Abstract
Bombinins H are mildly cationic antimicrobial peptides isolated from the skin of the anuran genus Bombina, the fire-bellied toad. Some members of this peptide family coexist in skin secretions as diastereomers in which a single d-amino acid (alloisoleucine or leucine) is incorporated as a result of the post-translational modification of the respective gene-encoded l-amino acid. Here we report on the antimicrobial properties and membrane interactions of bombinins H2 and H4. The latter differs from H2 by the presence of a d-alloisoleucine at the second N-terminal position. Specifically, we have evaluated the antimicrobial activity of H2 and H4 against a large panel of reference and clinical isolates of Gram-negative and Gram-positive bacteria; performed membrane permeation assays on both intact cells and model membranes (lipid monolayers and liposomes) mimicking the composition of the plasma membrane of Gram-negative/positive bacteria; used biochemical tools, such as trypsin-encapsulated liposomes and capillary electrophoresis, to monitor the peptides’ ability to translocate through the membrane of liposomes mimicking Escherichia coli inner membrane. The results revealed interesting relationships between the presence of a single d-amino acid in the sequence of an antimicrobial peptide and its target microbial cell selectivity/membrane-perturbing activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Naturally occurring gene-encoded antimicrobial peptides (AMPs) are an extremely diverse group of anti-infective molecules whose function is crucial for the host immune response (Boman 2003; Ganz 2003; Giuliani and Rinaldi 2010; Selsted and Ouellette 2005). Besides being key components of the innate immune system of all living organisms, rapidly killing a broad spectrum of microbes, they interact with the host itself, triggering events (e.g., anti-endotoxin activity, chemotactic activity) that complement their role as antibiotics (Beisswenger and Bals 2005; Hancock and Diamond 2000).
Furthermore, the mechanism of action of the majority of AMPs is based on the permeation of the microbial cell membrane, causing damage that is hard to fix (Mangoni et al. 2007). This limits the induction of microbial resistance to AMPs and makes them attractive molecules for the development of alternative anti-infective agents (Easton et al. 2009). The search for new therapeutics is strongly required because of the growing emergence of resistant pathogens to the commercially available antibiotics/antimycotics, most of which interfere with intracellular processes (Yeaman and Yount 2003). Although AMPs do possess a wide variety of structural and conformational features, many of them share selected properties such as being usually cationic at neutral pH, and displaying the tendency to form amphipathic structures upon interacting with the membrane of the pathogen (Dempsey et al. 2010; Shai 1999).
Among the natural sources for peptide antibiotics, the granular glands of amphibian skin constitute one of the richest storehouses (Rinaldi 2002; Simmaco et al. 1998). An interesting and unique family of AMPs is the short and mildly cationic bombinins H, from the skin secretion of Bombina genus, the fire-bellied toad (Simmaco et al. 2009). Their existence was predicted from the sequence of the bombinin precursors, obtained via cDNA cloning (Gibson et al. 1991; Simmaco et al. 1991). Bombinins H are 17–20 residue AMPs with an amidated C-terminus (Mangoni et al. 2000). However, their most surprising feature is the presence of a d-amino acid (alloisoleucine or leucine) at the second N-terminal position in some of them, as a consequence of post-translational modification (there is no codon for a d-amino acid) of the respective gene-encoded l-isoleucine or l-leucine (Kreil 1994; Mor et al. 1992). During this reaction, the chirality of the α-carbon is changed and the enzyme responsible for this l- to d-isomerization has been purified and characterized from the skin secretions of B. variegata (Jilek et al. 2005).
The discovery of a d-amino acid in ribosomally synthesized peptides of animal origin first occurred in the opioid skin peptides dermorphins and delthorphins from the South American frogs Phyllomedusa sauvagei and P. bicolour (Erspamer et al. 1989; Montecucchi et al. 1981), and later in other neuropeptides and toxins from invertebrates (Buczek et al. 2005; Kamatani et al. 1989; Kreil 1997; Kuwada et al. 1994; Torres et al. 2002). However, bombinins H are the first example of natural AMPs with a single d-amino acid in their sequence. Furthermore, in contrast with opioid peptides, the d-amino acid–containing AMPs coexist with their all-l counterparts.
