Abstract
Magnetotactic microorganisms are characterized by swimming in the direction of an applied magnetic field. In nature, two types of swimming polarity have been observed: north-seeking microorganisms that swim in the same direction as the magnetic field, and south-seeking microorganisms that swim in the opposite direction. The present work studies the reversal in the swimming polarity of the multicellular magnetotactic prokaryote Candidatus Magnetoglobus multicellularis following an isolation process using high magnetic fields from magnets. The proportion of north- and south-seeking organisms was counted as a function of the magnetic field intensity used during the isolation of the organisms from sediment. It was observed that the proportion of north-seeking organisms increased when the magnetic field was increased. The magnetic moment for north- and south-seeking populations was estimated using the U-turn method. The average magnetic moment was higher for north- than south-seeking organisms. The results suggest that the reversal of swimming polarity must occur during the isolation process in the presence of high magnetic fields and magnetic field gradients. It is shown for the first time that the swimming polarity reversal depends on the magnetic moment intensity of multicellular magnetotactic prokaryotes, and new studies must be undertaken to understand the role of magnetic moment polarity and oxygen gradients in determination of swimming polarity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnetotactic bacteria (MTB) are known for their production of magnetic nanoparticles in magnetosomes, enabling them to swim along geomagnetic field lines, an ability known as magnetotaxis. It is assumed that MTB are different in the two hemispheres of the Earth, because they always need to swim vertically downwards to oxic/anoxic sediment layers. As the vertical component of the geomagnetic field is upward in the Southern Hemisphere but downward in the Northern Hemisphere, two types of swimming polarity have been identified in MTB: north-seeking (NS) in the Northern Hemisphere, characterized by swimming in the same direction as the geomagnetic field, and south-seeking (SS) in the Southern Hemisphere, swimming in the direction antiparallel to the geomagnetic field (Bazylinski and Frankel 2004). In the presence of a magnet, NS MTB swim in the direction of the magnetic south pole while SS MTB swim toward the magnetic north pole. This swimming polarity is known as “polar magnetotaxis,” in contrast to “axial magnetotaxis” where MTB swim freely in both senses of the magnetic field lines, not towards a single magnetic pole. The discovery of MTB at the Geomagnetic Equator (Frankel et al. 1981) shows that NS and SS populations can coexist in that region and that their population densities are a function of the geomagnetic inclination (Torres de Araujo et al. 1990). This suggests that it is always possible to find a small fraction of NS MTB in the Southern Hemisphere and vice versa. However, Simmons et al. (2006) reported that large populations of SS MTB were found in marine sediments of the Northern Hemisphere, with the proportion of SS microorganisms correlated to the in situ environmental oxidation–reduction potential. Zhang et al. (2010) suggested that redox gradients combined with different magnetic field directions can control the swimming polarity of the MTB MO-1 culture. Recently, Pop et al. (2014) reported that the swimming polarity of the MTB Magnetospirillum gyrphiswaldense can be switched from axial to polar by growth of cultures in presence of oxygen gradients and magnetic fields, both oriented parallel or antiparallel, and even that the NS population can become SS upon a sudden increase in the local oxygen level. All those reports show that the swimming polarity depends on the relation between the direction of the magnetic field and the oxygen gradient.
In the present report, isolation of MTB using magnets is investigated. In our laboratory, such isolation is done using a small glass vessel with one or two capillary ends. To isolate SS MTB, a magnet is put in front of one capillary end with the magnetic north pole facing it (Fig. 1). If NS and SS MTB are present in the sediment, only SS MTB will swim into the capillary end facing the magnet while NS MTB will swim in the opposite direction to the other capillary end of the vessel. In fact, in Rio de Janeiro, Brazil, at the opposite capillary end a small number of NS MTB may be collected. However, in the water drop isolated in front of the magnet, both populations appear, with NS MTB being found at lower proportion. It is interesting to ask how the NS MTB population is mixed with SS MTB in the same capillary end, because in theory they must be found only at opposite ends of the concentrator. The present report aims to discuss this phenomenon, analyzing the isolation of MTB using different magnetic field intensities in the capillary tip. The multicellular magnetotactic prokaryote (MMP) Candidatus Magnetoglobus multicellularis (CMm) was used in the present study, because it is easier to quantify the amount of MMPs isolated (in comparison with MTB) and because it shows “polar magnetotaxis” (Abreu et al. 2007).
