Abstract
The main aim of this study was to investigate a possible functional connection between sigma-1 receptors and voltage-gated sodium channels (VGSCs) in human breast cancer cells. The hypothesis was that sigma-1 drugs could alter the metastatic properties of breast cancer cells via the VGSC. Evidence was found for expression of sigma-1 receptor and neonatal Nav1.5 (nNav1.5) expression in both MDA-MB-231 and MDA-MB-468 cells. Sigma-1 drugs (SKF10047 and dimethyltryptamine) did not affect cell proliferation or migration but significantly reduced adhesion to the substrate. Silencing sigma-1 receptor expression by siRNA similarly reduced the adhesion. Blocking nNav1.5 activity with a polyclonal antibody (NESOpAb) targeting an extracellular region of nNav1.5 also reduced the adhesion in both cell lines. Importantly, the results of combined treatments with NESOpAb and a sigma-1 drug or sigma-1 siRNA suggested that both treatments targeted the same mechanism. The possibility was tested, therefore, that the sigma-1 receptor and the nNav1.5 channel formed a physical, functional complex. This suggestion was supported by the results of co-immunoprecipitation experiments. Furthermore, application of sigma-1 drugs to the cells reduced the surface expression of nNav1.5 protein, which could explain how sigma-1 receptor activation could alter the metastatic behaviour of breast cancer cells. Overall, these results are consistent with the idea of a sigma-1 protein behaving like either a “chaperone” or a regulatory subunit associated with nNav1.5.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sigma receptors show an unusual promiscuous ability to associate with a surprisingly large number of critical cellular components. These include the IP3 receptor in endoplasmic reticulum, voltage sensitive ion channels and the cytoskeletal element ankyrin (for review, see Aydar et al. 2004). The sigma-1 receptor is a transmembrane protein that has been reported to be localised to the plasma membrane, perinuclear areas, endoplasmic reticulum and in regions of cell-to-cell communication (Aydar et al. 2004). The protein has two transmembrane regions and two steroid-binding domains. The latter forms a pocket that serves as a binding site for cholesterol, steroids and sphingolipids (Palmer et al. 2007; Fontanilla et al. 2009). A wide range of synthetic or natural compounds (‘sigma ligands’), including opiates, antipsychotics, psychostimulants, alkaloids and antidepressants, also bind to sigma-1 receptors (Palmer et al. 2008; Maurice and Su 2009). In vivo, endogenous dimethyltryptamine (DMT) interacts with sigma-1 receptors in the brain but the physiological significance of this is not clear (Fontanilla et al. 2009; Mavlyutov et al. 2012). The drug SKF10047 has been utilised extensively as a sigma-1 ligand and has significant preference for sigma-1 over sigma-2 receptors (Aydar et al. 2004). Importantly, sigma-1 has pathophysiological importance (Tsai et al. 2009).
Studies on sigma-1 receptor modulation of ion channels have deduced the signal transduction mechanism of sigma-1 receptors (1) to be membrane delimited; (2) to be independent of G-protein coupling and protein phosphorylation (Lupardus et al. 2000); (3) to be reconstitutable in a heterologous system (Aydar et al. 2002); (4) not to require cytoplasmic factors (Lupardus et al. 2000); (5) to necessitate the receptor and the ion channel to be in close proximity, probably to form a stable macro-molecular complex (Aydar et al. 2002). Modulation of the heart voltage-gated Na+ channel (Nav1.5) by sigma-receptors was demonstrated and this could be an important pathway through which drugs could alter cardiac excitability and rhythmicity (Johannessen et al. 2011). This was further corroborated by atomic force microscopy imaging of complexes between sigma-1 receptors and Nav1.5 channels revealing a fourfold symmetry (Balasuriya et al. 2012). In human breast cancer (BCa) MDA-MB-231 cells, which functionally express the voltage-gated sodium channel (VGSC) subtype Nav1.5, sigma-1 receptor was found to regulate current density, suggesting a functional interaction between the two proteins (Balasuriya et al. 2012). Despite such advances, however, the exact cellular mechanism of sigma-1 receptor action is still unclear and further experiments are required to clarify their role in physiological and pathophysiological cell signalling (Tsai et al. 2009). A model has been proposed in which the sigma-1 receptors are “silent” under normal physiological conditions, whereas in disease, the receptor behaves as a chaperone that binds ‘client’ proteins, modulates cellular activity and promotes survival (Su et al. 2010). This hypothesis has received some support from studies demonstrating that sigma-1 silencing would suppress recovery after experimental stroke (Ruscher et al. 2011) and promote retinal degeneration after acute damage to the optic nerve (Mavlyutov et al. 2012).
The role that sigma-1 receptors play in cancer is not clear, although it is frequently up-regulated in human cancer cells and tissues (Aydar et al. 2004, 2006). The most in-depth study on the role that sigma-1 receptors play in BCa was performed by Palmer et al. (2007). The results indicated that cancer cells express high levels of sigma-1 receptors compared with corresponding normal cells. Localisation of the sigma-1 receptor was investigated in MDA-MB-231 cells by immunocytochemistry and confocal microscopy. Expression was predominantly at the cell periphery but also in the endoplasmic reticulum (Palmer et al. 2007). Both sigma-1 and sigma-2 drugs and a sigma-1 receptor silencing construct reduced proliferation and adhesion of BCa cell lines compared to a non-cancerous BCa cell line. The effect on adhesion was found to be through a functional interaction with β-1 integrin (Palmer et al. 2007). Although the exact nature of how sigma-1 receptors could modulate adhesion and migration in BCa cells has not been determined, it has been suggested that voltage-gated sodium channels (VGSCs) known to be expressed in BCa cells could play an important role (Aydar et al. 2004). Several studies have shown that functional VGSC expression occurs in a variety of carcinomas and promotes metastatic behaviour. Such carcinomas include BCa (Fraser et al. 2005) as well as cancers of the prostate (Grimes et al. 1995; Laniado et al. 1997; Nakajima et al. 2009), lung (Onganer and Djamgoz 2005; Campbell et al. 2013), colon (House et al. 2010) and cervix (Diaz et al. 2007). It has been suggested, therefore, (1) that VGSC up-regulation is an early event in metastatic progression and (2) that VGSC expression is a ‘switch,’ necessary and sufficient for engaging cancer cells in a highly invasive state. Importantly, in BCa and colon cancer the predominant VGSC was found to be the neonatal splice variant of Nav1.5 (nNav1.5) and this has been proposed to be a novel mechanism targetable with small-molecule drugs, monoclonal antibody and natural neurotoxins (Onkal and Djamgoz 2009).