In this paper, we focussed our attention on a pair of bombinins H: the all l-peptide H2 (IIGPVLGLVGSALGGLLKKI-NH2) and its diastereomer H4, which differs from H2 by the presence of a d-alloisoleucine in position 2. Both peptides have a +3 net charge at pH 7. Previous studies on their antimicrobial properties in agar medium revealed a stronger activity for the d-amino acid–containing bombinin H4. In addition, mode of action studies pointed out that both peptides are capable of permeating the membrane of Gram-negative bacteria, causing the release of large cytosolic components, such as the β-galactosidase, with H4 having a faster killing kinetic (Mangoni et al. 2000). In order to enlarge our knowledge on the anti-infective properties of these two compounds and to explore whether the mechanism underlying their different antimicrobial activity is related to a different ability to permeate the microbial cell membrane, the following experiments were performed: (1) antimicrobial assays to determine the minimum inhibitory concentration (MIC) value on a large panel of reference and clinical isolates of Gram-negative and Gram-positive bacteria; (2) membrane permeation assays on both intact cells and model membranes (lipid monolayers and liposomes) mimicking the composition of the plasma membrane of Gram-negative/positive bacteria; (3) biochemical assays, using trypsin-encapsulated liposomes and capillary electrophoresis, to monitor the peptides’ ability to translocate through the membrane of liposomes mimicking the Escherichia coli inner membrane.
Although more quantitative information should be obtained in the future, considering simulation of peptide dynamics in lipid membranes as well, an interesting relationship between the target cell selectivity/membrane-perturbing activity and the presence of a single d-amino acid in the sequence of an AMP has emerged from these investigations.
Materials and methods
Materials
Synthetic bombinins H2 and H4 were purchased from GENEPEP (Prades-le-Lez, France). The purity of the peptides, their sequence and concentrations were determined as previously described (Mangoni et al. 2000). l-α-phosphatidylethanolamine (PE), l-α-phosphatidyl-dl-glycerol (PG), cardiolipin (CL), fluorescein isothiocyanate dextrans of 4, 20, and 70 kDa average molecular mass (FITC-D4/D20/D70), calcein, ammonium thiocyanate, and iron (III) chloride hexahydrate were obtained from Sigma. Trypsin (EC 3.4.21.4, sequencing grade) was purchased from Roche Diagnostics, Mannheim, Germany. Soybean trypsin inhibitor (SBTI) was obtained from ICN Biomedicals (Irvine, CA, USA). Imidazole was purchased from Merck (Whitehouse Station, NJ, USA). SYTOX™ Green was from Molecular Probes (Invitrogen, Carlsbad, CA, USA). All other chemicals used were of reagent grade.
Microorganisms
The following bacteria were used for the microbiological assays. Gram-negatives: Acinetobacter baumannii ATCC 19606, A. baumannii 1 (clinical isolate), Escherichia coli D21, E. coli O111:B4, E. coli ATCC 25922, Pseudomonas aeruginosa 3 (clinical isolate), P. aeruginosa ATCC 27853, and Yersinia pseudotuberculosis YPIII. Gram-positives: Staphylococcus aureus ATCC 29213, S. aureus Cowan I, S. epidermidis ATCC 12228 and the clinical isolates S. aureus 8, S. aureus 43300, S. aureus 11270, S. capitis n.1, S. epidermidis 18, and Enterococcus faecalis 9546.
Antibacterial activity of the peptides
Susceptibility testing of peptides was performed by adapting the microbroth dilution method outlined by the Clinical and Laboratory Standards Institute (CLSI 2006), using sterile 96-well plates (Falcon, Franklin Lakes, NJ, USA). Stock solutions of peptides were prepared in serial twofold dilutions in 20% ethanol; 5 μl was then added to 45 μl of Mueller-Hinton broth (MHB; Oxoid, Cambridge, UK), previously put into the wells of the microtiter plate. Afterwards, aliquots (50 μl) of bacteria in mid-log phase, at a concentration of 2 × 106 colony-forming units (CFU)/ml, were added to each well. The range of peptide dilutions used was 1.56–50 μM. Inhibition of growth was determined by measuring the absorbance at 595 nm with a microplate reader (Infinite M200, Tecan, Salzburg, Austria) after 18–20 h incubation at 37°C. Antibacterial activities were expressed as MIC, the minimum concentration of peptide causing 100% inhibition of bacterial growth.
