involvement of negatively charged residues in calcium phosphate sequestration. In Table 7, the calculations are made for the partition of the individual casein. The κ-casein is entirely in the free state and has a net negative charge of about − 3.6 at milk pH. In the bound state, β- and αS1-caseins have a small net negative charge but the αS2-casein has a large and positive net charge which, despite its low concentration, effectively neutralises the other two bound caseins.
Also shown in Table 6 are values for the average number of moles of divalent cations bound to casein but not part of the complexes with calcium phosphate, expressed per mole of casein (MCas) and the average number of moles of divalent cations bound to the strong calcium binding sites in whole casein (MSerPs). Strong calcium binding sites in Model 2 are available only in the free β-, αS1- and αS2-casein fractions. As shown in Table 6, the average net charge in the bound state is positive but close to zero. Even such a small charge could lead to a large internal electrical potential but in a porous colloid such as the casein micelle, the Donnan effect of the potential would draw in ions of opposite charge to keep the internal domain at or very close to zero net charge. The free casein carries a larger net negative charge than the bound caseins, particularly the αS1-casein (Table 7) resulting in an average of about − 7.8. Individual milks are quite variable, the net negative charge ranging from − 6.06 to − 9.10 (Table 6). In this group of milk samples, the pH range is limited (6.62–6.79) so the main factor influencing the average net charge of free and bound caseins is the divalent metal ion concentration, as shown in Fig. 6.
Model 2 calculations on the effect of free calcium and magnesium ion concentrations on the net charge on free or bound caseins in milk samples from16 individual cows (Bijl et al. 2014)
Model 2 was used to construct stability diagrams for all 18 milk samples. The salt concentrations and casein mole fractions were held constant, while the minimum concentration of sequestrant needed to ensure that the milk was stable was determined over the pH range 5.6–8.0. The stability curves of [Cas]min versus pH were quite variable due to the variability of the salt and total casein concentrations in the samples. The individual stability curves were closely fitted by Eq. (2) and the fitted parameters and standard errors are given in Table 8. All the fitted parameters were significant at the level P < 0.001. From the actual and plateau values of the concentration of strong binding sites, the stability ratios were calculated. These showed that half the samples were stable to alkaline pH adjustment (Rstab ≥ 1) and half became unstable, or metastable above a certain pH. Two of the samples (JL01 and EH01) were unstable at the natural pH of the milk; the maximum pH at which sample JL01 was calculated to be stable was 6.45 whereas it was 6.5 for sample EH01. The other 7 samples were found to be stable up to at least pH 7.4 (JL09, JL06, EH02, JL07, PM02, MV07 and MV08). A reasonable generalisation from these findings is that a milk with a casein concentration of at least 30 g L−1 is likely to be stable to alkaline pH adjustment.
Discussion
Model 2 incorporates a new and more general way of describing the interaction of calcium ions with caseins. It consolidates earlier evidence from measured binding isotherms (Dalgleish and Parker 1980; Parker and Dalgleish 1981) and 1H- and 31P-NMR spectroscopy (Baumy et al. 1989; Sleigh et al. 1983a; Thomsen et al. 1995; Wahlgren et al. 1993) that there are strong hydronium and divalent cation binding sites in casein involving the phosphate centres. The subsequent affinity of either hydronium or divalent cations for the remaining residues in the phosphate centre is assumed in the model to be the same as that of single phosphorylated residues. These simplifications are sufficient to provide an excellent fit to experimental binding isotherms of calcium ions to a casein phosphopeptide containing a single phosphate centre over a wide range of pH. Further studies are needed on other single phosphopeptides to test our assumptions more widely.