The aims of this study were (1) to investigate a possible functional link between sigma-1 receptors and nNav1.5 in BCa cells and (2) to test the possibility that the effects of sigma-1 drugs in altering the metastatic properties of BCa cells could occur via modulation of nNav1.5.
Methods
Cell culture
Human BCa cell lines (MDA-MB-468, MDA-MB-231 and MDA-MB-453) were obtained from the American Type Culture Collection and cultured in DMEM (Life Technologies) containing 5 % foetal bovine serum (FBS) and 4 mmol/l glutamine with penicillin-streptomycin. All cell lines were maintained in a 37 °C CO2 incubator in 100-mm culture dishes. Cells prepared for electrophysiological analysis were cultured without the addition of penicillin-streptomycin.
Pharmacological agents
Sigma-1 receptor ligands SKF10047 and 5-methyl-dimethytryptamine (5-CH3-DMT) were obtained from Sigma-Aldrich and diluted to a stock concentration of 10 mM in a 50/50 mixture of water/ethanol. As a control treatment all cells were treated with an equivalent 50/50 mixture of water/ethanol. Tetrotodoxin (TTX) was obtained from Tocris and a stock of 10 mM was prepared in sterile water.
Antibodies
A polyclonal antibody to nNav1.5 was produced in a similar manner as described previously (Chioni et al. 2005). Two rabbits were injected with a peptide derived from the published sequence of the nNav1.5 ion channel D1/S3:S4 extracellular loop (peptide sequence YVSENIKLGNLSALRC) using the Covalab polyclonal antibody service. Peptides were purified by RP-HPLC and stored dry at −20 °C. Identity of the peptide was confirmed by MALDI-TOF. The purity was estimated to be not less than 80 %. Rabbit anti-serum was generated towards the peptide covalently linked to maleimide-activated key-hole limpet heamacyanin (KLH) for improved immunogenicity. The C-terminal cysteine (not present in the nNav1.5 sequence) allowed coupling of the peptides to maleimide-activated KLH. Rabbits were immunised by injection with peptide–KLH conjugates mixed with Freund’s incomplete adjuvant. Subsequent booster injections were performed on days 21, 42 and 63. Animals were killed and bled on day 88. Antibodies were purified from the rabbit sera by immunopurification against the above peptide. The immunoaffinity column was prepared by coupling the peptide to 1 ml of activated Sepharose beads and purification from 5 ml of serum. The purified antibody was subsequently tested by ELISA against the immunixing peptide. The purified antibody concentration was 378.6 μg/ml. Production of the sigma-1 receptor antibody in rabbits was described previously (Palmer et al. 2007).
Gene silencing
A sigma-1 gene silencing vector was constructed using the pSilencer RNAi vector (Ambion) as described previously, which affects both sigma-1 receptor mRNA and protein levels without affecting basal gene expression levels (Palmer et al. 2007). As a control, a sigma-1 receptor RNAi randomised sequence was constructed in pSilencer. Transfection was accomplished using Lipofectamine 2000 (Invitrogen) and Opti-MEM (Life Technologies). Transfection efficiency was verified by cotransfecting a green fluorescence protein-expressing plasmid (pHr-GFP). Silencing efficiency was quantified by protein extraction from cultures 4 days post-transfection and Western blotting 5 μg of total cell protein onto nitrocellulose followed by detection using a sigma-1 receptor-specific antibody and an anti-α-actinin antibody (Sigma-Aldrich) as control for nonspecific effects on gene expression. Silencing efficiencies of >60 % were usually obtained.
Proliferation assay
Freshly harvested cells were diluted to 10,000 cells/ml in growth media and seeded into 96-well plates at 100 μl per well. Following an overnight incubation the media was removed and replaced with 100 μl of growth media containing SKF10047, N-methy-DMT or control treatment. The cells were incubated for 4 days at 37 °C and cell proliferation was measured by adding 10 μl of cell counting reagent (cc-8 Sigma-Aldrich) and incubation for 3 h at 37 °C. Subsequently the absorbance at 450 nm was measured with a Floustar Omega plate reader using wells with growth media and cell counting reagent only as a blank control.
Adhesion assay
Cells were seeded at 1.5 × 106 cells into 100-mm culture dishes in 10 ml of growth media. The cells were incubated overnight and the media was removed and replaced with fresh media containing sigma-1 drugs or control treatment. Following 24 h treatment at 37 °C, the cells were washed with PBS and incubated with PBS containing 10 mM EDTA for 15 min at 37 °C. The detached cells were collected by centrifugation at 1200 rpm for 5 min and the pellet was resuspended in 2 ml of growth media. The cells were counted and diluted to a concentration of 5 × 105 cells per ml. To 1 ml of cell suspension 3 μl of calcein, AM (Invitrogen 1 mM stock in DMSO), was added and incubated at 37 °C for 30 min. The cells were centrifuged twice and washed with growth media at 1200 rpm for 5 min and resuspended in 1 ml of growth media. Cells were plated into 96-well plates (100 μl per well) precoated with collagen. Plates were coated in a solution containing 125 μg/ml of collagen protein in PBS for 1 h at 37 °C before washing thrice with PBS and once with DMEM. Afterwards, non-adherent cells were removed by washing the wells three times with PBS, swirling the contents and inverting them onto filter paper. Finally 100 μl of phosphate-buffered saline (PBS) was added to each well and fluorescence was measured using a Fluostar Omega plate reader (excitation, 480 nm; emission, 520 nm) using wells containing PBS alone as a blank control.