Bactericidal activity
The bactericidal activity of H2 and H4 against the Gram-positive S. aureus ATCC 29213 and S. aureus Cowan I was evaluated as previously described (Mangoni et al. 2008). Briefly, exponentially growing bacteria in MHB were harvested by centrifugation and then resuspended in fresh MHB or phosphate-buffered saline (PBS) to obtain a density of 1 × 107 CFU/ml. Ten μl of the bacterial suspension was incubated at 37°C for 90 min in the presence of different concentrations of peptide (serial twofold dilutions ranging from 6.25 to 50 μM) dissolved in MHB or PBS, to a final volume of 100 μl.
Following incubation, appropriate aliquots were plated onto Luria-Bertani agar plates to accurately determine the 99.9% killing. The number of surviving bacteria was counted after overnight incubation at 37°C. Bactericidal activity was expressed as the peptide concentration necessary to induce a reduction in the number of viable bacteria of ≥3 log10 CFU/ml (Maisetta et al. 2006). Controls were run without peptide and in the presence of peptide solvent (20% ethanol) at a final concentration of 1%.
Bacterial membrane permeabilization
To assess the ability of bombinins H to alter the bacterial membrane permeability, 1 × 106 cells in 100 μl of PBS were mixed with 1 μM SYTOX™ Green for 5 min in the dark. After adding peptide, the increase in fluorescence, due to the binding of the dye to intracellular DNA, was measured at 37°C in the microplate reader (excitation and emission wavelengths were 485 and 535 nm, respectively). The peptide concentrations ranged from 3.125 to 50 μM. Controls were given by cells without peptide, whereas the maximum membrane perturbation was obtained after lysing bacteria in 1% (v/v) Triton X-100.
Measurement of penetration of bombinins H2 and H4 in lipid monolayers
Insertion of H2 and H4 into monolayers formed of either PE/PG (7:3, w/w) and PG/CL (6:4, w/w) was evaluated as an indication of the peptides’ ability to bind and penetrate microbial targets’ plasma membranes. To this end, lipid mixtures in chloroform were spread at an air/buffer (50 mM potassium phosphate, pH 7, with 0.1 mM EDTA) interface, and penetration was monitored by measuring surface pressure (π) with a Wilhelmy wire attached to a microbalance (DeltaPi, Kibron, Helsinki) connected to a PC and using circular glass wells (subphase volume 0.5 ml). After evaporation of lipid solvent and stabilization of monolayers at different initial surface pressures (π 0), the peptide (1 μM, final concentration) was injected into the subphase, and the increment in surface pressure of the lipid film upon intercalation of the peptide dissolved in the subphase was followed for the next 35 min. The difference between the initial surface pressure and the value observed after the penetration of bombinins H into the film was taken as Δπ. Surface activity of H2 and H4 at the air/buffer interface was also evaluated in the absence of lipids, by injecting increasing amounts of the peptide into the subphase and measuring the variation of π 0 with time. All measurements were performed at room temperature.
Preparation of calcein/dextran-loaded liposomes and leakage measurement
Calcein-loaded liposomes of different compositions (PE/PG, 7:3 w/w and PG/CL, 6:4 w/w) were prepared as previously described (Rinaldi et al. 2002). Briefly, phospholipids were dissolved in chloroform and mixed with a calcein solution (60 mM in phosphate buffer, pH 7.0). The mixture was then sonicated until becoming homogeneous.