Materials and methods
CMm MMPs are found at Araruama Lagoon (22°50′S; 42°13′W), a hypersaline (~55 ‰) coastal lagoon of Rio de Janeiro State, Brazil. Samples of water and sediment containing the microorganism were collected at depth of 1 m and, in the laboratory, transferred into aquariums with approximately 1/3 sediment and 2/3 water. The aquariums were near to a window to illuminate them by sunlight. To collect MMPs, water and sediment were collected using a Pasteur pipette from a zone somewhat below the sediment surface in the aquarium and transferred to a glass container (Fig. 1), as described by Lins et al. (2003). This glass container has two lateral capillary ends, in one of which the microorganisms are concentrated using a permanent magnet. The north pole of the magnet was placed near to and at the level of the capillary end to collect an optimal amount of SS MMPs. To investigate whether the magnetic field intensity determines the presence of NS organisms in the same capillary end, the distance of the magnet from the capillary end was changed and the magnetic field measured at the position of the capillary end (digital gaussmeter Globalmag model TLMP-Hall-05k-T1), yielding 480 Oe at 1 cm, 130 Oe at 2 cm, 50 Oe at 3 cm, 28 Oe at 4 cm, and 15 Oe at 5 cm. Ten different isolation procedures were undertaken for each distance.
Counting procedure
After 15 min, a drop of water was collected from the capillary end using a dropper. The dropper tip was taken out from the capillary perpendicular to the magnet to avoid the drop being exposed to higher magnetic fields. The drop was then placed on a coverslip. A digital microscope (Celestron no. 33340) was used to observe and record the MMPs in the drop. A pair of coils was adapted to the sides of the digital microscope and connected to a direct-current (DC) power supply to generate a uniform magnetic field of about 5 Oe in the objective lens region (magnetic field measured with a digital gaussmeter Globalmag model TLMP-Hall-050). The magnification used was 10×. The entire water drop border was recorded, making it possible to count the amount of SS and NS MMPs isolated in the same drop, since the film was stationary or could be separated into frames to count individual MMPs. As mentioned above, ten drops were analyzed for each magnet distance. After counting, the proportion of SS and NS organisms was calculated and statistical analysis carried out on each set of proportions for each magnet distance.
Effect of magnetic gradient
Assuming that a small proportion of MMPs is NS, there is a possibility that they will appear in the same drop because of the force due to the magnetic field gradient from the magnet. To investigate whether this applies, isolation was carried out with the magnet south pole facing the capillary end and a magnetic field of 460 Oe (Video 1) and another with the magnet north pole facing the capillary end and a magnetic field of 460 Oe (Video 2). Another video was recorded with the magnetic north pole facing the capillary end and a magnetic field of 15 Oe (Video 3), to show the effect of different magnetic field intensities.
The effect of different isolation times (5, 10, and 15 min) on the NS proportion was analyzed. The magnetic field in the capillary end was 460 Oe, and the procedure for counting the number of SS and NS MMPs was the same as described above.