Transverse migration assay
The assay was performed using 8-μm-pore, 24-well polycarbonate transwell filters (Corning Inc., UK). Twenty-four hours prior to the assay the cells were starved by growing them in serum-free medium. The cells were harvested using PBS containing 10 mM EDTA and resuspended in serum-free media at 2 × 106 cells/ml. The bottom chamber contained 500 μl of growth medium. To the top chamber, 100 μl of cells was added with or without sigma-1 drugs. The plates were incubated at 37 °C in a CO2 incubator for 24 h. The media from the top chamber was aspirated and the chamber washed with PBS. The bottom chamber was aspirated and washed with PBS and then replaced with 500 μl of PBS-EDTA containing 10 μM calcein-AM and incubated at 37 °C in a CO2 incubator for 1 h. Subsequently, the plate was shaken for 10 min at room temperature. The top chamber was removed and the plate was read at 480 nm excitation, 520 nm emission.
Patch clamp recording
Experiments were performed on MDA-MB-468 cells that had not been electrophysiologically characterised before. Details of the patch pipettes, solutions and the whole-cell recording protocols have been described previously (Grimes et al. 1995; Laniado et al. 1997; Fraser et al. 2005). Patch pipettes (tip resistances, ~5 MΩ) were filled with a solution designed to block the outward K+ currents; the composition was as follows (in mM): NaCl 5, CsCl 145, MgCl2 2, CaCl2 1, HEPES 10 and EGTA 11, adjusted to pH 7.4 with 1 M CsOH. The estimated intracellular free calcium concentration was ~15 nM (Laniado et al. 2001). Cells were triturated 1–2 h prior to patching and whole-cell membrane currents were recorded from cells that appeared ‘isolated’ in culture, using an Axopatch 200B amplifier (Axon Instruments, CA, USA). Analogue signals were filtered at 5 kHz using a lowpass Bessel filter and series resistance errors were compensated by ~95 %. Electrophysiological signals were sampled at 50 kHz and digitised using an interface (Digidata 1200). Data acquisition and analysis of whole-cell currents were performed using pClamp software (Axon Instruments). A holding potential of −100 mV was applied. Standard voltage-clamp protocols were used to study the electrophysiological characteristics of the Na+ currents. Tetrodotoxin was bath applied and effects on VGSCs were studied using a single-step voltage pulse protocol (from −100 to −10 mV for 10 ms), repeated at 10-s intervals.
Western blotting
Protein samples (concentration, 1 mg/ml) were mixed with SDS sample buffer (Sigma-Aldrich), heated to 95 °C for 5 min and 5 μg of protein were loaded per lane and separated on acrylamide 4–20 % gradient Tris–glycine minigels (Invitrogen). The proteins were transferred to nitrocellulose membrane for 2 h (for sigma-1 receptor) or 6 h (for nNav1.5) at 4 °C; transfer was verified using Ponceau red (Sigma-Aldrich). Blots were ‘blocked’ with a solution containing 2.5 % skimmed milk and 2.5 % bovine serum albumin (BSA) in PBS overnight at 4 °C before probing with specific antibodies. The antibodies were diluted to 1 μg/ml in PBS containing 0.1 % Tween (PBST) with 0.5 % skimmed milk and 0.5 % BSA and incubated with the blot for 4 h at room temperature. Following four 10-min washes with PBST (with constant agitation) at room temperature, the blots were incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibodies (at the manufacturers’ suggested concentrations) as appropriate and diluted in PBST with 0.5 % skimmed milk and 0.5 % BSA for 2 h at room temperature. The blots were again washed as before and then developed with an enhanced chemiluminescence Western blot kit (Amersham). All blots were quantified using the NIH IMAGE programme.
Immunocytochemistry
Cells were seeded into 35-mm dishes containing glass coverslips coated with poly-l-lysine at 1 × 106 cell cells/dish. Following an overnight incubation at 37 °C wells were washed three times with HBSS. Cells were fixed with 3 % paraformaldehyde at room temperature for 12 min and subsequently washed three times with PBS. Cells were permeabilised with 0.1 % saponin for 10 min at room temperature followed by a further three washes with PBS. The cells were blocked with 5 % BSA in PBS for 1 h. Primary antibodies to sigma-1 or nNav1.5 were added overnight at a concentration of 5 μg/ml at 4 °C. Control antibodies, which included purified rabbit IgG, were also used. Following three washes with PBS containing 0.05 % Tween, the cells were stained with an anti-rabbit antibody with flourochromes attached overnight at 4 °C. Following a further three washes, the cells were drained of liquid and mounted with VectorShield and analysed by florescence microscopy.
Surface expression of nNav1.5
Surface expression of nNav1.5 was determined by seeding cells into 24-well plates coated with poly-l-lysine at 5 × 105 cells cells/well. Following an overnight incubation at 37 °C wells were washed three times with Hanks-buffered saline solution (HBSS) and subsequently 5 μg rabbit anti-nNav1.5 antibody in 1 ml of HBSS containing 5 % BSA was added for 1 h at 37 °C. The cells were washed three times with HBSS and incubated with a 1/100 dilution of anti-rabbit IgG Texas Red (Sigma-Aldrich) in 1 ml of HBSS containing 5 % BSA for 1 h at 37 °C. The cells were washed three times in HBSS and then fluorescence was measured using a Floustar Omega plate reader.
Immunoprecipitation
Protein extracts were prepared from cells using RIPA buffer (Triton-X at 1 %) containing a proteinase cocktail (Roche) and adjusted to a concentration of 1 mg/ml. A mouse anti-Nav1.5 was added at 1 μg/ml for 4 h at 4 °C. Subsequently beads with protein-A were added for 4 h at 4 °C. Immunoprecipitation washes were carried out with a kit from Pierce utilising spin columns. Following pull-down and washing of the beads, the samples were mixed with SDS-PAGE sample buffer (Sigma) and loaded onto SDS-PAGE gels as described previously. As controls, the immunoprecipitation was performed in the absence of immunoprecipitation antibody and with beads without protein A attached (to verify nonspecific binding of proteins).