Liposomes were prepared by reverse phase evaporation (Szoka and Papahadjopoulos 1978). Free calcein was removed through gel filtration (Sephadex G-50), with a 1.5 × 15 cm column, equilibrated and eluted with 50 mM potassium phosphate buffer, pH 7.4 containing 0.1 mM EDTA. The lipid concentration was determined by the Stewart method (Stewart 1980). Calcein loaded in liposomes is self-quenched (Allen 1981) and its leakage was monitored as a relief of quenching (excitation and emission wavelengths were 490 and 517 nm, respectively) with a Perkin-Elmer LS 55B spectrofluorimeter. The maximum fluorescence intensity corresponding to 100% calcein release was determined by addition of Triton X-100 (0.2%, v/v, final concentration), which produced the total destruction of the vesicles. The leakage value was calculated according to the equation:
where F and F t denote the fluorescence intensity before and after the addition of the detergent, respectively, and F 0 represents the fluorescence of intact vesicles.
Liposomes loaded with FITC-D4/D20/D70 were prepared as reported elsewhere (Rinaldi et al. 2001). The release of dextran from loaded vesicles upon interaction with bombinin H2 and H4 was examined fluorimetrically; excitation and emission wavelengths were 494 and 520 nm, respectively. In a typical experiment, an aliquot of the peptide solution in 20% (v/v) ethanol was incubated with a suspension of dextran-loaded vesicles in 50 mM buffer (sodium phosphate, pH 7.4 with 0.1 mM EDTA, SPB), at a final lipid concentration of 50 μM. The mixture (2 ml, final volume) was stirred gently for 10 min in the dark and centrifuged at 27,000g for 30 min. The supernatant was recovered, and its fluorescence intensity was measured. The total dextran release was determined by the addition of 20 μl of 10% (v/v) Triton X-100 to the vesicle suspension. The leakage value was calculated as described above for calcein. All experiments were carried out at room temperature.
Preparation and degradation of peptides by trypsin-loaded liposomes
Trypsin-loaded liposomes were prepared from a 7:3 (w/w) mixture of PE and PG. The lipid mixture in chloroform was vacuum-dried and hydrated with 5 mM SPB containing 100 μg/ml trypsin, to a final lipid concentration of 2.5 mg/ml. After five cycles of freeze-thawing, liposomes were extruded 21 times through two stacked 400 nm filters, as described previously (MacDonald et al. 1991). Free trypsin was removed by gel filtration on a Sephadex G-50, 1.5 × 15 cm column, equilibrated and eluted with 5 mM SPB. Liposomes were stored at 4°C and used within 48 h. Trypsin-loaded liposomes were suspended in 5 mM SPB supplemented with 10 μg/ml of imidazole, an internal standard for capillary zone electrophoresis (CZE), and with or without 20 μg/ml of SBTI, at a lipid concentration of 250 μg/ml. The concentration of SBTI was sufficient to effectively inhibit all trypsin activity after liposomes were lysed by adding an aliquot of Triton X-100 (0.1% v/v, final concentration), as reported in Den Hertog et al. (2004). The suspension of lipid vesicles was subsequently incubated with 60 μM of each bombinin H or 40 μM of the control membrane-inactive peptide cystatin cysS1–15 (SSSKEENRIIPGGIY), corresponding to the first 15 N-terminal amino acids of cystatin S (Den Hertog et al. 2004). At different time intervals, 50 μl aliquots were withdrawn, boiled for 5 min to inhibit all trypsin activity, lysed in 0.1% Triton X-100, and analyzed by CZE. Degradation of peptides implied digestion, by trypsin, of the peptide internalized within the liposome.
Capillary zone electrophoresis
Purity and degradation of peptides was analyzed by CZE on a BioFocus 2000 Capillary Electrophoresis System (Bio-Rad, Hercules, CA, USA) equipped with an uncoated fused silica capillary of 50 μm internal diameter and a length of 24 cm. Samples were loaded by pressure injection at 20 lbf in−2 s−1 (where 1 lbf in−2 = 6.9 kPa), and peptide separation was performed according to the manufacturer’s instructions at 10 kV (anode at the detector side) and 20°C, using 0.1 M phosphate buffer, pH 2.5, as the electrolyte. Online UV detection was performed at 200 nm. The running time of the analysis was 20 min, and data were analyzed using BioFocus Integrator software. The level of peptide degradation was quantified in the electropherogram by comparing the relevant peak heights of the sample with that of the internal standard imidazol.