Phosphate centres are also known to sequester the calcium phosphate nanoclusters. The evidence for this in bovine milk comes from a wide range of studies including the non-perturbative technique of 31P-NMR-spectroscopy applied to casein micelles and individual nanoclusters which shows that a substantial proportion of the phosphorylated residues in casein micelles are immobilised by sequestration (Belton et al. 1985; De Sa Peixoto et al. 2017; Gonzalez-Jordan et al. 2015; Holt et al. 1996). Identification of sequences that remain bound to the calcium phosphate after exhaustive proteolytic digestion (Gagnaire et al. 1996; Holt et al. 1986; Ono et al. 1994) or after disulphide reduction and treatment with 6-M urea (Aoki et al. 1986, 1990, 1992) confirm that a strong link can be formed by the most highly phosphorylated sequences in caseins to the calcium phosphate. In the urea treatment experiments, size fractionation reveals that the 6-M urea solution contains oligomeric material comprising caseins bound to calcium phosphate (fraction 1) and fraction 2, comprising a mixture of free caseins without any sequestered Pi. The urea fractionation technique was expertly deployed by Aoki and co-workers to follow the effects of variables on the relative sizes of fractions 1 and 2. The fraction 1 was reported to be 57.3% of the combined area of fractions 1 and 2, as measured by absorption at 280 nm and contained no κ-casein, less β-casein and more of the αS1- and αS2-caseins than whole casein (Aoki et al. 1986). These results for the bound fraction are in better agreement with Model 2 than with Model 1 but are lower than most of our findings. Working with various artificial micelle-forming systems it was shown that the relative size of fraction 1 increased with the calcium phosphate content and, at a given content, increased with the degree of phosphorylation of different caseins (Aoki et al. 1987, 1986, 1990, 1991, 1992). The effect of the number of phosphorylated residues on the strength of binding to calcium phosphate was also investigated using phosphoforms from human caseins (Aoki et al. 1992). No 1-P and 2-P phosphoforms of human casein and only a small proportion of the 3-P but a high proportion of 4-P and 5-P phosphoforms were found to be associated with the calcium phosphate after treatment with 6-M urea. Most humans secrete milk caseins that are mainly β-casein 0-5P phosphoforms with lower proportions of αS1-casein 0-8P phosphoforms and κ-casein (Froehlich et al. 2011; Liao et al. 2017; Poth et al. 2008). Positional isomers of the 3P form of human β-casein were not detected so it is likely that a strong divalent cation binding site is generated by the preferred sites of phosphorylation at residues S-23, S-24 and S-25 of the mature polypeptide chain. Given the existence of the strong divalent cation binding site in the 3-P phosphoform, and the weak binding of the 3-P phosphoform in the presence of the urea, it may be necessary to modify Model 2 to allow the affinity of binding to increase with the degree of phosphorylation.
The effect of cooling to 4 °C on native casein micelle size and structure has been studied (Creamer et al. 1977; Davies and Law 1983; Liu et al. 2013; Ono et al. 1990; Zhang et al. 2018). Although the whole micelle size distribution appears to be affected, the smallest size class remaining in the supernatant after ultracentrifugation shows a substantial increase in β-casein concentration but no corresponding rise in sequestered calcium or phosphate. The implication is that some or all of the free β-casein dissociates from the micelles into single molecules or oligomers. After 20 h of storage at 4 °C the supernatant β-casein increased to 31.8% of the total β-casein (Aoki et al. 1990). Creamer et al. (1977) reported a similar value of up to 26% within 24 h. These values are in better agreement with the calculations of Model 2 than with Model 1. A much higher figure of 44.7% after 40 h storage at 4 °C has also been reported (Davies and Law 1983).
In a controlled dissociation experiment where the concentration of colloidal Pi was held constant and the free calcium ion concentration was varied by dialysis, caseins dissociated from casein micelles to a variable extent (Holt et al. 1986). At the lowest free calcium ion concentration studied of 0.4 mM, 68% of casein remained in the ultracentrifugal pellet whereas the same centrifugation procedure pelleted 97% of casein at a free calcium ion concentration of 3 mM or more. The magnitude of dissociation by the individual caseins was αS2- < αS1 < β- reflecting their sensitivity to calcium ions but κ-casein also dissociated, presumably because it was bound to other dissociated caseins. This experiment supports the conclusions from the urea treatment and cold dissociation experiments and the predictions of Model 2 rather than Model 1 that a substantial fraction of caseins in the casein micelle are not involved in sequestering the nanoclusters of calcium phosphate.
