Abstract
Black pepper is a source of effective antioxidants. It contains several powerful antioxidants and is thus one of the most important spices for preventing and curtailing oxidative stress. There is considerable interest in the development of a drug-delivery systems that would result in the selective delivery of antioxidants to tissues in sufficient concentrations to ameliorate oxidant-induced tissue injuries. Liposomes are biocompatible, biodegradable and nontoxic artificial phospholipid vesicles that offer the possibility of carrying hydrophilic, hydrophobic and amphiphilic molecules. This article focuses on the use of liposomes for the delivery of antioxidants in the prevention or treatment of pathological conditions related to oxidative stress. Liposome formulations of piperine were analyzed with various spectroscopic methods. The formulation with the highest entrapment efficiency (90.5 %) was formulated with an L-α-phosphatidylcholine dipalmitoyl (DPPC):piperine, 30:1 molar ratio, and total lipid count of 19.47 mg/ml in the final liposomal preparation. The liposome formulation was found to be stable after storage at 4 °C, protected from light, for a minimum of 3 weeks. The incremental process of piperine penetration through the phospholipid membrane was analyzed using the FT-IR, UV-Vis and NMR methods. Temperature stability studies carried out at 37 °C showed the highest percentage of piperine release in the first 3 h of incubation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many food ingredients contain unsaturated fatty acids that are quite susceptible to quality deterioration, especially under oxidative stress. For this reason, efforts to reduce oxidation have increased. Most often, the best strategy is the addition of antioxidants. According to recent developments, free radicals are involved in many diseases (Neeraj et al. 2013). Also, different free radicals produced in the body attack membranes, causing the oxidation of lipids, reduction of the fluidity of biomembranes, loss of different enzyme activities and loss of receptor activities. They also cause cell inactivation by damaging the proteins present in the membranes. Some free radicals are the causal agent of cancer. First, the free radicals attack cell DNA. The attacked DNA causes mutational changes, which finally induce cancer (Valko et al. 2007; Flora 2007; Haulica et al. 2000; Gorrini et al. 2013). Due to such diseases, natural antioxidants, such piperine, eugenol, thymol, resveratrol and many others, are of interest to scientists in order to cure cellular degeneration (Brewer 2011; Zarai et al. 2013; Ahmad et al. 2012). Piper nigrum L. (Black pepper, P. nigrum) is considered the king of spices throughout the world. It contains lignans, alkaloids, flavonoids, aromatic compounds and amides. It also contains essential oil up to 3.5 %, and this oil is constituted of sabinene, pinene, phellandrene, linalool and limonene. It also has piperine, which is a weakly basic substance. Chavicine is an isomer of piperine (Upadhyay et al. 2013). Piperine and chavicine are not responsible for the aroma of black pepper, but piperine imparts its pungency. Piper nigrum L. is a member of the Piperaceae family. The piper genus includes more than 1000 species, but the most well known are P. nigrum, P. longum and P. betle—51 cultivars of P. nigrum have been reported from tropical and subtropical regions of India (Ahmad et al. 2012). P. nigrum fruits are also used to produce white and green peppers and are valued because of the presence of piperine, including its different isomers (Namjoyan and Hejazi 2012). Black pepper can be used for different purposes, e.g., in human diets, as medicine, as a preservative and as a biocontrol agent (Ahmad et al. 2012; Ulbricht et al. 2008; Hu et al. 2005). This plant and its active component piperine can stimulate the digestive enzymes of the pancreas and intestines and also increase biliary bile acid secretion when orally administrated (Ulbricht et al. 2008). Some reports have demonstrated that black pepper consumption in humans increases the orocecal transit time (Vazquez-Olivencia et al. 1992). Piperine prevents and minimizes diarrhea produced by various oils and chemicals and also reduces intestinal fluid accumulation in mouse intestines (Vasavirama and Upender 2014). P. nigrum and its active derivatives, especially peppercorn extract, have been reported to inhibit tumor formation in experimental models (Brewer 2011 ; Hirokawa et al. 2006). Such antitumor activity, to a lesser extent, was also reported as a consequence of oral administration (La et al. 2012). The alcoholic extract of peppercorn and piperine was effective in immunomodulatory and antitumor activity and Dalton’s lymphoma (Damanhouri and Ahmad 2014). Appiah et al. (2009) observed that piperine is involved in antimetastatic activity. In their experiment they documented that in mouse models, when exposed to melanoma cells (B16F-10), the active agent piperine prevented and inhibited lung metastasis and finally concluded that piperine dramatically reduced tumor nodule formation. Moreover, piperine from P. nigrum reduced lung cancer by modulating lipid peroxidation and the activation of antioxidative protection enzymes (Pradeep and Kuttan 2002).
Liposomes are spherical vesicles whose membranes consist of one (unilamellar) or more (oligolamellar, multilamellar) bilayers (Sharma and Sharma 1997). They can range in size from around 25 nm to more than 1000 nm in diameter (Smith and Smith 2012). The polar heads of phospholipids are hydrophilic, and they are aligned with and face the liquid exterior and interior of the liposome. The hydrophobic regions (tails) of the phospholipid molecules are aligned inside the lipid membrane. Water-soluble drugs can be enclosed by the aqueous center of the liposome, whereas lipid-soluble drugs are incorporated into the lipid bilayer of the liposome. Many drugs are insoluble and need to be dissolved in a solvent before they can be administered. Incorporating the insoluble drug into liposomes obviates the necessity for a solvent, and it allows the liposomal drug to be administered over a shorter period without triggering a toxic reaction (Smith and Smith 2012).
The understanding of drug-liposome interactions at the molecular level is not an easy task because, depending on their hydrolipophilic characteristics, drugs can interact with either the polar headgroups or the hydrophobic hydrocarbon chains of the lipid bilayer constituents, or both parts of the membrane, and specific changes in the liposome morphology or drug conformation can occur (Rodrigues and Gameiro 2003).