Results
Antimicrobial activity
In our previous studies, the antimicrobial activity of H2 and H4 was tested on standard Gram-negative and Gram-positive bacterial strains using the inhibition zone assay in agar medium (Mangoni et al. 2000). Here, to deepen our understanding of the antibacterial activity of these two peptides, we analyzed their ability to inhibit the microbial growth in liquid medium, employing the commonly used serial twofold dilution method. This allowed us to determine the MIC values, as defined in the “Materials and methods” section. A large panel of standard and clinical isolates of Gram-negative and Gram-positive bacterial strains, most of which have not been studied previously, was investigated. The results are reported in Table 1. They indicated that bombinin H4 had the same or a higher activity than that of H2 against Gram-negative bacteria. In contrast, the opposite behavior was detected toward Gram-positive species, with bombinin H2 having a two- to fourfold lower MIC—and thus a stronger activity—than H4 against almost all of the tested microorganisms. Note that our previous data using the inhibition zone assay had shown a slightly higher activity of H4 than H2 against the Gram-positive S. aureus Cowan I (Mangoni et al. 2000). This could be due to a different solubility and diffusion of the peptides through a solid medium.
We next examined the bactericidal activity of the two diastereomers against some representative Gram-positive bacterial strains (i.e., S. aureus ATCC 29213 and S. aureus Cowan I), by counting the number of viable cells after 90 min of incubation with the peptide, in both MHB and PBS. Specifically, PBS was used to assay the bactericidal activity of bombinins H in a medium whose ionic strength (150 mM sodium salt compared to 100 mM of MHB) was closer to that of biological fluids (Li et al. 2003). Surprisingly, both peptides caused 99.9% killing of the microbial population at the same concentration (Table 2). When tested in MHB, the two diastereomers displayed a bactericidal activity at 50 μM (Table 2), a peptide concentration fourfold higher than the corresponding MICs of H2, and equal or twofold higher the MIC, in the case of H4 (Table 1). When tested in PBS, both peptides were found to be more active, with a bactericidal activity at 12.5 and 25 μM, against S. aureus ATCC 29213 and S. aureus Cowan I, respectively. Interestingly, these two peptide concentrations were equal or twofold higher than the corresponding MICs of H2 on these two strains, but they were twofold lower than the MICs of H4 (Table 1).
Mode of action studies on intact cells
As mentioned above, previous reports had emphasized the ability of these two bombinins H to permeate the membrane of Gram-negative bacteria with a stronger effect for the d-amino acid–containing peptide H4 (Mangoni et al. 2000).
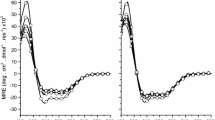
Here, the activity of the two diastereomers on the membrane permeability of Gram-positive bacteria, such as S. aureus ATCC 29213, was studied by measuring the intracellular influx of SYTOX™ Green (Fig. 1). This cationic dye (mw ≈ 600), which is excluded by intact membranes but not by those having lesions of a size large enough to allow its entrance (Marcellini et al. 2009), dramatically increases its fluorescence when bound to intracellular nucleic acids. As indicated in Fig. 1b, bombinin H4 augmented the membrane’s permeability to SYTOX™ Green to a larger extent with respect to H2 (Fig. 1a). In addition, H4 caused the complete alteration of the plasma membrane’s structure, at 2 × MIC (Fig. 1b). Conversely, in the case of bombinin H2 (Fig. 1a), a peptide concentration fourfold higher than the MIC was not enough to completely disintegrate the membrane.