It is unlikely that cooling to 4 °C would disrupt the linkage between casein and calcium phosphate so the fraction of β-casein released into the continuous phase provides a lower bound to the free β-casein content in these milks. However, both enzymic digestion and the urea treatment method destroy casein micelle structure completely. In experiments that alter the micellar structure, the extent of sequestration could be increased or decreased by the method of investigation. With this caveat in mind the results from the urea treatment experiments point to the conclusion that there is an equilibrium between caseins in the free and bound states and that an increase in the degree of phosphorylation favours the sequestration reaction. Nevertheless, experiments to measure the affinity of the sequestration reaction under closely controlled conditions using pure single phosphoforms by a non-perturbing method are highly desirable.
Model 2 incorporates a more sophisticated description of milk stability than Model 1in the form of a stability diagram. It provides a measure of stability, the stability ratio Rstab, which depends on pH, the concentrations of salts and casein. Metastable milks are not necessarily unstable since the metastable complexes of caseins and calcium phosphate may have a high degree of kinetic stability even though they are not in the lowest free energy state. Model 2 does not, at present, make any predictions about other types of instability resulting from, for example, heat or pressure treatment, renneting or the addition of non-solvents but the model does provide a good starting point for such calculations.
The stability of milk has much in common with the stability of other biofluids even though the concentrations of phosphoproteins and sequestered calcium phosphate in these other biofluids may be less than in milk by at least 3 orders of magnitude (Holt et al. 2014). Indeed, it has been argued that the remarkable thermodynamic properties of a biofluid containing sequestered calcium phosphate allow soft and mineralised tissues to co-exist in the same organism with relative ease (Holt 2013; Lenton et al. 2015a).
Conclusions
Models 1 and 2 provide broadly similar predictions of the salt partition in milk but Model 2 provides a better way of calculating the binding of divalent cations to caseins. Moreover, the method is capable of being adapted and applied to other types of phosphoproteins.
Model 2, when applied to 16 individual milks (Bijl et al. 2014) predicted that on average 67 ± 10% of caseins with a strong divalent cation binding site are in the bound state whereas Model 1 predicts that 80–90% of caseins with phosphate centres are bound . Thus, Model 2 provides better agreement with experiment than Model 1. It is assumed in Model 2 that all caseins are equally distributed between the free and bound states, apart from κ-casein which is assumed to be entirely free. This assumption can be readily replaced by separate figures for individual caseins once reliable data become available. Clearly, this area of work is important for our understanding of casein micelle structure and there is a need for less perturbative, preferably quantitative, methods that generate thermodynamic information on the strength of the nanocluster complexes formed by calcium phosphate sequestration.
References
Aoki T, Kako Y, Imamura T (1986) Separation of casein aggregates cross-linked by colloidal calcium phosphate from bovine casein micelles by high-performance gel chromatography in the presence of urea. J Dairy Res 53:53–59
Aoki T, Yamada N, Tomita I, Kako Y, Imamura T (1987) Caseins are cross-linked through their ester phosphate groups by colloidal calcium-phosphate. Biochem Biophys Acta 911:238–243