Magnetic moment estimation
To determine whether the SS and NS populations isolated in the same drop have the same size and magnetic moment, isolations were done with magnetic fields of 480 and 15 Oe. To estimate the magnetic moment of the MMPs, the U-turn method was used to calculate the U-turn time t u. A pair of coils connected to a DC power supply were set on the stage of an inverted microscope (Nikon Eclipse TS100), and the coverslip with the drop was placed in the middle of the coils (Fig. 2). The lens had magnification of 40×, allowing measurement of the MMP radius R. The magnetic field generated by the coils was 11 Oe. An electric circuit for changing the voltage polarity (current reversal) was connected between the power supply and coils, leading to inversion of the magnetic field direction when a button was pushed. After two magnetic field inversions, the MMPs performed U-turn trajectories (Fig. 3). The magnetic moment (m) can be estimated using the formula (Esquivel and Lins de Barros 1986)
where t u is the U-turn time, η is the viscosity of the medium (about 10−3 Pa s), R is the microorganism radius, B is the external magnetic field, k is the Boltzmann constant, and T is the temperature (about 300 K). To calculate t u, the following procedure was undertaken: U-turn trajectories were recorded at rate of 82 fps using the inverted microscope with a digital camera (Lumera Infinity 1). The coordinates of the U-turn trajectories were obtained using ImageJ software (NIH, USA) (Fig. 4a). In the experimental setup, the external magnetic field is applied in the x direction, meaning that the x coordinate as a function of time must be two straight lines with different slopes (Fig. 4b). The U-turn time t u is the time necessary for the change of slope and can be calculated from the derivative dx/dt (Fig. 4c). As t u depends on the radius of the microorganism, a table of theoretical values for t u/R 3, as a function of m and maintaining η, B, and T constant, permits determination of the value of m for each MMP via comparison with the experimental values of t u/R 3. All graphs were produced using Microcal Origin software, and statistical analysis carried out using GraphPad InStat software.
Experimental setup used to record the U-turn movement. A pair of coils was adapted to the stage of an inverted microscope, and the coils were connected to a power supply through a circuit that permits change of the magnetic field polarity. MMPs (SS or NS) are first concentrated at a border of the drop then stimulated to swim when the magnetic field polarity is inverted. A second inversion in the magnetic field polarity produces the U-turn. The drop was positioned in the middle of the pair of coils, and U-turns were recorded using a video camera attached to the microscope
Example of procedure to measure U-turn time t u. a The coordinates of the U-turn trajectory are obtained using ImageJ software. The example corresponds to the U-turn in Fig. 3. b As the magnetic field is oriented along the x-axis, the U-turn x-coordinate must be a linear function of time, and during the U-turn the slope must change. c The U-turn time, t u, corresponds to the time interval for the change of slope in the x-coordinate. The initial (T i) and final (T f) instants of the change of slope process are indicated in the figure. t u is calculated from the difference between T f and T i, i.e., t u = T f − T i. In this example, t u = 0.16 s
Results and discussion
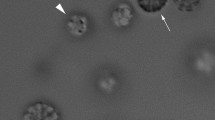
The results showed that NS and SS populations were present in the same water drop after isolation from the capillary end in front of a magnetic north pole. Normally, one would expect to detect only the SS population at that capillary end because MMPs show polar magnetotaxis. Figure 5d, f and Videos 2 and 3 show that the amount of NS MMPs increased when the magnetic field used for isolation was increased. Let us assume that NS MMPs are commonly present in water sediment. If this is the case, their presence in Video 2 must be because an attractive force produced by the magnetic field gradient carried them to the capillary end. On the other hand, several NS MMPs must be isolated when the magnetic south pole is facing the capillary end. This, however, is not observed in Fig. 5b and Video 1. The corresponding isolation with the magnetic south pole showed very few microorganisms, in contrast to the presence of several NS MMPs isolated when using the magnetic north pole (Video 3). So, it can be concluded that, in natural MMP populations, NS microorganisms are present at very low proportions (less than 1%), in contrast to SS microorganisms. From Videos 2 and 3 and Fig. 5d, f, one can conclude that the MMPs reaching the capillary end are SS when the magnetic north pole is used and that a proportion of them become NS inside the drop during isolation and transportation to the microscope slide.