Effect of bombinins H2 and H4 on the membrane permeation of S. aureus ATCC 29213. Cells (1 × 107 CFU/ml) were incubated with 1 μM SYTOX™ Green in PBS. Once basal fluorescence reached a constant value, the peptide was added (arrow, t = 0) and changes in fluorescence were monitored (λexc = 485 nm, λems = 535 nm) and plotted as the percentage of fluorescence relative to that of bacteria fully permeated by the addition of Triton X-100 (1%, v/v, final concentration). All readings were normalized by subtracting fluorescence values of control (bacteria without peptide). Data points represent the average values of three independent experiments, with SD not exceeding 5%. Peptide concentrations used for bombinin H2 (a) and H4 (b) were as follows: 3.125 μM (open triangle), 6.25 μM (filled square), 12.5 μM (open circle), 25 μM (filled circle), and 50 μM (filled triangle)
Mode of action studies on model membranes
Peptide insertion into PE/PG and PG/CL monolayers
Monomolecular lipids have been increasingly used as suitable model systems to analyze the interaction of peptides (and proteins) with biological membranes (Brockman 1999; Maget-Dana 1999; Zhao and Kinnunen 2002). We therefore used PE/PG (7:3, w/w) and PG/CL (6:4, w/w) monolayers to mimic the lipid composition of the bacterial plasma membrane of the Gram-negative E. coli and the Gram-positive S. aureus, respectively (Epand and Epand 2009).
The surface activity of H2 and H4, recorded in the absence of lipids, is shown in Fig. 2. Both peptides were found to be highly surface active, with surface pressure rising rapidly with increasing peptide concentration. Maximum surface pressure reached 30.4 and 33.9 mN/m for H2 and H4, respectively, at a peptide concentration of 2 μM. Of note, H4 was always found to be more surface active than H2, especially at lower peptide concentrations.
The surface activity of bombinins H2 and H4. The adsorption of peptides at the air/buffer (potassium phosphate 50 mM, pH 7, with 0.1 mM EDTA) interface was monitored as increasing surface pressure (π 0) over time. The maximum values of these surface pressures were plotted against peptide’s final subphase concentration. Values are means ± SD of three independent measurements
Bombinins H2 and H4 efficiently and readily penetrated into PG/CL and PE/PG monolayers, as demonstrated by the increase in film surface pressure (Fig. 3a, b).
Insertion of H2 and H4 into lipid monolayers. Increments of surface pressure of PG/CL (a) and PE/PG (b) monolayers due to the addition of 1.0 μM H2 and H4 into the subphase are illustrated as a function of initial surface pressure. Typical kinetics of surface pressure increase related to H2 penetration into a PG/CL film (c; π 0 = 11.8, with 1.0 μM peptide) and of H4 into a PE/PG monolayer (d; π 0 = 14.2, with 1.0 μM peptide) are shown as representative of general trends. X-axis shows elapsed time (s). Peptide injection into the subphase took place at ≈200 s (arrow)
Through analysis of measurements in terms of Δπ versus π 0, the critical surface pressure corresponding to the lipid lateral packing density preventing the intercalation of H2 and H4 into the PG/CL film could be derived by extrapolating the Δπ − π 0 slope to Δπ = 0, giving a value ≈43 mN/m for both peptides (Fig. 3a). In the case of the PE/PG monolayer, this value increased to 44 and 47 mN/m for H2 and H4, respectively (Fig. 3b). It is well appreciable that in the case of both PE/PG and PG/CL films, H4 displayed a constantly stronger intercalation activity with respect to H2, although this difference was not dramatic. Such differences were not found when the intercalation of H2 and H4 was tested in neutral dipalmitoylphosphatidylcholine (DPPC) monolayers (data not shown), showing that the lipid composition of the model membrane drove the different behavior of H2 and H4, and that the stronger film intercalation activity detected for H4 was not (only) due to its inherently greater surface activity. The kinetics of the insertion of the peptides into lipid monolayers was also analyzed and found to be comparable for both H2 and H4 and both monolayer compositions (Fig. 3c, d). In general, the kinetics were characterized by a very fast initial peptide intercalation subsequent to the peptide’s injection into the subphase, followed by a rather stable value of π, with a few signs of relaxation or peptide’s reorganization. This general kinetics pattern was apparently independent from the specific peptide tested and from initial surface pressure (Fig. 3c, d).
The very rapid kinetics of intercalation recorded resembles that found previously for the frog skin-derived AMP temporins (Zhao and Kinnunen 2002) and fits well with the strong surface activity of bombinins H, as discussed above.