Aoki T, Yamada N, Kako Y (1990) Relation between the colloidal calcium-phosphate cross-linkage and release of beta casein from bovine casein micelles on cooling. Agric Biol Chem 54:2287–2292
Aoki T, Sakamoto H, Kako Y (1991) Cross-linking of caseins by colloidal calcium phosphate in the presence of urea. Int Dairy J 1:67–75
Aoki T, Umeda T, Kako Y (1992) The least number of phosphate groups for cross-linking of casein by colloidal calcium phosphate. J Dairy Sci 75:971–975
Baumy JJ, Guenot P, Sinbandhit S, Brulé G (1989) Study of calcium binding to phosphoserine residues of β-casein and its phosphopeptide (1–25) by 31P NMR. J Dairy Res 56:403–409
Belton PS, Lyster RLJ, Richards CP (1985) The P-31 nuclear magnetic resonance spectrum of cows milk. J Dairy Res 52:47–54
Bhattacharyya J, Das KP (1999) Molecular chaperone-like properties of an unfolded protein, alpha(s)-casein. J Biol Chem 274:15505–15509
Bijl E, van Valenberg HJF, Huppertz T, van Hooijdonk ACM (2013) Protein, casein, and micellar salts in milk: current content and historical perspectives. J Dairy Sci 96:5455–5464
Bijl E, de Vries R, van Valenberg H, Huppertz T, Van Hooijdonk T (2014) Factors influencing casein micelle size in milk of individual cows: genetic variants and glycosylation of kappa-casein. Int Dairy J 34:135–141
Borgia A, Borgia MB, Bugge K, Kissling VM, Heidarsson PO, Fernandes CB, Sottini A, Soranno A, Buholzer KJ, Nettels D, Kragelund BB, Best RB, Schuler B (2018) Extreme disorder in an ultrahigh-affinity protein complex. Nature 555:61
Clegg RA, Holt C (2009) An E. coli over-expression system for multiply-phosphorylated proteins and its use in a study of calcium phosphate sequestration by novel recombinant phosphopeptides. Protein Expr Purif 67:23–34
Creamer LK, Berry GP, Mills OE (1977) A study of the dissociation of beta -casein from the bovine casein micelle at low temperature. N Z J Dairy Sci Technol 12:58–66
Cross KJ, Huq NL, Reynolds EC (2016) Casein phosphopeptide-amorphous calcium phosphate nanocomplexes: a structural model. Biochemistry 55:4316–4325
Dalgleish DG (2011) On the structural models of bovine casein micelles-review and possible improvements. Soft Matter 7:2265–2272
Dalgleish DG, Parker TG (1980) Binding of calcium ions to bovine alpha-S1-casein and precipitability of the protein-calcium ion complexes. J Dairy Res 47:113–122
Davies DT, Law AJR (1977) Improved method for quantitative fractionation of casein mixtures using ion-exchange chromatography. J Dairy Res 44:213–221
Davies DT, Law AJR (1980) Content and composition of protein in creamery milks in southwest scotland. J Dairy Res 47:83–90
Davies DT, Law AJR (1983) Variation in the protein-composition of bovine casein micelles and serum casein in relation to micellar size and milk temperature. J Dairy Res 50:67–75
Davies DT, White JCD (1960) The use of ultrafiltration and dialysis in isolating the aqueous phase of milk and in determining the partition of milk constituents between the aqueous and disperse phases. J Dairy Res 27:171–190
de Kruif CG (2014) The structure of casein micelles: a review of small-angle scattering data. J Appl Crystallogr 47:1479–1489
de Kruif CG, Holt C (2003) Casein micelle structure, functions and interactions. In: Fox PF, McSweeney PLH (eds) Advanced dairy chemistry, vol 1A. Proteins. Kluwer Academic/Plenum, New York, pp 675–698
De Sa Peixoto P, Silva JVC, Laurent GP, Schmutz M, Thomas D, Bouchoux A, Gesan-Guiziou G (2017) How high concentrations of proteins stabilize the amorphous state of calcium orthophosphate: a solid-state NMR study of the casein case. Langmuir