Novel microcarrier technologies supporting targeted drug delivery to tissues are needed to improve the therapeutic index of the carried drugs. In 1974, Gregoriadis et al. suggested liposomes as drug carriers in chemotherapy (Gregoriadis et al. 1974). Since then, the interest in liposomes has gradually increased, and liposome systems are extensively studied as drug carriers. Three basic requirements have to be met for liposomes to become effective targeted drug delivery systems: (1) prolonged blood circulation, (2) sufficient tissue accumulation and (3) controlled drug release and uptake by tumor cells with a release profile matching the pharmacodynamics of the drug (Andresen and Jensen 2005).
The physicochemical properties of the lipids composing the liposomes, such as membrane fluidity, surface charge, permeability and size, determine the interactions of liposomes with blood components and other tissues after systemic administration (Sharma and Sharma 1997).
Vesicle size and composition are critical parameters for determining the circulation half-life of liposomes. Size influences the degree of drug encapsulation in liposomes. The geometry, size and properties of liposomes in an aqueous environment have to be described to enable potential applications of liposome systems as drug carriers.
In pathological conditions, rapidly growing solid tumors are surrounded by angiogenic blood vessels, which are abnormally constructed with large vascular fenestrae and compromised lymphatic drainage. The liposomes and other nanoparticles up to 200 nm in size penetrate the tumor endothelia and extravasate into the tumor interstitium, whereas penetration through regular, healthy vasculature is limited to species <1–2 nm in size (Andresen and Jensen 2005).
The present work is intended to elucidate the penetration of piperine through liposomal membranes as a result of rising temperatures. The temporal and temperature stability of liposomal piperine in a sodium phosphate buffer at pH 7.0 was characterized by NMR, UV and FT-IR methods. In comparison with free piperine, the temporal stability of liposomal piperine was evaluated at 37 °C. The influence of temperature on the stability of encapsulated piperine was studied as temperature increased from 20 to 47 °C, crossing the three phases of phospholipids (tilted gel L β′, rippled gel P β′ and liquid-crystalline L ∝ phases).
Materials and methods
Materials
L-α-phosphatidylcholine dipalmitoyl (1,2-dihexadecanoyl-sn-glycerol-3-phosphocholine) (DPPC, purity 99 %), piperine (97 %) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich, Schnelldorf, Germany. Chloroform, dichloromethane, hydrochloric acid, phosphate-buffered saline (PBS buffer pH 7.0: K2HPO4, NaH2PO4) and ethanol were supplied by POCH, Gliwice, Poland. Deuterium oxide (D2O) 99 %, dimethyl sulfoxide-d 6 99.96 atom % D, chloroform-d 99 %, stab. with Ag and sodium 4,4-dimethyl-4-silapentane sulfonate (DSS) were purchased from ARMAR Chemicals, Döttingen, Switzerland.
Methods
Liposome preparation
Small liposomes (DPPC, DPPC/piperine) with a diameter of 100–120 nm were obtained using the modified reverse-phase evaporation method (mREV) (Pentak 2014) by applying 2 ml buffer (PBS buffer with pH 7.0) and 4 ml organic solution prepared from methylene chloride and chloroform. A DPPC:drug molar ratio 30:1 was used; 0.18 ml piperine (1.42 mg/ml in CDCl3) was added to the preparation mixture. The concentration of the lipid in the sample was 2.64 × 10−2 M. The preparation process was carried out at 20 °C. The average time of liposome preparation did not exceed 12 min. For NMR and FT-IR measurements deuterated solvents were applied. Liposome entrapped piperine was separated from free piperine by dialysis in Float-A-Lyzer G2 (Spectra/Por) tubing with several buffer changes at 4 °C.
NMR measurements of liposomes
1H NMR spectra and T1 measurements were obtained using the 9.4-T Bruker Avance UltraShield (400.130 MHz for 1H) (Karlsruhe, Germany) with the use of a 5-mm inverse broadband probe (BBI). Water suppression for T1 measurements using a 3–9–19 pulse sequence with gradients was used. The peak integrals were fitted to the following function: I[t] = I[0] + P* exp(−t/T1) (Bruker uxnmrt1 fitting function).
1H NMR spectra were recorded at a temperature range of 25–47 °C. Two-dimensional NOE or Nuclear Overhauser Effect SpectroscopY (NOESY) experiments were obtained using the 11.74-T Bruker Ascend UltraShield (500.180 MHz for 1H) (Karlsruhe, Germany) using a 5-mm multinuclear broadband probe (PABBO BB/19F-1H/D Z-GRD) carried out using 500-ms mixing times for the detection of build-up NOEs. The 2D NOE spectra were recorded at 25 °C. Sample temperature was controlled by air and monitored by the Bruker thermal control system. The samples were heated at a rate of up to 1.0 °C min−1 and were left for approximately 15 min to achieve equilibrium, which was monitored based on the free induction decay (FID) signal. The temperature was maintained at ± 0.1 °C. Water suppression was obtained by presaturation. For 1H NMR spectra 32 transients were accumulated with a 1H pulse length of 10.70 µs and 4-s relaxation delay, 2.044-s acquisition time, 32,768 date points and 0.30-Hz line broadening. 1H chemical shift values were referred to DSS (for DPPC/piperine) or TMS (for piperine solution in chloroform) as an external reference, respectively. The spectra were processed using TopSpin 3.1 (Bruker, Karlsruhe) software. Apparatus error was ±0.001 ppm.