Data analysis
Statistical significance was analysed by one-way ANOVA. Differences were taken as significant for P < 0.05.
Results
Initial characterisation
Sigma-1 receptor protein expression was investigated in MDA-MB-468, MDA-MB-231 and MDA-MB-453 cells (Fig. 1a). Both MDA-MB-231 and MDA-MB-468 cells are known to be highly invasive/metastatic both in vitro and in vivo. We therefore decided to investigate the role of the sigma-1 receptor on in vitro metastasis. Previously, sigma-1 receptor silencing constructs were validated in MDA-MB-231 cells (Palmer et al. 2007). These vectors were transfected into MDA-MB-468 and MDA-MB-231 cells and after 48 h the level of mRNA silencing was determined in comparison to a control vector (Fig. 1a, b). Transfection efficiency was determined by cotransfection of a plasmid expressing GFP (pHr-GFP). For transfection efficiency of >70 %, the silencing efficiency ranged between 62 and 86 %. nNav1.5 protein and functional expression in MDA-MB-231 cells has been shown previously (Fraser et al. 2005). Here, we extended these studies to MDA-MB-468 cells. nNav1.5 protein expression was detected in the MDA-MB-468 cell line at the expected size of ~200 kDa (Fig. 1c). Functional expression was confirmed in 57 % (26 out of 46) cells tested (Fig. 1d, e). Peak current density was 2.0 ± 0.3 pA/pF (n = 26). Removing the Na+ in the bathing solution completely eliminated the inward current (n = 3; not shown). The inward current was blocked by TTX in a dose-dependent manner (Fig. 1f). The dose dependence was consistent with the VGSC being TTX resistant, as would be expected for nNav1.5 (Fraser et al. 2005). Previously we demonstrated that the non-metastatic BCa cell line (MCF10A) expressed a lower amount of sigma-1 receptor when compared to the metastatic cell line MDA-MB-231 (Aydar et al. 2006).
Expression of sigma-1 receptors and VGSCs in BCa cell lines. a MDA-MB-468, MDA-MB-468 control siRNA, MDA-MB-468 sigma-1 receptor siRNA, MDA-MB-231 and MDA-MB-453. Sizes of molecular weight markers in KDa are shown to the left of the blot. Expected sizes of α-actinin and sigma-1 receptor proteins are indicated to the right of the blot. b Silencing of sigma-1 receptors in MDA-MB-231 cells. Sigma-1 receptor siRNA, sigma-1 receptor control siRNA. Expected sizes of α-actinin and sigma-1 receptor proteins are indicated to the right of the blot. c Expression of nNav1.5 in MDA-MB-468 cells. nNav1.5 probed with a specific antibody to nNav1.5. Sizes of molecular weight markers in KDa are shown to the left of the blot. d Current traces showing activation of the VGSC in MDA-MB-468 cells over the −80 to 0 mV range. For clarity only every second trace is shown. e Current-voltage relationship for the VGSC expressed in the MDA-MB-468 cells (n = 9). f Dose-dependent effect of tetrodotoxin (TTX) on the current expressed in MDA-MB-468 cells (n = 5 for both)
Immunolocalisation
Cellular localisation of nNav1.5 and sigma-1 receptors in MDA-MB-231 cells has been previously reported (Fraser et al. 2005; Palmer et al. 2007). We extended these studies to MDA-MB-468 cells (Fig. 2). The sigma-1 receptor was observed throughout the cytoplasm and at or close to the plasma membrane. nNav1.5 protein also was visualised in the cytoplasm and also at or close to the plasma membrane, although the majority of staining appeared to be the latter. On the other hand, when the cells were not permeabilised, nNav1.5 staining was extremely weak (data not shown). These observations are consistent with the electrophysiology and the nNav1.5 antibody binding to an extracellular domain.
Immunocytochemical localisation and analysis of nNav1.5 and sigma-1 receptor expression in MDA-MB-468 cells. Total magnification is shown to the left of the images. a Permeabilised MDA-MB-468 cells probed with sigma-1 receptor antibody. b Permeabilised MDA-MB-468 cells probed with antibodies to nNav1.5
Proliferation and migration
Although sigma ligands have been reported to affect cellular proliferation, in the vast majority of cases this was likely due to the action of the ligands used having a mixed specificity for both sigma-1 and sigma-2 receptors (Aydar et al. 2004). The effects of sigma-1 drugs on the proliferation of MDA-MB-231 and MDA-MB-468 cells were determined here using agents specific for sigma-1. Cells were treated with SKF10047 or 5-methyl-DMT at concentrations of 50, 20, 5 and 1 μM (Fig. 3). No significant difference was observed between drug-treated cells and untreated controls even after 4 days of incubation. Additionally no change in viability was detected when cells were stained with trypan blue.
An important factor in metastasis is the ability of cells to migrate from the site of the primary tumour to form secondary cancer lesions. The effects of sigma-1 drugs on the migration of MDA-MB-231 and MDA-MB-468 cells were determined. Cells were treated with SKF10047 or 5-methyl-DMT at concentrations of 50, 20, 5 and 1 μM (Fig. 4). No significant difference was observed between sigma-1 drug-treated cells and untreated cells.
Lack of effect of sigma-1 drugs on migration of MDA-MB-468 (a, b) and MDA-MB-231 cells (c, d). Cells were treated with SKF10047 (a, c) or 5-methyl-DMT (b, d) at concentrations of 50, 20, 5 and 1 μM as indicated. Migrated cells were labelled with calcein-AM. Migration was quantified as the fluorescent emission measured at 520 nm
It was concluded that sigma-1 receptors do not play a significant role in the proliferative activities or migration of MDA-MB-231 and MDA-MB-468 cells.