Fang ZH, Bovenhuis H, Delacroix-Buchet A, Miranda G, Boichard D, Visker MHPW, Martin P (2017) Genetic and nongenetic factors contributing to differences in alpha-S-casein phosphorylation isoforms and other major milk proteins. J Dairy Sci 100(7):5564–5577
Farrell H Jr, Cooke P, Wickham E, Piotrowski E, Hoagland P (2003) Environmental influences on bovine κ-casein: reduction and conversion to fibrillar (amyloid) structures. J Protein Chem 22:259–273
Froehlich JW, Chu CS, Tang N, Waddell K, Grimm R, Lebrilla CB (2011) Label-free liquid chromatography-tandem mass spectrometry analysis with automated phosphopeptide enrichment reveals dynamic human milk protein phosphorylation during lactation. Anal Biochem 408:136–146
Froloff N, Windemuth A, Honig B (1997) On the calculation of binding free energies using continuum methods: application to MHC class I protein-peptide interactions. Protein Sci 6:1293–1301
Gagnaire V, Pierre A, Molle D, Léonil J (1996) Phosphopeptides interacting with colloidal calcium phosphate isolated by tryptic hydrolysis of bovine casein micelles. J Dairy Res 63:405–422
Gebauer D, Coelfen H (2011) Prenucleation clusters and non-classical nucleation. Nano Today 6:564–584
Gonzalez-Jordan A, Thomar P, Nicolai T, Dittmer J (2015) The effect of pH on the structure and phosphate mobility of casein micelles in aqueous solution. Food Hydrocolloids 51:88–94
Gower LB (2008) Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem Rev 108:4551–4627
Heck JML, Schennink A, van Valenberg HJF, Bovenhuis H, Visker M, van Arendonk JAM, van Hooijdonk ACM (2009) Effects of milk protein variants on the protein composition of bovine milk. J Dairy Sci 92:1192–1202
Hill AV (1910) Proceedings of the physiological society: January 22, 1910. J Physiol 40:i–vii
Holt C (1985) The milk salts: their secretion, concentrations and physical chemistry. In: Fox PF (ed) Developments in dairy chemistry: lactose and minor constituents, vol 3. Elsevier, London, pp 143–181
Holt C (1997) The milk salts and their interaction with casein. In: Fox PF (ed) Advanced dairy chemistry, vol 3. Lactose salts and vitamins. Water, Chapman and Hall, London, pp 233–254
Holt C (2004) An equilibrium thermodynamic model of the sequestration of calcium phosphate by casein micelles and its application to the calculation of the partition of salts in milk. Eur Biophys J Biophys Lett 33:421–434
Holt C (2013) Unfolded phosphopolypeptides enable soft and hard tissues to coexist in the same organism with relative ease. Curr Opin Struct Biol 23:420–425
Holt C, Dalgleish DG, Jenness R (1981) Inorganic constituents of milk.2. Calculation of the ion equilibria in milk diffusate and comparison with experiment. Anal Biochem 113:154–163
Holt C, Davies DT, Law AJR (1986) Effects of colloidal calcium phosphate content and free calcium ion concentration in the milk serum on the dissociation of bovine casein micelles. J Dairy Res 53:557–572
Holt C, Wahlgren NM, Drakenberg T (1996) Ability of a beta-casein phosphopeptide to modulate the precipitation of calcium phosphate by forming amorphous dicalcium phosphate nanoclusters. Biochem J 314:1035–1039
Holt C, Timmins PA, Errington N, Leaver J (1998) A core-shell model of calcium phosphate nanoclusters stabilized by beta-casein phosphopeptides, derived from sedimentation equilibrium and small-angle X-ray and neutron-scattering measurements. Eur J Biochem 252:73–78
Holt C, de Kruif CG, Tuinier R, Timmins PA (2003) Substructure of bovine casein micelles by small-angle X-ray and neutron scattering. Colloids Surf A 213:275–284
Holt C, Sorensen ES, Clegg RA (2009) Role of calcium phosphate nanoclusters in the control of calcification. FEBS J 276:2308–2323
Holt C, Lenton S, Nylander T, Sorensen ES, Teixeira SCM (2014) Mineralisation of soft and hard tissues and the stability of biofluids. J Struct Biol 185:383–396