FT-IR measurements
Fourier transform infrared spectra were obtained using a PerkinElmer Spectrum One FT-IR spectrometer (Waltham, MA) equipped with an IF KT-3 (Krakow, Poland) automatic temperature controller. Spectra were recorded for both dried and fully hydrated samples of liposomes. For the dried samples, spectra were recorded at room temperature. The lipid dispersions were placed in a demountable cell between two ZnSe windows separated by a 50-µm-thick Teflon spacer. For temperature regulation, the cell was placed in a thermostatized jacket with internal temperature measurement. An external water bath was used for temperature control. The temperature was maintained at ± 0.1 °C. All the spectra were acquired after equilibration of the liposome samples for 15 min at each desired temperature ranging from 20 to 47 °C using an automatic temperature controller. Data acquisition was performed with 1–3 °C intervals. Usually ten interferograms were collected, Fourier transformed and averaged. Spectra in the region 4000–400 cm−1, at a resolution of one data point per 2 cm−1, were obtained. Moreover, the FT-IR spectrum of buffer was also obtained under identical instrumental conditions. Peak positions were determined with one significant digit with Spectrum v3.01 spectral processing software.
UV measurements
UV spectra of piperine alone and with liposomes were recorded at λ max 340 nm with the Lambda Bio 40 spectrometer (Perkin Elmer, Walthman, MA) equipped with a PTP-1 Peltier System (PerkinElmer, Walthman, MA) automatic temperature controller. The temperature was maintained at ±0.1 °C. All spectra were acquired after equilibration of the liposome samples for 15 min at each desired temperature ranging from 20 to 47 °C using an automatic temperature controller. The spectral analysis was performed with UV WinLab PerkinElmer software.
Measurement of encapsulation efficiency of liposomal piperine
Immediately after preparing the liposomal piperine, the sample was dialyzed in order to separate the encapsulated piperine from its unencapsulated form. For separating the unencapsulated molecules of piperine from liposomes, 4 ml of liposomal piperine dispersion was placed in a Float-A-Lyzer G2 (Spectra/Por) dialysis tube of cellulose ester (Spectra/Por®, MWCO 8000–10,000 Daltons, Spectrum, Canada), which was then immersed in 50 ml buffer at 4 °C and magnetically stirred at 200 rpm. Samples taken from the recipient solution at predetermined times were replaced with equal volumes of fresh buffer and spectrometrically assayed at 340 nm for the piperine content. The mass of encapsulated piperine was analyzed according to the piperine standard curve through the measurement of maximum absorption of piperine at 340 nm by a UV-Vis spectrometer. The encapsulation efficiency of liposomal piperine can be calculated from the ratio of the amount of encapsulated piperine to that of the initial amount added.
Stability measurements of liposomal piperine
The stability of liposomal piperine at 37 °C in a PBS buffer of pH 7.0 was investigated by recording the absorption spectrum of encapsulated piperine for 7 h on a UV-Vis spectrometer. The stability of free piperine was also tested for 7 h at 37 °C for comparison. The stability of piperine-free (blank) liposomes was investigated as a control (Supplementary Fig. I).
In the study of the effect of temperature on the stability of encapsulated piperine, the maximum absorption at 340 nm of liposomal piperine was recorded at a temperature range of 20–47 °C. The sample was kept at the specific temperature for 15 min before each measurement.
For piperine release from liposomes, in a 3-week period, the study was carried out by comparing to values for the freshly prepared liposome formulations. The piperine release measurements were performed according to the method reported by Jin and Hou (2005). Release of encapsulated piperine can be given as:
Here, R is the release percentage; the subscripts of [P] (piperine), such as f, f0 and t, represent the free piperine after release, initial free piperine before release and total piperine, respectively. In Eq. (1), [P]t was equal to [P]t0/β, where [P]t0 is the concentration of total piperine in the original liposomes, and β is the dilution folds (β = 40). The encapsulation fraction (E) represented the ratio of encapsulated piperine to total piperine.
DPPH scavenging activity measurements of liposomal piperine
The radical scavenging activities of liposomal piperine and free piperine were examined according to the DPPH method (Zarai et al. 2013; Brand-Williams et al. 1995; Kerdudo et al. 2014). DPPH has a characteristic maximum absorption at 517 nm. A 0.3 mM DPPH solution was prepared in ethanol. Piperine was dissolved in ethanol and then diluted in water to obtain a final piperine concentration of 5.38 µg/ml; 0.5 ml of the DPPH solution was then added to 3.0 ml of the piperine solution. For the studies on liposomes, 0.5 ml of DPPH (0.3 mM) was mixed with 3.0 ml of liposomal dispersion. DPPH activity was measured at 517 nm for both systems (the piperine solution and liposomal piperine) at 37 °C. The results are expressed as the percentage of DPPH scavenging activity, calculated according to the following formula (2) (Niu et al. 2012a):
For each measurement, a blank with solvents only was used. All tests were carried out in triplicate.
Results and discussion
Analysis of the mechanisms of the molecular interactions between piperine and DPPC liposome
The FT-IR technique was applied with the aim of understanding the mechanisms of the molecular interactions between piperine molecules and DPPC liposome. The most significant frequencies of piperine, blank DPPC liposome and liposomal piperine in the KBr disc are shown in Table 1.
The shape of the absorption band is temperature dependent. The broad band centered at ~3400 cm−1 represents O–H stretching in water molecules associated with the membranes via hydrogen bonding. After the incorporation of piperine into DPPC liposome, the % of transmittance of this band gradually decreases along with the rise in temperature. This band also becomes broader. This effect can be related to hydrogen bond formation between piperine and DPPC molecules. The principal band between 2800 and 3000 cm−1 represents the C–H stretching modes with the maxima of peaks at 2850 and 2918 cm−1, corresponding to symmetric and antisymmetric stretching in the CH2 groups of alkyl chains, respectively, with a minor contribution from symmetric and antisymmetric stretching vibration in the CH3 groups at ~2873 and ~2957 cm−1, respectively.