Adhesion
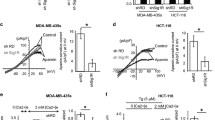
The ability of cancer cells to detach from a primary tumour and subsequently re-attach to other tissues is an important determinant of their metastatic potential. The effects of sigma-1 drugs on adhesion of MDA-MB-231 and MDA-MB-468 cells were thus determined. Cells were treated with SKF10047 or 5-methyl-DMT at concentrations of 50, 20, 5 and 1 μM (Fig. 5). SKF10047 at concentrations of 20 and 50 μM reduced the adhesion of MDA-MB-468 cells by 35 and 40 %, respectively (Fig. 5a; P < 0.05; n = 5). Similarly, treatment with 5-methyl-DMT at concentrations of 20 and 50 μM reduced the adhesion of MDA-MB-468 cells by 46 and 52 %, respectively (Fig. 5b; P < 0.05; n = 5). Similar results were obtained for MDA-MB-231 cells with SKF10047, as reported previously (Fig. 5e, f) (Palmer et al. 2007). Cellular adhesion was determined in MDA-MB-468 cells also following sigma-1 receptor gene silencing. Compared with the control silencing construct, a significant reduction in adhesion of 30 % was observed (P < 0.05; n = 5) (Fig. 5c). Additionally, 50 μM SKF10047 had no effect on the adhesion of sigma-1 receptor silenced MDA-MB-468 cells (Fig. 5c). Similar results were obtained for MDA-MB-231 cells (Fig. 5g). It was concluded that the effect of SKF10047 on cellular adhesion occurred through the sigma-1 receptor and not through some other non-specific mechanism.
Effect of sigma-1 drugs and sigma-1 receptor gene silencing on adhesion of MDA-MB-468 (a–d) and MDA-MB-231 cells (e–h). Cells were treated with SKF10047 (a, e) or 5-methyl-DMT (b, f) at concentrations of 50, 20, 5 and 1 μM, as indicated. Following sigma-1 receptor gene silencing, cellular adhesion was determined in comparison to cells transfected with a control silencing construct (c, g). Additionally, adhesion of control and sigma-1 receptor silenced cells was determined following a 24-h treatment with SKF10047. A comparison of the effect of 10 μM TTX or 50 μM SKF10047 was made (d, h). Experiments with a significant difference from the control treatment are marked with an asterisk (P < 0.05). Adhesion was quantified as the fluorescence emission measured at 520 nm
The effects of SKF10047 on cellular adhesion were also studied in relation to VGSC activity (Fig. 5d, h). For the MDA-MB-468 cell line, 20 μM SKF10047 elicited 45 % reduction in adhesion while 10 μM TTX elicited a 62 % reduction. For the MDA-MB-231 cell line, similar concentrations of SKF10047 and TTX elicited 37 and 55 % reduction in adhesion, respectively. These effects were statistically significant (P < 0.05, n = 6) and raised the possibility that VGSC activity could be involved in the action of SKF10047 since it is clear that the reduction in adhesion seen by application of SKF10047 was in theory able to be reduced further (e.g., by sigma-1 SiRNA). Given that application of TTX reduces the adhesion to a greater extent than SKF10047, this gives weight to our theory that the sigma-1 receptor is acting through modulation of nNav1.5 since dual application of SKF10047 and sigma-1 receptor SiRNA did not elicit a significantly greater reduction in adhesion compared to single treatments.
Sigma-1—VGSC (nNav1.5) interaction
Sigma-1 receptors have been demonstrated to modulate Nav1.5 channels in cardiomyocyes or when heterologously coexpressed in HEK-293 cells (Johannessen et al. 2011). In an initial attempt to determine whether the effect of sigma-1 receptor drugs on the adhesion of MDA-MB-231 and MDA-MB-468 cells involved VGSC activity, we performed a number of experiments with a combination of treatments with SKF10047 and the polyclonal antibody to nNav1.5 which is known to be a channel blocker (Chioni et al. 2005). Thus, the ability of the “blocking” polyclonal antibody to nNav1.5 to reduce the adhesion of MDA-MB-231 and MDA-MB-468 cells was first determined (Fig. 6). Cells were incubated with 100, 20, 5, 1 and 0.2 ng/ml of antibody for 24 h and then cellular adhesion was determined. Significant, dose-dependent reduction in adhesion was observed for MDA-MB-468 cells with 100, 20 and 5 ng/ml antibody causing a reduction of 45, 40 and 29 %, respectively (P < 0.05 for all; n = 5; Fig. 6a). Incubation of cells with a control IgG antibody at the same concentrations had no effect (Fig. 6a). Additionally, preincubation of the nNav1.5 polyclonal antibody with an excess of the immunisation peptide blocked the effect of the antibody (Fig. 6a). The effect on adhesion of combination treatments with SKF10047 (50 μM) and nNav1.5 antibody (20 ng/ml) was also determined. No significant difference was observed in the adhesiveness of MDA-MB-468 cells treated with SKF10047 or nNav1.5 antibody alone or a combination of both (Fig. 6c). Finally, silencing the sigma-1 receptor in MDA-MB-468 cells also reduced the adhesion and application of nNav1.5 antibody (20 ng/ml) to the cells had no further effect (Fig. 6e). Comparable results were obtained for MDA-MB-231 cells (Fig. 6d, f). These results were consistent with nNa1.5 playing a significant role in the mechanism of action of the sigma-1 receptor.
Combination treatments with sigma-1 drugs and nNav1.5 blocking antibody on adhesion of MDA-MB-468 (a, c, e) and MDA-MB-231 cells (b, d, f). The ability of a polyclonal antibody to nNav1.5 to reduce the adhesion of MDA-MB-468 and MDA-MB-231 cells was determined (a, b). Cells were incubated with 100, 20, 5, 1 and 0.2 ng/ml of nNav1.5 polyclonal antibody (n = 5). Control-a (Con-a) consisted of 100 ng/ul of rabbit IgG in PBS containing azide. Control-b (Con-b) consisted of 20 ng/ml of nNav1.5 antibody pre-incubated with 100 ug/ml of nNav1.5 peptide for 1 h at room temperature. The effect on adhesion on a combination of treatments with SKF10047 (50 μM) and nNav1.5 polyclonal antibodies (20 ng/ml) was determined (c, d). The effect on adhesion on a combination of sigma-1 receptor gene silencing and nNav1.5 polyclonal antibodies (20 ng/ml) was determined (e, f). Experiments with a significant difference from the respective control treatment are marked with an asterisk (P < 0.05). Adhesion was quantified as the fluorescence emission measured at 520 nm
We then demonstrated that nNav1.5 is in a complex with the sigma-1 receptor in both MDA-MB-231 and MDA-MB-468 cells (Fig. 7a, b). Immunoprecipitation was utilised, protein extracts were prepared from the two cell lines and a mouse anti-Nav1.5 antibody was used to pull down the Nav1.5 protein and any proteins in a complex with the Nav1.5 protein. The immuneprecipitated complexes were separated by SDS-PAGE and transferred by Western blot to nitrocellulose membranes. The blots were probed with antibodies to the sigma-1 receptor (produced in rabbits). Following development bands at the expected size of the sigma-1 were visualised indicating the Nav1.5 in these cells is in a complex with the sigma-1 receptor.