Effect of magnetic field intensity on number of isolated SS and NS MMPs. a, c, e Isolation process with different magnet configurations. b, d, f Opposite sides of the isolated drop observed in the microscope; the arrow at the top indicates the magnetic field direction during the observation. This magnetic field is generated by coils as in Fig. 2. In this configuration, SS MMPs should accumulate at the left drop border and NS MMPs at the right drop border. a, b The magnet is 1 cm away from the concentrator tip with the magnetic south pole (MS) facing the tip (B ≈ 460 Oe). It is observed in b that no MMPs were isolated. This result shows that NS MMPs are not commonly present in the sample. c, d The magnetic north pole (MN) is facing the tip, 1 cm away from the concentrator tip (B ≈ 460 Oe). d SS and NS MMPs are isolated in the same drop, but the proportion of SS MMPs is higher than that of NS MMPs. e, f The MN is 6 cm away from the concentrator tip (B ≈ 15 Oe). NS MMPs are still observed but in much fewer number compared with d. b, d Show that the sample is composed of SS MMPs and that they convert to NS MMPs during the isolation process
During the present research, several experiments were done to observe the inversion of swimming polarity in individual MMPs, using strong magnetic fields during the observation process under the microscope. Magnetic fields of about 50 or 100 Oe, generated with coils or magnets, were unable to invert the swimming polarity in observation times of about 30 min (data not shown). These experiments show that the inversion of swimming polarity occurs only during the isolation process in the concentrator tip. Perhaps we did not observe the change of swimming polarity because an abrupt change in the local oxygen gradient is essential, as happens at the moment of drop extraction from the capillary tip.
The proportion of NS and SS individuals in the same drop is a function of the strength of the magnetic field at the capillary end (Fig. 6), with increasing NS proportion as the magnetic field is increased. The proportion measured for different isolation times was the same, meaning that the proportion is not the result of an accumulative process. To determine whether the two populations are different, the radii and magnetic moments were determined for two different isolation procedures (Table 1). For NS and SS populations isolated using a magnetic field of 480 Oe, the mean radii were statistically different amongst themselves, while the mean radius of the SS population isolated using a magnetic field of 15 Oe was statistically similar to that of the SS population isolated using 480 Oe [analysis of variance (ANOVA) test: p < 0.05]. To determine whether the two populations had different magnetic moments, the U-turn method was used. As shown by the results in Table 1, the mean magnetic moments were statistically different for the SS and NS populations but statistically similar for the two SS populations (ANOVA test: p < 0.05). The magnetic moments obtained for the SS and NS populations (Table 1) are in good agreement with the magnetic moment estimated previously using the U-turn method (Perantoni et al. 2009). Table 1 shows that the NS population has higher magnetic moment than the SS population. The ratio of the lower to higher average values was about 0.73 ± 0.10. One can consider that the magnetic moment for NS MMPs results from SS MMPs that were remagnetized. Winklhofer et al. (2007) and Acosta-Avalos et al. (2012) showed that remagnetization of MMPs produces a degree of magnetic optimization (defined as the ratio among lower and higher magnetic moment) of about 0.85 ± 0.01 (Winklhofer et al. 2007) or 0.90 ± 0.07 (Acosta-Avalos et al. 2012). The magnetic moment ratio obtained here of about 0.7 is far from that expected theoretically and experimentally, indicating that the magnetic moments of NS MMPs are not the result of remagnetization. Moreover, CMms show positive correlation between magnetic moment and volume (Perantoni et al. 2009). Our results show that the NS and SS populations had different distributions of radius, meaning that NS MMPs should have higher magnetic moments than SS MMPs, ruling out the remagnetization hypothesis, because that process does not change the size of the microorganism.