The main differences between the FT-IR spectra of the DPPC liposome and DPPC/piperine liposome obtained in a temperature range of 20–47 °C involve the bands in the regions characteristic for polar head vibrations and the upper part of the hydrophobic region of the membrane. The shape and position of the analyzed bands [1734.5 cm−1 \(\nu \, ( {\text{C=O)}}\) (Fig. 1a); 1469.5 cm−1 scissoring mode (Fig. 1b); 1213 cm−1 \(\nu_{{as}}\, \text{PO}_{2}^{ - }\) (Fig. 1c); 1084.5 cm−1 \(\nu_{{s}}\, \text{PO}_{2}^{ - }\) (Fig. 1d); \(\nu_{{as}} (\text{N}^{ + } -\text{CH}_{3} )\) (Fig. 2)] change with rising temperature.
It is assumed that the observed changes can be a consequence not only of increasing temperature, but also of the movement of the piperine molecules in the phospholipid membrane.
Directly after incorporation of piperine molecules into the lipid membrane, a significant spectral shift of the lipid main bands was observed. One such example was the spectral shift of the band at 1734.5 cm−1 (for pure DPPC liposome), assigned to the \(\nu \, ( {\text{C=O)}}\) stretching mode of the ester carbonyl groups toward the higher frequency of 1738 cm−1 after the incorporation of piperine molecules into the liposome membrane. No significant changes were observed in the 20–39 °C temperature range for this band. However, at 40 °C, close to the main phase transition temperature, this band shifted to the lower frequency of 1733 cm−1 (Fig. 1a). These temperature changes are similar to the one observed in the case of the PO2 − group (Fig. 1c; antisymmetric stretching mode) but with smaller amplitudes, which suggests that piperine, at temperature range 20–39 °C, interacts more selectively with PO2 − groups and only partly with the C=O one (Fig. 1). The shift of the observed band to a lower frequency of 1733 cm−1 at a temperature >40 °C confirms the gradual penetration of the membrane by piperine molecules in the direction of its polar region.
The sharp bands at 1634.5, 1612.5 and 1583.5 cm−1 might be assigned to the –C=C– vibration in the phenol of piperine. These bands are shifted toward higher frequencies (to 1659, 1654.5 and 1648 cm−1) and overlapping in the DPPC/piperine complex. This suggests that after dispersion of piperine in DPPC the van der Waals interactions between the aromatic rings in piperine molecules are reduced.
The band at 1468 cm−1 represents the scissoring mode (Fig. 1b). The changes in the region corresponding to this mode are diagnostic of lipid hydrocarbon phase transitions—alkyl chain packing arrangements—as they reflect concomitant increases inn the hydrocarbon chain mobility and gauche rotamer proportion. The position of the δCH2 vibration is sensitive to the type of lateral alkyl chain packing. An examination of the contours of this band at the onset of the hydrocarbon chain-melting process can provide useful information about the intermolecular interactions. In a gel phase, this band is relatively sharp and intensive, indicating hexagonal packing. With increasing temperature, the intensity of the δCH2 band decreases and becomes broader, and the center of the band shifts in the direction of lower frequencies (Fig. 1b). These observations indicate a loss of hexagonal chain order. A decrease in the organization of the membrane’s structure (especially visible for this band at the pre-transition temperature of 35 °C) is conducive to the penetration of piperine particles in the direction of its polar region.
The most important band probing the headgroup of DPPC interactions with piperine directly is the aforementioned PO2 − antisymmetric stretching mode, which is located at 1246 cm−1, symmetric stretching mode at 1092 cm−1 partially overlapped with the band representing the C–O–P–O–C stretching modes (1064 cm−1) and the band representing the antisymmetric N+–CH3 stretching vibrations (~971 cm−1) (Fig. 2). The bands show the different behaviors with temperature. For example, an increase in the temperature shifted the band located at 1092 cm−1 toward lower frequencies, which indicates an interaction via hydrogen bonds between the PO2 − group of the lipid and piperine molecules. This suggests decreasing H-bonding between DPPC molecules.
On the basis of the results obtained, it can be concluded that first, at the tilted gel phase (L β′), piperine molecules move in the direction of the PO2 − group and interact (temperature range 20–35 °C), and then at the ripple gel phase (P β′) (35–41 °C) they start the interactions in the hydrophilic lipid zone with the N+(CH3)3 group.
FT-IR spectroscopic analyses of thermal phase transitions
Both hydrophobic and hydrophilic molecules can be encapsulated into the liposome. Pawlikowska et al. (2012) concluded from the NMR analyses that small flavonoids with Mw ~270 g/mol are preferentially located in the upper part of the phosphatidylcholine membrane interacting with headgroups through hydrogen bonds. Piperine is insoluble in the pH 7.0 sodium phosphate buffer used in the present work. Therefore, it is assumed that 90.5 % of encapsulation of piperine with high hydrophobicity results from its complete incorporation inside the hydrophobic phospholipid bilayer of the liposome.
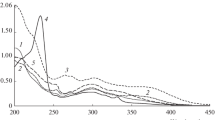
Figure 3 shows the variations in the frequency of the CH2 symmetric stretching vibration band of the blank liposome (DPPC) and liposome containing 3.39 mol % piperine, as a function of temperature. The frequency (2850 cm−1) of the methylene symmetric stretching vibrational mode is known to be sensitive to changes in the conformational order of lipid hydrocarbon chains, and it can therefore be used to monitor the progress of lipid gel/liquid-crystalline phase transitions (Pare et al. 2001; Hirsh et al. 1998; Popova and Hincha 2011; Exerowa 2002; Crowe and Hoekstra 1989). This phase transition involves the conversion of a relatively ordered gel-state bilayer in which the hydrocarbon chains exist predominantly in their rigid, extended, all-trans conformation to a relatively disordered liquid-crystalline bilayer in which the hydrocarbon chains contain a number of gauche conformers and the lipid molecules exhibit greatly increased rates and amplitudes of intra- and intermolecular motion (Lewis and McElhaney 2013).