Immunoprecipitation of the sigma-1 receptor by nNav1.5 and effect of SKF10047 and sigma-1 gene silencing on surface expression of nNav1.5 in the MDA-MB-468 (a, c) and MDA-MB-231 cell line (b, d). a, b Cells were grown for 48 h and protein was extracted as detailed in the methods. Protein extracts were mixed with an antibody to Nav1.5 (anti-mouse Nav1.5) for 2 h. Subsequently Sepharose beads with protein A attached were added for 2 h. The beads were sedimented and washed three times. Subsequently, the beads were resuspended in SDS-sample loading buffer and heated at 95 °C for 5 min. The samples were separated on polyacrylamide gel electrophoresis and the proteins were transferred to nitrocellulose membranes and probed with an antibody to the sigma-1 receptor (anti-rabbit Sigma-1 receptor). Lanes Control reactions without a Nav1.5 immunoprecipitation antibody; 3 μl of immunoprecipitation extract; 10 μl of immunoprecipitation extract; whole cell extract; immunoprecipitation with beads without protein A. Sizes of molecular weight markers in KDa are shown to the right of the blots. c, d Cells were seeded into 24-well dishes coated with poly-l-lysine. Cells were treated with 50 uM SKF10047 for 24 h. Subsequently, the cells were stained for surface expression of nNav1.5 followed by an anti-rabbit IgG antibody labelled with Texas Red. The results were quantified in a fluorescence plate reader (n = 5). Following the assay cells were labelled with calcein-AM in order to verify that each dish contained equivalent numbers of cells. A similar experiment was performed for cells transfected with a sigma-1 receptor silencing construct for 48 h compared to those cells transfected with a control plasmid
In addition, the effects of SKF10047 and sigma-1 receptor gene silencing on the surface expression of nNav1.5 were determined (Fig. 7c, d). Treatment of MDA-MB-468 and MDA-MB-231 cells with 50 μM SKF10047 for 24 h caused a 40 and 31 % reduction in the plasma membrane expression of nNav1.5 protein, respectively. For 50 μM 5-methyl-DMT, there was 52 and 44 % reduction in expression, respectively. Similar results were obtained from sigma-1 receptor-silenced cells. Thus, a reduction of 46 and 47 % in the cell-surface expression of nNav1.5 protein was observed upon sigma-1 silencing in MDA-MB-468 and MDA-MB-231 cells, respectively. Finally, treatment of sigma-1-silenced cells with SKF10047 did not produce any further effect. It was concluded that sigma-1 receptor modulated (up-regulated) the plasma membrane expression of nNav1.5 in BCa cells.
Discussion
This article attempts to tie together several pieces of experimental evidence and provide a plausible explanation for the mechanism through which sigma-1 receptor drugs are able to alter the metastatic behaviour of BCa cells. It has been established by many researchers that sigma-1 receptors are able to modulate ion channels in a variety of cell types (Aydar et al. 2002; Lupardus et al. 2000; Johannessen et al. 2011). In particular, the sigma-1 receptor was found to regulate the current density of VGSCs in MDA-MB-231 cells (Balasuriya et al. 2012). Here, we have demonstrated that modulation of sigma-1 receptors in BCa cell lines can partially affect their metastatic behaviour (with respect to adhesiveness) and that this effect could be mediated via functional nNav1.5 expression. It is now well established that VGSC (nNav1.5) up-regulation occurs in metastatic BCa cells and this promotes the cells metastatic behaviour in vitro and in vivo (Fraser et al. 2005; Brackenbury et al. 2007; Nelson et al. 2015). Although the exact mechanism of sigma-1 receptor modulation of ion channels is not clear, it has been suggested that sigma-1 receptors form a complex with VGSCs (Balasuriya et al. 2012). Given these facts, our hypotheses are (1) that sigma-1 receptors form a functional signalling complex with VGSCs in BCa cell lines and (2) that, through this complex, sigma-1 receptors are able to modulate VGSC plasma membrane expression and hence the cells’ metastatic behaviour.
We have previously reported on the expression and activity of sigma-1 receptors in MDA-MB-231 cells (Palmer et al. 2007). We now provide evidence for the expression of sigma-1 receptors in two further BCa cell lines: MDA-MB-468 and MDA-MB-453. Further to this, in MDA-MB-468 cells, sigma-1 receptor protein expression was found to be localised throughout the cell, except within the nucleus, consistent with previous studies on MDA-MB-231 cells (Palmer et al. 2007). In addition, functional expression of nNav1.5 channels in MDA-MB-231 cells has been reported (Fraser et al. 2005). We now provide both immunocytochemical and electrophysiological evidence that the MDA-MB-468 cell line (when grown in 5 % FBS) also expresses a TTX-resistant VGSC, most likely to be nNav1.5.