Horne DS (1998) Casein interactions: casting light on the black boxes, the structure in dairy products. Int Dairy J 8:171–177
Huppertz T, Gazi I, Luyten H, Nieuwenhuijse H, Alting A, Schokker E (2017) Hydration of casein micelles and caseinates: implications for casein micelle structure. Int Dairy J 74:1–11
Ibsen CJS, Gebauer D, Birkedal H (2016) Osteopontin stabilizes metastable states prior to nucleation during apatite formation. Chem Mater 28(23):8550–8555
Ingham B, Erlangga GD, Smialowska A, Kirby NM, Wang C, Matia-Merino L, Haverkamp RG, Carr AJ (2015) Solving the mystery of the internal structure of casein micelles. Soft Matter 11:2723–2725
Jahnen-Dechent W, Heiss A, Schaefer C, Ketteler M (2011) Fetuin-A regulation of calcified matrix metabolism. Circ Res 108:1494–1509
Ketto IA, Knutsen TM, Øyaas J, Heringstad B, Ådnøy T, Devold TG, Skeie SB (2017) Effects of milk protein polymorphism and composition, casein micelle size and salt distribution on the milk coagulation properties in Norwegian Red cattle. Int Dairy J 70:55–64
Lam E, Holt C, Edwards P, McKinnon I, Otter D, Li N, Hemar Y (2017) The effect of transglutaminase treatment on the physico-chemical properties of skim milk with added ethylenediaminetetraacetic acid. Food Hydrocolloids 69:329–340
Lenton S, Nylander T, Teixeira SCM, Holt C (2015a) A review of the biology of calcium phosphate sequestration with special reference to milk. Dairy Sci Technol 95:3–14
Lenton S, Seydel T, Nylander T, Holt C, Hartlein M, Teixeira S, Zaccai G (2015b) Dynamic footprint of sequestration in the molecular fluctuations of osteopontin. J R Soc Interface 12(110):20150506
Lenton S, Nylander T, Holt C, Sawyer L, Härtlein M, Müller H, Teixeira SCM (2016) Structural studies of hydrated samples of amorphous calcium phosphate and phosphoprotein nanoclusters. Eur Biophys J Biophys Lett 45:405–412
Liao Y, Weber D, Xu W, Durbin-Johnson BP, Phinney BS, Lonnerdal B (2017) Absolute quantification of human milk caseins and the whey/casein ratio during the first year of lactation. J Proteome Res 16:4113–4121
Little EM, Holt C (2004) An equilibrium thermodynamic model of the sequestration of calcium phosphate by casein phosphopeptides. Eur Biophys J Biophys Lett 33:435–447
Liu DZ, Weeks MG, Dunstan DE, Martin GJO (2013) Temperature-dependent dynamics of bovine casein micelles in the range 10–40 degrees C. Food Chem 141:4081–4086
Lyster RLJ (1981) Calculation by computer of individual concentrations in a simulated milk salt solution.2. An extension to the previous model. J Dairy Res 48:85–89
Marchin S, Putaux J-L, Pignon F, Léonil J (2007) Effects of the environmental factors on the casein micelle structure studied by cryo transmission electron microscopy and small-angle X-ray scattering/ultrasmall-angle X-ray scattering. J Chem Phys 126:045101
Mazzali M, Kipari T, Ophascharoensuk V, Wesson JA, Johnson R, Hughes J (2002) Osteopontin—a molecule for all seasons. QJM 95:3–13
McMahon DJ, McManus WR (1998) Rethinking casein micelle structure using electron microscopy. J Dairy Sci 81:2985–2993
McMahon DJ, Oommen BS (2012) Casein micelle structure, functions and interactions. In: Fox PF, McSweeney PLH (eds) Advanced dairy chemistry, vol 1A. Proteins: basic aspects. Springer, New York, pp 185–210
Mekmene O, Gaucheron F (2011) Determination of calcium-binding constants of caseins, phosphoserine, citrate and pyrophosphate: a modelling approach using free calcium measurement. Food Chem 127:676–682
Mekmene O, Le Graet Y, Gaucheron F (2009) A model for predicting salt equilibria in milk and mineral-enriched milks. Food Chem 116:233–239
Ono T, Murayama T, Kaketa S, Odagiri S (1990) Changes in the protein-composition and size distribution of bovine casein micelles induced by cooling. Agric Biol Chem 54:1385–1392