Lipid melting curves determined as the temperature-dependent increase in the position of the CH2 symmetric stretching vibration band \((\nu_{s} \text{CH}_{2} )\) of DPPC (o) and DPPC/piperine (◓) of the fatty acyl chains in FT-IR spectra. The insert figure represents the main phase transition temperature (Tc) determined from the first derivative of the melting curves a DPPC; b DPPC/piperine
In the case of the blank liposome, a sharp increase in the CH2 symmetric stretching frequency of 1 cm−1 (ΔW = 1) is observed at the temperature of the main phase transition. Increases of this order of magnitude invariably accompany the melting of the hydrocarbon chains at the gel to liquid/crystalline phase transition of phospholipid bilayers. The DPPC/piperine dispersion exhibits twice the change in position of the CH2 symmetric stretching band (ΔW = 2) and a much broader range of temperature of the main phase transition than for the DPPC liposome dispersion. A relatively small increase (0.5 cm−1) in CH2 symmetric stretching band frequency occurs at temperatures near 35 °C, which corresponds to the pre-transition phase range.
Determined experimentally by the FT-IR method, the main phase transition temperatures (Tc) of piperine liposome and blank liposome are 40.67 and 41.28 °C, respectively. The encapsulation of piperine lowers, to an insignificant degree, the Tc values of liposomes. Niu et al. (2012b) indicated that only when the molar concentration of encapsulated compound in liposomes is greater than 5 % is the change of Tc value bigger than 1 °C. The molar concentration of liposomal piperine is about 3.39 % in the current system, so that there is no distinct difference in Tc values of piperine liposome compared to blank liposome. The close Tc values of liposomes with and without piperine suggest that piperine does not strongly disrupt the packing of the phospholipid acyl chains.
Piperine-membrane interaction by 1H NMR
Lipid dynamics in biological membranes have been a subject of enormous interest to the scientific community over many years. This is in part because the physicochemical properties of membranes, which are critical in determining their function as selective permeability barriers, arise from interactions that occur on the molecular length scale and over the time scale for molecular dynamics (Gawrisch 2005).
Molecular self-diffusion, reorientational motions and spectral densities of atomic species reveal a variety of time scales playing a role in membrane dynamics. The mechanisms of lipid motion strongly depend on the time scale, from fast ballistic translation at the scale of picoseconds (effective diffusion coefficients of the order of 10−5 cm2/s) to diffusive flow of a few lipids forming nanodomains at the scale of hundreds of nanoseconds (diffusion coefficients on the order of 10−8 cm2/s) (Yang et al. 2014).
Nuclear Overhauser effect spectroscopy (NOESY) has been used for the investigation of bilayer organization. Phospholipids exist in multiple conformations with rapid transitions between them; therefore, the current system does not represent stable molecular arrangements with fixed distances. Phospholipid molecules are intrinsically flexible and change their conformation with correlation times from pico- to nanoseconds (Brown et al. 1995). The distances to lipid segments of nearest-neighbor lipids in the bilayer change within nano- to microseconds (Pastor and Feller 1996; Huster and Arnold 1999). The NOESY experiment reflects the probability of a close approach between protons of neighboring lipid molecules. Despite all of the chaos, lipid molecules, on average, maintain their orientation in the bilayer, and membranes have a defined hydrophobic-hydrophilic interface.
NOESY spectroscopy in combination with T1 measurements has been shown to be a valuable tool for lipid-lipid and drug-lipid interactions.
The incorporation of piperine (Fig. 4) within the lipid bilayer causes chemical shift changes in the lipid peaks and NOESY cross peaks between drug and lipid resonances.
Structure and assignments of piperine. 1H NMR (400 MHz, CDCl3): δ [ppm] = 1.597 (m, 4H, 3 + 5); 1.664 (m, 2H, 4); 3.582 (d, 4H, 2 + 6); 5.975 (s, 2H, 7″); 6.435 (d, 1H, 2″); 6.758 (m, 3H, 3″, 4′, 5′); 6.892 (d, 1H, 4″); 6.979 (s, 1H, 6″); 7.399 (qd, 1H, 3′). Bold values indicate the position of a proton
In the following, the complexation-induced chemical shifts (Δδ) observed in the 1H NMR spectra for the DPPC/piperine will be discussed in reference to the 1H NMR spectrum of pure DPPC (Supplementary Fig. II).
The strongest shifts of lipid resonances were found for the protons in the area of the glycerol backbone (i Δδ 0.309 ppm, h1 Δδ 0.115 ppm, f Δδ 0.162 ppm) and polar headgroup (e Δδ 0.130 ppm, g Δδ 0.160 ppm).
In the case of piperine, the strongest shift is assigned to proton H3′ (Δδ−0.026 ppm), as its chemical shift is typical for a β-proton in α/β-unsaturated carbonyl compounds (shielding effect). Significantly smaller shifts were found for the protons H3″, 4′, 5′ (Δδ 0.016 ppm) and H2′ (Δδ 0.011 ppm). This may be connected with the deshielded effect caused by the decrease of the electron density around the protons.
In the 2D NOESY data (Fig. 5), cross peaks of the piperidine ring protons (H4, H3, H5) to the quaternary ammonium group and the α methylene protons of the alkyl chains were found.