Two drugs (SKF1047 and 5-methyl-DMT) with significant selectivity for sigma-1 receptors over sigma-2 receptors showed no observable effect on the proliferation of the MDA-MB-231 and MDA-MB-468 cells. These results are consistent with earlier suggestions that many of the anti-proliferative effects of sigma drugs are due to binding to the sigma-2 receptor (Aydar et al. 2004). Both drugs also showed no effect on the migration of MDA-MB-231 and MDA-MB-468 cells suggesting that sigma-1 receptors do not play a significant role in cellular motility. This result was rather surprising and would require further exploration in light of the fact that 4-IBP, another sigma-1 receptor agonist, was found to decrease the migration of glioblastoma cells (Megalizzi et al. 2007). Previously, SKF10047 was found to promote the detachment of MDA-MB-231 cells plated on collagen in a ‘single cell’ adhesion assay (Palmer et al. 2007, 2008). In the present study, a florescence-based ‘population’ adhesion assay was used that measured the ability of cells to attach to collagen-coated plates. The results showed both SKF10047 and DMT as well as sigma-1 receptor silencing significantly reduced adhesion of both BCa cell lines. In addition, application of SKF10047 to cells with sigma-1 receptor silenced did not produce any effect. This suggested strongly that the sigma-1 receptor controlled the adhesiveness. The VGSC blocker TTX and a polyclonal antibody blocking nNav1.5 activity also reduced the adhesion of both BCa cell lines used. Systematic combination treatments involving TTX, nNav1.5 antibody, SKF10047 and sigma-1 receptor gene silencing suggested consistently that the sigma-1 modulation of adhesion occurred through the VGSC pathway. Possible interaction between nNav1.5 and sigma-1 receptor was investigated. Immunoprecipitation showed for both MDA-MB-231 and MDA-MB-468 cell lines that nNav1.5 and sigma-1 receptor were in a protein complex. This is in agreement with past findings using atomic force microscopy showing that sigma-1 receptors and Nav1.5 form a complex with a fourfold symmetry (Balasuriya et al. 2012). It is not yet known whether the interaction between sigma-1 receptor and nNav1.5 is direct (i.e., protein–protein) or indirect (e.g., involving a small intermediary molecule). Neverthelss, one consequence of this association appeared to be promotion of plasma membrane expression of nNav1.5 and adhesion to the substrate. Interestingly, both such effects are known to be controlled by the VGSC beta subunit(s) (Chioni et al. 2009).
Conclusions and future perspectives
We have found significant evidence that sigma-1 receptors are expressed in a number of metastatic BCa cell lines and that in both MDA-MB-231 and MDA-MB468 cells sigma-1 receptor increases cellular adhesion to the substrate through a functional interaction with nNav1.5 channels. This appears to involve a close physical interaction between sigma-1 receptors, possibly in macromolecular complex within the cells. Our data add weight to the model in which the sigma-1 receptor is “silent” under normal physiological conditions, whereas in disease, sigma-1 receptors behave as a “chaperone” that binds and traffics client protein(s) so as to modulate the pathophysiology (Su et al. 2010). In the case of MDA-MB-231 and MDA-MB-468 cells, the sigma-1 receptor translocates nNav1.5 protein to the plasma membrane and reduces adhesion, thereby enhancing the metastatic potential. These effects are consistent with the interaction occurring early in metastasis and, indeed, House et al. (2010) have shown that VGSC expression is upstream of a network of genes controlling the invasiveness in colon cancer. Studies of Kv11.1 (hERG) and sigma-1 receptor are also consistent with the idea of sigma-1 receptor behaving either like a chaperone and/or a channel regulatory auxillary subunit through protein–protein interaction (Crottes et al. 2011; Kinoshita et al. 2012). This hypothesis was further strengthened by a recent report showing that cocaine exposure induces a persistent protein–protein association between the sigma-1 receptor and Kv1.2 channel. This phenomenon was associated with a redistribution of both proteins from the intracellular compartments to the plasma membrane (Kourrich et al. 2013).
The significant involvement of the sigma-1 receptor in VGSC expression/activity may have wide-reaching clinical implications since VGSCs, and in particular nNav1.5, have already been proposed as a novel anti-metastatic target in a range of cancers (Fraser et al. 2014; Onkal and Djamgoz 2009; Djamgoz and Onkal 2013).
References
Aydar E, Palmer CP, Klyachko VA, Jackson MB (2002) The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron 34:399–410
Aydar E, Palmer CP, Djamgoz MB (2004) Sigma receptors and cancer: possible involvement of ion channels. Cancer Res 64:5029–5035
Aydar E, Onganer P, Perrett R, Djamgoz MB, Palmer CP (2006) The expression and functional characterization of sigma (sigma) 1 receptors in breast cancer cell lines. Cancer Lett 242:245–257
Balasuriya D, Stewart AP, Crottes D, Borgese F, Soriani O, Edwardson JM (2012) The sigma-1 receptor binds to the Nav1.5 voltage-gated Na+ channel with 4-fold symmetry. J Biol Chem 287:37021–37029
Brackenbury WJ, Chioni AM, Diss JK, Djamgoz MB (2007) The neonatal splice variant of Nav1.5 potentiates in vitro invasive behaviour of MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat 101:149–160
Campbell TM, Main MJ, Fitzgerald EM (2013) Functional expression of the voltage-gated Na+-channel Nav1.7 is necessary for EGF-mediated invasion in human non-small cell lung cancer cells. J Cell Sci 126:4939–4949
Chioni A-M, Fraser SP, Foran P, Wilkin GP, Diss JKJ, Djamgoz MBA (2005) A novel polyclonal antibody specific for the Nav1.5 voltage-gated Na+ channel ‘neonatal’ splice form. J Neurosci Methods 147:88–98