Ono T, Ohotawa T, Takagi Y (1994) Complexes of casein phosphopetides and calcium phosphate prepared from casein micelles by tryptic digestion. Biosci Biotechnol Biochem 58:1376–1380
Parker TG, Dalgleish DG (1981) Binding of calcium ions to bovine beta-casein. J Dairy Res 48:71–76
Poth AG, Deeth HC, Alewood PF, Holland JW (2008) Analysis of the human casein phosphoproteome by 2-D electrophoresis and MALDI-TOF/TOF MS reveals new phosphoforms. J Proteome Res 7:5017–5027
Pouget EM, Bomans PHH, Goos JACM, Frederik PM, de With G, Sommerdijk NAJM (2009) The initial stages of template-controlled CaCO3 formation revealed by cryo-TEM. Science 323:1455–1458
Rollema HS (1992) Casein association and micelle formation. In: Fox PF (ed) Advanced dairy chemistry, vol 1. Elsevier Science Publishers, Barking, pp 111–140
Schmidt DG (1982) Association of caseins and casein micelle structure. In: Fox PF (ed) Developments in dairy chemistry, vol 1. Elsevier, London, pp 61–86
Sleigh RW, Mackinlay AG, Pope JM (1983) NMR-studies of the phosphoserine regions of bovine alpha-S1-casein and beta-casein—assignment of P-31 resonances to specific phosphoserines and cation binding studied by measurement of enhancement of H-1 relaxation rate. Biochem Biophys Acta 742:175–183
Thomsen JK, Jakobsen HJ, Nielsen NC, Petersen TE, Rasmussen LK (1995) Solid-state magic-angle spinning 31P-NMR studies of native casein micelles. Eur J Biochem 230:454–459
Thorn DC, Meehan S, Sunde M, Rekas A, Gras SL, MacPhee CE, Dobson CM, Wilson MR, Carver JA (2005) Amyloid fibril formation by bovine milk kappa-casein and its inhibition by the molecular chaperones alpha(s-) and beta-casein. Biochemistry 44:17027–17036
Thorn DC, Ecroyd H, Sunde M, Poon S, Carver JA (2008) Amyloid fibril formation by bovine milk alpha(s2)-casein occurs under physiological conditions yet is prevented by its natural counterpart, alpha(s1)-casein. Biochemistry 47:3926–3936
Thorn DC, Ecroyd H, Carver JA, Holt C (2015) Casein structures in the context of unfolded proteins. Int Dairy J 46:2–11
Treweek TM, Thorn DC, Price WE, Carver JA (2011) The chaperone action of bovine milk alpha(S1)- and alpha(S2)-caseins and their associated form alpha(S)-casein. Arch Biochem Biophys 510:42–52
Vekilov PG (2010) Nucleation. Cryst Growth Des 10:5007–5019
Wahlgren NM, Dejmek P, Drakenberg T (1993) Binding of Mg2+ and Ca2+ to beta casein A(1)- a multinuclear magnetic resonance study. J Dairy Res 60:65–78
Waugh DF (1971) Formation and structure of casein micelles. In: McKenzie HA (ed) Milk proteins chemistry and biology, vol II. Academic Press, New york and London, pp 3–85
White JCD, Davies DT (1958) The relation between the chemical composition of milk and the stability of the caseinate complex.1. General introduction, description of samples, methods and chemical composition of samples. J Dairy Res 25:236–255
White JCD, Davies DT (1963) The determination of citric acid in milk and milk sera. J Dairy Res 30:171–189
Yong YH, Foegeding EA (2010) Caseins: utilizing molecular chaperone properties to control protein aggregation in foods. J Agric Food Chem 58:685–693
Zhang Y, Liu D, Liu X, Hang F, Zhou P, Zhao J, Zhang H, Chen W (2018) Effect of temperature on casein micelle composition and gelation of bovine milk. Int Dairy J 78:20–27
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bijl, E., Huppertz, T., van Valenberg, H. et al. A quantitative model of the bovine casein micelle: ion equilibria and calcium phosphate sequestration by individual caseins in bovine milk. Eur Biophys J 48, 45–59 (2019). https://doi.org/10.1007/s00249-018-1330-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-018-1330-2