A 2D NOESY 1H NMR spectrum of DPPC/piperine liposomes recorded with a mixing time of 500 ms. Assignment of 1H-resonances, δ [ppm]: polar headgroup (g–quaternary ammonium group (–N+(CH3)3), 3.216; e–(–CH2–N+(CH3)3), 3.637; j–(–CH2–CH2–N+(CH3)3), 4.250); hydrogen belt: (f–(–CH2–OP–), 3.939; h1–(–PO–CH2–) and (–OCO–CH2–), 4.155; h2–(–OCO–CH2–), 4.250; i–(–OCO–CH), 5.533); hydrophobic core (a–terminal methylene group, 0.883; b–(CH2)n of β–alkyl chains, 1.268; c–protons of acyl chains (CH2–CH2–CO), 1.664; d–α methylene protons (–CH2–CO), 2.334)
The NOESY spectrum confirms the attachment of the piperidine ring because of the strong cross signal between protons H2/6 and proton H2′. It also shows the close overlap of H4′ and H5′ with their respective cross signals to H2′ and H3′.
Observed on the NOESY spectrum, the signal at 7.373 ppm from H3′ protons is connected to two other protons: first with the proton H4′ at 6.774 ppm and second with the H2′ proton at 6.446 ppm. The latter shows a sharp doublet with a spin coupling of 15.32 Hz. Proton H4′ resonates very closely to H5′. The three proton signals at 6.986 (H6″), 6.895 (H4″) and 6.774 (H3″) ppm constitute the very typical pattern of a 1,2,4-trisubstituted aromatic compound (Berger and Sicker 2009). The mentioned cross peaks are due to scalar coupling (COSY-type), not NOESY, as these protons are vicinal.
Based on the NMR and FT-IR data, the piperidine ring may be localized in the chain/glycerol/headgroup region (oriented along the bilayer), while the dioxolane ring remained in the aliphatic area of the alkyl chains (Fig. 6b).
a Relaxation time T1 [s] of the –N+(CH3)3 resonances in the 1H NMR spectra of the DPPC (o) and DPPC/piperine (◓) liposomes at a temperature range between 25 and 47 °C. b Hypothetical schematic of the molecular reorientation arrangement from the horizontal to vertical position of DPPC polar headgroups in different phases with respect to piperine molecules
1H NMR spectra of the liposomes were recorded at intervals of 2 or 3 °C between 25 and 47 °C. The T1 relaxation times of Me3N+ (3.58 ppm) resonances were measured at each step of heating. Variations of T1 values with temperature are shown in Fig. 6a.
The results obtained suggest that T1 is proportional to the statistical average of lateral phospholipid-piperine contacts. The bursting of hydrogen bonds between DPPC molecules as a result of increasing temperatures causes a loosening of the membrane and the slow penetration of piperine along the normal membrane surface—in the direction of the polar region. Such a change in the environment of –N+(CH3)3 groups causes the relaxation time to be lengthened. The longest relaxation time of the –N+(CH3)3 groups was observed in the temperature range of 35–40 °C, which corresponds to the membrane changes occurring during the ripple gel phase. This time is close to 1.5 times longer in the case of liposomes built of DPPC/piperine in comparison with blank liposomes. In the tilted gel phase (L β′) and liquid-crystalline (L α) phase, the changes in the T1 values take the same course. Only the aforementioned temperature range of 35–40 °C is significant. It is only in this temperature range that analyzed liposomes maintain their distinct character, which confirms the hypothesis of the mobility of piperine molecules in the membrane as a result of bursting hydrogen bonds.
Effect of temperature on the stability of liposomal piperine
Observed by the FT-IR method, the thermotropic phase transitions of phospholipids from the gel phase to fluid liquid crystalline phase have a huge impact on the temperature stability of liposomal piperine. It is thus an important value to investigate the effect of temperature on the stability of liposomal piperine, which is trapped in the phospholipid bilayer of liposomes. Figure 7a compares the values of the maximum absorption of liposomal piperine and free piperine at a temperature range of 20–47 °C.
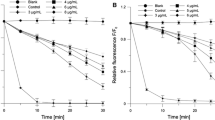
a The maximum absorption of liposomal piperine and free piperine at various temperatures. b Relative intensity of the maximum absorption of liposomal piperine and free piperine at various times, T = 37 °C. c Piperine release from the liposomes stored for a period of 20 days (n = 3). d The effect of liposomal piperine incubation at 37 °C on the percentage of its permeation through the phospholipids membrane
Upon increasing the temperature from 20–47 °C, the absorption values of liposomal piperine are higher than those of free piperine. The markedly higher heat stability of liposomal piperine than free piperine supports the protective effect of liposomal piperine from liposome encapsulation. In the liquid crystalline phase (>41 °C) of the phospholipid bilayer, the liposomes have weaker protection than in the rigid gel phase. The marked temperature indicates the highest permeability of liposomal membranes as a result of their dynamics. It must also be noted that free piperine appears to degrade at the same rate as liposomal piperine after 41 °C.
Stability of liposomal piperine at 37 °C
The changes in the relative intensity of the characteristic maximum absorption of piperine at 340 nm as a function of time is used to evaluate the stability of liposomal piperine and free piperine at 37 °C. As seen in Fig. 7b, no reduction of relative intensity of maximum absorption of liposomal piperine was observed, in contrast to free piperine. After 1 h incubation, the values of the relative intensity of encapsulated piperine are about 4 % higher than the values of the unencapsulated piperine. During subsequent hours of incubation, the differences in the relative intensity are even higher and amount to 2 h:8 %, 3 h:19.5 %, 4 h:29 %, 5 h:32.8 %, 6 h:31.8 % and 7 h:30.4 %, respectively.
Piperine release from liposomes depending on time
Figure 7c shows in vitro drug release from liposomes stored for a 20-day period. It was observed that the percent of piperine release varies between about 3.47–4.33 %. The liposomal drug release process reached its maximum value after 2 weeks and maintained it throughout the following 7 days of observation.