Chioni AM, Brackenbury WJ, Calhoun JD, Isom LL, Djamgoz MB (2009) A novel adhesion molecule in human breast cancer cells: voltage-gated Na+ channel beta1 subunit. Int J Biochem Cell Biol 41:1216–122710
Crottes D, Martial S, Rapetti-Mauss R, Pisani DF, Loriol C, Pellissier B et al (2011) Sig1R protein regulates hERG channel expression through a post-translational mechanism in leukemic cells. J Biol Chem 286:27947–27958
Diaz D, Delgadillo DM, Hernandez-Gallegos E, Ramirez-Dominguez ME, Hinojosa LM, Ortiz CS, Berumen J, Camacho J, Gomora JC (2007) Functional expression of voltage-gated sodium channels in primary cultures of human cervical cancer. J Cell Physiol 210:469–478
Djamgoz MB, Onkal R (2013) Persistent current blockers of voltage-gated sodium channels: a clinical opportunity for controlling metastatic disease. Recent Pat Anticancer Drug Discov 8:66–84
Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE (2009) The hallucinogen N, N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 323:934–937
Fraser SP, Diss JKJ, Chioni A-M, Mycielska ME, Pan H, Yamaci RF et al (2005) Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res 11:5381–5389
Fraser SP, Özerlat-Gunduz I, Brackenbury WJ, Fitzgerald EM, Campbell T, Coombes RC, Djamgoz MBA (2014) Regulation of voltage-gated sodium channel expression in cancer: hormones, growth factors and auto-regulation. Philos Trans R Soc Lond B Biol Sci 369:20130105
Grimes JA, Fraser SP, Stephens GJ, Downing JEG, Laniado ME, Foster CS et al (1995) Differential expression of voltage-activated Na+ currents in two prostatic tumour cell lines: contribution to invasiveness in vitro. FEBS Lett 369:290–294
House CD, Vaske CJ, Schwartz AM, Obias V, Frank B, Luu T et al (2010) Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res 70:6957–6967
Johannessen M, Fontanilla D, Mavlyutov T, Ruoho AE, Jackson MB (2011) Antagonist action of progesterone at sigma-receptors in the modulation of voltage-gated sodium channels. Am J Physiol Cell Physiol 300:C328–C337
Kinoshita M, Matsuoka Y, Suzuki T, Mirrielees J, Yang J (2012) Sigma-1 receptor alters the kinetics of Kv1.3 voltage gated potassium channels but not the sensitivity to receptor ligands. Brain Res 1452:1–9
Kourrich S, Hayashi T, Chuang JY, Tsai SY, Su TP, Bonci A (2013) Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell 152:236–247
Laniado ME, Lalani E-N, Fraser SP, Grimes JA, Bhangal G, Djamgoz MBA et al (1997) Expression and functional analysis of voltage-activated Na+ channels in human prostate cancer cell lines and their contribution to invasiveness in vitro. Am J Pathol 150:1213–1221
Laniado ME, Fraser SP, Djamgoz MBA (2001) Voltage-gated K+ channel activity in human prostate cancer cell lines of markedly different metastatic potential: distinguishing characteristics of PC-3 and LNCaP cells. Prostate 46:262–274
Lupardus PJ, Wilke RA, Aydar E, Palmer CP, Chen Y, Ruoho AE, Jackson MB (2000) Membrane-delimited coupling between sigma receptors and K+ channels in rat neurohypophysial terminals requires neither G-protein nor ATP. J Physiol 526(Pt 3):527–539
Maurice T, Su TP (2009) The pharmacology of sigma-1 receptors. Pharmacol Ther 124:195–206
Mavlyutov TA, Epstein ML, Liu P, Verbny YI, Ziskind-Conhaim L, Ruoho AE (2012) Development of the sigma-1 receptor in C-terminals of motoneurons and colocalization with the N, N’-dimethyltryptamine forming enzyme, indole-N-methyl transferase. Neuroscience 206:60–68
Megalizzi V, Mathieu V, Mijatovicz T, Gailly P, Debeir O, De Nevez N et al (2007) 4-IBP, a σ1 receptor agonist, decreases the migration of human cancer cells, including glioblastoma cells, in vitro and sensitizes them in vitro and in vivo to cytotoxic insults of proapoptotic and proautophagic drugs. Neoplasia 9:358–369
Nakajima T et al (2009) Eicosapentaenoic acid inhibits voltage-gated sodium channels and invasiveness in prostate cancer cells. Br J Pharmacol 56:420–431
Nelson M, Yang M, Dowle AA, Thomas JR, Brackenbury WJ (2015) The sodium channel-blocking antiepileptic drug phenytoin inhibits breast tumour growth and metastasis. Mol Cancer 14:13
Onganer PU, Djamgoz MBA (2005) Small-cell lung cancer (human): potentiation of endocytic membrane activity by voltage-gated Na+ channel expression in vitro. J Membr Biol 204:67–75
Onkal R, Djamgoz MBA (2009) Molecular pharmacology of voltage-gated sodium channel expression in metastatic disease: clinical potential of neonatal Nav1.5 in breast cancer. Eur J Pharmacol 625:206–219
Palmer CP, Mahen R, Schnell E, Djamgoz MB, Aydar E (2007) Sigma-1 receptors bind cholesterol and remodel lipid rafts in breast cancer cell lines. Cancer Res 67:11166–11175
Palmer CP, Mycielska ME, Burcu H, Osman K, Collins T, Beckerman R, Perrett R, Johnson H, Aydar E, Djamgoz MB (2008) Single cell adhesion measuring apparatus (SCAMA): application to cancer cell lines of different metastatic potential and voltage-gated Na+ channel expression. Eur Biophys J 37:359–368
Ruscher K, Shamloo M, Rickhag M, Ladunga I, Soriano L, Gisselsson L, Toresson H, Ruslim-Litrus L, Oksenberg D, Urfer R, Johansson BB, Nikolich K, Wieloch T (2011) The sigma-1 receptor enhances brain plasticity and functional recovery after experimental stroke. Brain 134:732–746
Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE (2010) The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci 31:557–566
Tsai S-Y, Hayashi T, Mori T, Su T-P (2009) Sigma-1 receptor chaperones and diseases. Cent Nerv Syst Agents Med Chem 9:184–189
Author information
Authors and Affiliations
Corresponding author
Additional information
Special Issue: Ion Channels, Transporters and Cancer.
Rights and permissions
About this article
Cite this article
Aydar, E., Stratton, D., Fraser, S.P. et al. Sigma-1 receptors modulate neonatal Nav1.5 ion channels in breast cancer cell lines. Eur Biophys J 45, 671–683 (2016). https://doi.org/10.1007/s00249-016-1135-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-016-1135-0