Peptide-induced liposome permeabilization
The ability of H2 and H4 to destabilize bacterial membranes by a nonstereospecific process was confirmed by employing calcein-loaded PE/PG and PG/CL liposomes.
Different concentrations of each peptide were added to a suspension of lipid vesicles (50 μM final lipid concentration), and membrane permeabilization was monitored by following fluorescence recovery due to calcein leakage from the liposomes. Figure 4 (panels a and c) shows the kinetics and the dose-response of peptide-induced calcein release from PE/PG liposomes. The data highlighted a comparable membrane-perturbing activity for both bombinins H with a slightly stronger effect for the isoform H4, at a peptide concentration range of 1–2.5 μM. For both peptides, the vesicle-permeabilization activity increased in a concentration-dependent manner and reached the maximum effect at a peptide concentration of 2.5 μM. Similarly to what was seen with calcein-loaded vesicles, when the peptides were analyzed for their ability to cause the release of fluorescently labeled dextrans of different size (4/20/70 kDa) from PE/PG liposomes, both H2 and H4 displayed basically the same activity, with the probe being released in a manner that depended significantly on the size of the solute (Table 3).
Calcein leakage from PE/PG (7:3, w/w; panels a and c) or PG/CL (6:4, w/w; panels b and d) vesicles, after bombinin H2 (left panels) and H4 (right panels) treatment. Calcein release from liposomes (50 μM final lipid concentration) was detected fluorimetrically (λexc = 490 nm, λems = 517 nm). The percentage of leakage was calculated as described in “Materials and methods”. Data points represent the average values of three independent measurements, with SD not exceeding 3%. Peptide concentrations used were as follows: 0.25 μM (open circle), 0.5 μM (asterisk), 0.75 μM (filled circle), 1 μM (open square), 1.5 μM (filled square), 2.5 μM (open triangle), 3 μM (filled diamond), 3.5 μM (open diamond), 7.5 μM (cross symbol), 10 μM (filled triangle), 15 μM (filled inverted triangle)
The results obtained with PG/CL liposomes are reported in Fig. 4 (panels b and d). Both bombinins H displayed a generally lower activity on this membrane composition than that recorded for PE/PG liposomes. Interestingly, in this case, bombinin H4 appeared to be remarkably more potent than the all-L H2, giving rise to a total membrane permeation at a peptide concentration (3.5 μM) that was approximately fourfold lower than that needed for H2. These observations are in line with the results of the membrane-permeability assay described above, using intact cells (Fig. 1) and are consistent with the suggestion that the two isomers bind and perturb the membrane of Gram-positive bacteria with a different effectiveness.
Peptide translocation through a PE/PG membrane
To get insight into the ability of these two frog skin peptides to translocate through a model of E. coli inner membrane, an enzyme digestion assay was developed. As described in “Materials and methods,” to this end, trypsin-loaded PE/PG liposomes were incubated with peptides. In the absence of liposomes, both bombinins H were susceptible to trypsin action (two trypsin cleavage sites are present at the C-terminal region of each peptide), as proved by the appearance of novel peaks in the electropherograms of the peptide samples upon enzyme treatment (data not shown).
When trypsin-loaded liposomes were used, in the case of bombinin H2, only two very small peaks, at a migration time in capillary electrophoresis (t m ) of approximately 9 and 11 min, could be detected within 2 h of incubation with the peptide (Fig. 5a, gray and white bars, respectively), whereas in the presence of SBTI, no peptide degradation was discerned at all (Fig. 5b). On the other hand, with bombinin H4, a partial degradation of the peptide was detected within the first 60 min of incubation with the liposomes, as indicated by the presence of three peaks at 4, 9, and 11 min t m (Fig. 5c, light gray, gray and white bars, respectively). Note that the peak at 4 min t m was not present in the electropherograms of H2. In addition, the amount of one specific degradation product (peak at 9 min t m ) increased with the incubation time, making up to 30% of total digestion products after 2 h (Fig. 5c). In the presence of SBTI, H4 degradation was strongly reduced (Fig. 5d), probably due to the influx of SBTI through the peptide-induced membrane cracks and, therefore, to the inhibition of trypsin activity inside the liposome.