The percent of piperine permeation through the membrane phospholipid at 37 °C as a function of time is presented in Fig. 7d. Figure 7d shows that the highest percentage of piperine permeation through the phospholipid membranes occurs in the first 3 h of incubation and amounts to 3.12 %. For the following 3 h of the analysis, the percent of permeation decreases close to 2.4 times and amounts to only 1.29 %.
After 7 h of incubation, the value of the % of piperine permeation is 4.6 %. These results reveal that the encapsulation of piperine into liposomes obtained by the modified reverse-phase evaporation method (mREV) can effectively improve the stability of piperine better than free piperine.
DPPH-scavenging activity of liposomal piperine at 37 °C
The DPPH assay has been used to estimate the radical scavenging activity of piperine. The radical scavenging activity is dependent on the number and position of the phenolic OH groups. Piperine, which does not have any OH groups, shows negligible radical scavenging activity, which is consistent with the results obtained for flavones (Sinha and Joshi 2014).
Upon reduction by an antioxidant, the characteristic maximum absorption of DPPH decreases proportionally to the increase of the amount of the non-radical form of DPPH. After an incubation period of 30 min, the absorbance was measured at 517 nm. Figure 8 shows the decrease of relative intensity of maximum absorption of DPPH at 517 nm as a function of time for liposomal piperine in comparison with free piperine at 37 °C. In the case of free piperine, the decrease of relative intensity is significantly higher than in the case of the liposomal piperine.
The decrease of the absorbance of reduced DPPH was used to evaluate the radical-scavenging potential of the sample. After 1 h, the calculated values of % scavenging are 0.61 and 16.43 % for liposomal piperine and free piperine, respectively. After 9 h of incubation, the value increase amounts to 7.33 and 46.84 % for liposomal piperine and free piperine, respectively. The small values of % scavenging in the current work result from: (1) the build of the piperine molecule (lack of OH groups), (2) low piperine permeability through liposomal membranes and (3) the high polarity of the sodium phosphate buffer (in comparison with organic solvents wherein more than 50 % of other natural antioxidant scavenging has been registered by Priyadarsini et al. 2003; Shang et al. 2010; Zarai et al. 2013). As discussed above, the high protection of piperine from liposomes is partly a result of molecularly H-bonded piperine within the hydrophobic core of liposome and partly a result of molecularly H-bonded piperine within the headgroup. Such a strong incorporation of piperine in the area between the hydrophobic and hydrophilic parts of the membrane prevents it from having any direct contact with DPPH molecules.
Conclusion
The objective of this study was to perform a detailed measurement of the localization of piperine molecules in lipid membranes. The physical and chemical properties of the liposomes containing piperine, an evaluation of their stability and the antioxidant efficiency of the entrapped drug were also investigated. The results showed that the liposomes containing piperine had good stability and high entrapment efficacy.
The conducted study demonstrated that piperine molecules are anchored inside the phospholipid bilayer, and along with an increase in temperature they move toward the hydrophilic area of the membrane.
The NMR relaxation results and NOESY experiment are in good agreement with the FT-IR data used to determine the localization of piperine in the phospholipids membrane.
The UV-Vis study indicates that liposome encapsulation can significantly slow down the degradation speed of piperine.
However, the long-term stability of these liposomal formulations is still a challenge.
References
Ahmad N, Fazal H, Abbasi BH, Farooq S, Ali M, Khan MA (2012) Biological role of Piper nigrum L. (Black pepper): a review. Asian Pac J Trop Biomed 2:1945–1953
Andresen TL, Jensen SS (2005) Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res 44:68–97
Appiah I, Milovanovic S, Radojicic A, Nikolic-Kokic A, Orescanin-Dusic Z, Slavic M, Trbojevic S, Skrbic R, Spasic MB, Blagojevic D (2009) Hydrogen peroxide affects contractile activity and anti-oxidant enzymes in rat uterus. Br J Pharmacol 158:1932–1941
Berger S, Sicker D (2009) Classics in spectroscopy, isolation and structure elucidation of natural products. Wiley-VCH, Weinheim
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT—food. Sci Technol 28:25–30
Brewer MS (2011) Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr Rev Food Sci F 10:221–247
Brown ML, Venable RM, Pastor RW (1995) A method for characterizing transition concertedness from polymer dynamics computer simulations. Biopolymers 35:31–46
Crowe JH, Hoekstra FA (1989) Membrane phase transitions are responsible for imbibitional damage in dry pollen. Proc Nat Acad Sci USA 86:520–523
Damanhouri ZA, Ahmad A (2014) A review on therapeutic potential of Piper nigrum L. (black pepper): the king of spices. Med Aromat Plants 3:3–6
Exerowa D (2002) Chain-melting phase transition and short-range molecular interactions in phospholipid foam bilayers. Adv Colloid Interfac 96:75–100
Flora SJ (2007) Role of free radicals and antioxidant in health and disease. Cell Mol Biol 15:1–2
Gawrisch K (2005) The structure of biological membranes. In: Yeagle PL (ed) The dynamics of membrane lipids. CRC Press, New York, pp 540–560
Gorrini Ch, Harris IS, Mak TW (2013) Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov 12:931–947