These results show that exposure of the CMm organisms to high magnetic fields from a magnet, during the isolation procedure, induced a change in the swimming polarity, suggesting that the MMPs that change swimming polarity from SS to NS are selected owing to their higher magnetic moment. Our results do not allow us to determine whether the magnetic moment polarity also changed. Experiments done by Blakemore et al. (1980) showed that alternating 50-Hz magnetic fields above 1 kOe could invert the magnetic moment polarity and swimming polarity of MTB, showing a relationship among them. Penninga et al. (1995) used strong magnetic fields (of about 700 Oe) in pulses of 1–5 ms to invert the magnetic moment polarity of MTB. They observed that, for MTB, the minimum magnetic field to invert the polarity was the same as the coercive field (about 310 Oe). For MMPs, Penninga et al. (1995) measured the coercive field as 200 Oe and observed that MMPs can be demagnetized, which is distinct from MTB. MMP demagnetization was also reported by Rodgers et al. (1990) and Keim et al. (2006), where alternating 60-Hz magnetic fields were used, the demagnetized MMPs being unable to respond magnetotactically. In the present experiments, MMPs showed magnetotaxis with both swimming polarities after isolation from the capillary tip. No demagnetized MMPs were observed. The magnetic fields where NS MMPs were observed (28, 50, and 130 Oe) are lower than the coercive field of 200 Oe, and our experimental manipulation of the drop during the isolation procedure avoids exposure to higher magnetic fields from the magnet. These facts suggest that the magnetic moment polarity of the MMP was not inverted and that the inversion of swimming polarity must be related to another mechanism. Pop et al. (2014) showed that swimming polarity can be changed by oxygen gradients, relative to the magnetic field direction, meaning that, to change the swimming polarity, it is not necessary to change the polarity of the MTB magnetic moment. Our results indicate that the intensity of the magnetic moment m is important too in the determination of the swimming polarity in the presence of nonuniform high-intensity magnetic fields.
It has been shown that the swimming polarity of MTBs depends on the magnetic moment polarity (Blakemore et al. 1980; Penninga et al. 1995) and aerotaxis determined by the presence of oxygen gradients (Pop et al. 2014). In particular, Pop et al. (2014) showed that the change in swimming polarity is related to the relative orientation among the magnetic field and oxygen gradient. During the isolation procedure used in our experiment, there is no way the MMP magnetic moment m could change its polarity without being demagnetized, but in the process of isolating the water drop from the capillary end, the dropper moves the drop through an air environment and magnetic field gradient, creating an oxygen gradient through the drop (Cai et al. 2008). If this sudden oxygen gradient in the water drop promotes a change in swimming polarity, it may be hoped that all MMPs would have the same probability to change polarity. However, our results suggest that only those microorganisms with sufficient magnetic moment m could change from SS to NS (Table 1).
The same behavior is observed in noncultured MTB, but their number and size make it difficult to quantify the number of SS and NS organisms and determine the magnetic moment for each population.
In conclusion, the swimming polarity reversal of MMPs during the isolation process with the application of magnetic fields from magnets and with intensities much higher than that of the geomagnetic field was studied for the first time. The results show that the swimming polarity reversal depends on the intensity of the MMP magnetic moment. More studies are required to understand the relation between the magnetic moment polarity, magnetic moment intensity, and oxygen gradient in determining the swimming polarity.
References
Abreu F, Martins JL, Silveira TS, Keim CN, Lins de Barros HGP, Gueiros Filho FJ, Lins U (2007) ‘Candidatus Magnetoglobus multicellularis’, a multicelular, magnetotactic prokaryote from a hypersaline environment. IJSEM 57:1318–1322
Acosta-Avalos D, Azevedo LMS, Andrade TS, Lins de Barros H (2012) Magnetic configuration model for the multicelular magnetotactic prokaryote Candidatus Magnetoglobus multicellularis. Eur Biophys J 41:405–413