Gregoriadis G (1974) Drug entrapment in liposomes; possibilities for chemotherapy. Biochem Soc Trans 2:117–119
Haulica I, Boisteanu D, Bild W (2000) Free radicals between health and disease. Rom J Physiol 37:15–22
Hirokawa Y, Nheu T, Grimm K (2006) Sichuan pepper extracts block the PAK1/Cyclin D1 pathway and the growth of NF1-deficient cancer xenograft in mice. Cancer Biol Ther 5:305–309
Hirsh DJ, Lazaro N, Wright LR, Boggs JM (1998) A new monofluorinated phosphatidylcholine forms interdigitated bilayers. Biophys J 75:1858–1868
Hu Z, Yang X, Ho PC, Chan SY, Heng PW, Chan E, Duan W, Koh HL, Zhou S (2005) Herb-drug interactions: a literature review. Drugs 65:1239–1282
Huster D, Arnold K (1999) Investigation of lipid organization in biological membranes by two-dimensional nuclear overhauser enhancement spectroscopy. J Phys Chem B 103:243–251
Jin Y, Li M, Hou X (2005) Pyrocatechol violet as a marker to characterize liposomal membrane permeability using the chelation and the first-order derivative spectroscopy. J Pharmaceut Biomed 37:379–382
Kerdudo A, Dingas A, Fernandez X, Faure Ch (2014) Encapsulation of rutin and naringenin in multilamellar vesicles for optimum antioxidant activity. Food Chem 159:12–19
La L, Fu Q, Liu Y (2012) Piperine suppresses tumor growth and metastasis in vitroand in vivo in a 4T1 murine breast cancer model. Acta Pharmacol Sin 33:523–530
Lewis RN, McElhaney RN (2013) Membrane lipid phase transitions and phase organization studied by fourier transform infrared spectroscopy. Bioch Biophys Acta 1828:2347–2358
Namjoyan F, Hejazi H (2012) Evaluation of drying process on the composition of black pepper ethanolic extract by high performance liquid chromatography with diode array detector. Jundishapur J Nat Pharm Prod 7:163–167
Neeraj J, Sing S, Sing J (2013) Role of free radicals and antioxidants in human health and disease. Int J Curr Res Rev 5:14–22
Niu Y, Ke D, Yang Q, Wang X, Chen Z, An X, Shen W (2012a) Temperature-dependent stability and DPPC scavengign activity of liposomal curcumin at pH 7.0. Food Chem 135:1377–1382
Niu Y, Wang X, Chai S, Chen Z, An X, Shen W (2012b) Effects of curcumin concentration and temperature on the spectroscopic properties of liposomal curcumin. J Agr Food Chem 60:1865–1870
Pare Ch, Lafleur M, Liu F (2001) Differential scanning calorimetry and 2H nuclear magnetic resonance and fourier transform infrared spectroscopy studies of the effects of transmembrane α-helical peptides on the organization of phosphatidylcholine bilayers. Biochim Biophys Acta 1511:60–73
Pastor RW, Feller SE (1996) A molecular perspective from computation and experiment. In: Merz KM, Roux B (eds) Biological membranes. Birkhäuser, Boston, pp 3–30
Pawlikowska-Pawlega B, Misiak LE, Zarzyka B, Paduch R, Gawron A, Gruszecki WI (2012) Localization and interaction of genistein with model membranes formed with dipalmitoylphosphatidylcholine (DPPC). Biochim Biophys Acta 1818:1785–1793
Pentak D (2014) Physicochemical properties of liposomes as potential anticancer drugs carriers. Interaction of etoposide and cytarabine with the membrane: spectroscopic studies. Spectrochim Acta A 122:451–460
Popova AV, Hincha DK (2011) Thermotropic phase behavior and headgroup interactions of the nonbilayer lipids phosphatidylethanolamine and monogalactosyldiacylglycerol in the dry state. BMC Biophys 4:1–11
Pradeep CR, Kuttan G (2002) Effect of piperine on the inhibition of lung metastasis induced B16F-10 melanoma cells in mice. Clin Exp Metastas 19:703–708
Priyadarsini KI, Maity DK, Naik GH, Kumar MS, Unnikrishnan MK, Satav JG (2003) Role of phenolic O–H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radical Biol Med 35:475–484
Rodrigues C, Gameiro P (2003) Interaction of rifampicin and isoniazid with large unilamellar liposomes: spectroscopic location studies. Biochim Biophys Acta 1620:151–159
Shang YJ, Jin XL, Shang XL, Tang JJ, Liu GY, Dai F (2010) Antioxidant capacity of curcumin-directed analogues: structure-Activity relationship and influence of microenvironment. Food Chem 119:1435–1442
Sharma A, Sharma US (1997) Liposomes in drug delivery: progress and limitations. Int J Pharm 154:123–140
Sinha R, Joshi A (2014) Localization and interaction of hydroxyflavones with lipid bilayer model membranes: a study using DSC and multinuclear NMR. Eur J Med Chem 80:285–294
Smith HJ, Smith JR (2012) Multi-drug liposomes to treat tumors, Patent Application Publication, Pub. No.: US 2012/0231066 A1, United States
Ulbricht C, Chao W, Costa D (2008) Clinical evidence of herb-drug interactions: a systematic review by the natural standard research collaboration. Curr Drug Metab 9:1063–1120
Upadhyay V, Sharma N, Joshi HM (2013) Development and validation of rapid RPHPLC, method for estimation of piperine in Piper nigrum L. Int J Herb Med 1:6–9
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell B 39:44–84
Vasavirama K, Upender M (2014) Piperine: a valuable alkaloid from piper species. Int J Pharm Pharm Sci 6:34–38
Vazquez-Olivencia W, Shah P, Pitchumoni CS (1992) The effect of red and black pepper on orocecal transit time. J Am Coll Nutr 11:228–231
Yang J, Calero C, Marti J (2014) Diffusion and spectroscopy of water and lipids in fully hydrated dimyristoylphosphatidylcholine bilayer membranes. J Chem Phys 140:104901–104913
Zarai Z, Boujelbene E, Salem NB (2013) Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. Lwt-Food Sci Technol 50:634–641
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pentak, D. In vitro spectroscopic study of piperine-encapsulated nanosize liposomes. Eur Biophys J 45, 175–186 (2016). https://doi.org/10.1007/s00249-015-1086-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-015-1086-x