Bazylinski DA, Frankel RB (2004) Magnetosome formation in prokaryotes. Nat Rev Microbiol 2:217–230
Blakemore RP, Frankel RB, Kalmijn AJ (1980) South-seeking magnetotactic bacteria in the Southern hemisphere. Nature 286:384–385
Cai J, Wang L, Wu P, Li Z, Tong L, Sun S (2008) Study on oxygen enrichment from air by application of the gradient magnetic field. J Magn Magn Mater 320:171–181
Esquivel DMS, Lins de Barros HGP (1986) Motion of magnetotactic microorganisms. J Exp Biol 121:153–163
Frankel RB, Blakemore RP, Torres de Araujo FF, Esquivel DMS, Danon J (1981) Magnetotactic bacteria at the geomagnetic equator. Science 212:1269–1270
Keim CN, Martins JL, Lins de Barros H, Lins U, Farina M (2006) Structure, behavior, ecology and diversity of multicelular magnetotactic prokaryotes. In: Schuler D (ed) Magnetoreception and magnetosomes in bacteria. Springer-Verlag, Berlin, pp 103–132
Lins U, Freitas F, Keim CN, Lins de Barros H, Esquivel DMS, Farina M (2003) Simple homemade apparatus for harvesting uncultured magnetotactic microorganisms. Braz J Microbiol 34:111–116
Penninga I, de Waard H, Moskowitz BM, Bazylinski DA, Frankel RB (1995) Remanence measurements on individual magnetotactic bacteria using a pulsed magnetic field. J Magn Magn Mater 149:279–286
Perantoni M, Esquivel DMS, Wajnberg E, Acosta-Avalos D, Cernicchiaro G, Lins de Barros H (2009) Magnetic properties of the microorganism Candidatus Magnetoglobus multicellularis. Naturwissenschaften 96:685–690
Pop F, Armitage JP, Schuler D (2014) Polarity of bacterial magnetotaxis is controlled by aerotaxis through a common sensory pathway. Nat Commun 5:5398
Rodgers FG, Blakemore RP, Blakemore NA, Frankel RB, Bazylinski DA, Maratea D, Rodgers C (1990) Intercellular structure in a many-celled magnetotactic prokaryote. Arch Microbiol 154:18–22
Simmons SL, Bazylinski DA, Edwards KJ (2006) South-seeking magnetotactic bacteria in the Northern hemisphere. Science 311:371–374
Torres de Araujo FF, Germano FA, Gonçalves LL, Pires MA, Frankel RB (1990) Magnetic polarity fractions in magnetotactic bacterial populations near to geomagnetic equator. Biophys J 58:549–555
Winklhofer M, Abraçado LG, Davila AF, Keim CN, Lins de Barros HGP (2007) Magnetic optimization in a multicellular magnetotactic organism. Biophys J 92:661–670
Zhang WJ, Chen C, Li Y, Song T, Wu LF (2010) Configuration of redox gradient determines magnetotactic polarity of the marine bacteria MO-1. Environ Microbiol Rep 2:646–650
Acknowledgements
The authors thank Dr. Henrique Lins de Barros for fruitful discussion and suggestions, Dr. Steven Frederick Durrant from UNESP, Sorocaba, SP, Brazil for correcting the English of the manuscript, and the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico—CNPq, Brazil and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro—FAPERJ, Rio de Janeiro, Brazil for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
249_2017_1199_MOESM1_ESM.pdf
Table II: Values for the radius R, U-turn time t u, ratio t u/R 3, and corresponding values for the magnetic moment m, calculated using Eq. (1), ordered according to the magnetic field used in the isolation process and the type of swimming polarity. The corresponding statistics are summarized in Table I (PDF 130 kb)
Video 1: Isolation of MMPs with the magnetic south pole facing the capillary end. The magnetic field measured at the capillary end was 460 Oe. The video starts at the drop border where SS MMPs should accumulate (3gp 3330 kb)
Video 2: Isolation of MMPs with the magnetic north pole facing the capillary end. The magnetic field measured at the capillary end was 460 Oe. The video starts at the drop border where SS MMPs should accumulate (3gp 4650 kb)
Video 3: Isolation of MMPs with the magnetic north pole facing the capillary end. The magnetic field measured at the capillary end was 15 Oe. The video starts at the drop border where SS MMPs should accumulate (3gp 3789 kb)
Rights and permissions
About this article
Cite this article
de Melo, R.D., Acosta-Avalos, D. The swimming polarity of multicellular magnetotactic prokaryotes can change during an isolation process employing magnets: evidence of a relation between swimming polarity and magnetic moment intensity. Eur Biophys J 46, 533–539 (2017). https://doi.org/10.1007/s00249-017-1199-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-017-1199-5