Detection of bombinin H2 (left panels) and H4 (right panels) translocation into PE/PG (7:3, w/w) liposomes, using the trypsin digestion assay. Trypsin-loaded liposomes (250 μg/ml) were incubated with 60 μM peptides either in the absence (panels a and c) or in the presence (panels b and d) of SBTI. At different time points (0, 60, and 120 min), aliquots were taken, boiled for 5 min, and lysed in 0.1% Triton X-100. Samples were analyzed by CZE using imidazole as internal standard. Peptide degradation is indicated by the appearance of peptide fragments with different t m . Black bars indicate the peaks area at 7 min t m , corresponding to the intact peptides. Light gray, gray, and white bars indicate peaks at 4, 9, and 11 min t m , respectively. All values are the averages of three different experiments with SD not exceeding 2.5% and are expressed as percentages with respect to the internal standard
Discussion
The secretory glands of amphibian skin produce a multitude of bioactive peptides, many with antimicrobial activities, thus representing important constituents of the innate immune system of these animals (Boman 2000; Zasloff 2002). In particular, from the Bombina genus, several bombinin peptides have been discovered, as well as the structurally unrelated bombinins H, whose peculiar characteristic is the presence of a d-amino acid in the sequence of some of them. Most AMPs are unfolded in solution and acquire their active conformation only upon binding to their target membrane (Bechinger and Lohner 2006). Bombinins H2 and H4 are not an exception to this rule. Molecular dynamic simulations have shown that both peptides have an intrinsic propensity to fold when still in water, with H4 being less constrained and more flexible due to the inability of its d-alloisoleucine in position 2 to establish intramolecular contacts (Bozzi et al. 2008).
Environmental conditions, as well as the composition and the physical state of the phospholipid bilayer may be crucial for the ultimate peptide structure and mechanism of action. Circular dichroism (CD) spectra have shown that in a membrane-mimicking environment, such as trifluoroethanol, both H2 and H4 adopt predominantly an amphiphatic α-helical structure (Bozzi et al. 2008; Mangoni et al. 2000). More detailed CD and FTIR analyses of H2 and H4 structural features in lipid bilayers of different composition have confirmed that both peptides adopt mainly an α-helical structure—with H2 being slightly more helical than H4 in all membranes—and display a β-sheet component ranging from 14 to 33% (Mangoni et al. 2006). The same study also revealed that H2, but not H4, has the tendency to aggregate in membranes, in the form of aggregated strands (Mangoni et al. 2006).
In the present work, we have found that the two diastereomers H2 and H4 do possess different antimicrobial activity, with H4 being generally more active on Gram-negative bacteria (either standard or clinical strains), but less active on the majority of Gram-positive bacteria with respect to H2, when the microbroth dilution method is used to determine the MIC. Presumably, when the peptides are used in a bacterial growth medium at low concentrations, H2 is more potent than H4 in blocking those cellular functions that are involved in the control of the microbial growth, in Gram-positive bacteria, without significantly affecting the integrity of the plasma membrane and hence the bacterial viability. Nevertheless, a different outcome is found when the two peptides are tested for their bactericidal activity against a representative Gram-positive bacterial species, i.e., S. aureus. Indeed, when bacteria are incubated in MHB (the same growth medium used for MIC assays), 99.9% killing of the microbial population is caused by H4 at a concentration equal or twofold higher than its MIC (Table 2), while in the case of H2, a peptide concentration fourfold higher the corresponding MIC is necessary to get the same bactericidal effect. This could be due to the formation of larger membrane ruptures by H4, compared to H2, which is in agreement with the higher affinity of H4 towards PG/CL phospholipid monolayers (Fig. 3) and to its higher membrane-perturbing activity for this lipid composition. These latter effects are highlighted by the results of the permeabilization of PG/CL liposomes when monitoring the calcein release (Fig. 4), and by the results of the permeabilization of S. aureus cell membrane when monitoring the intracellular influx of SYTOX Green (Fig. 1).
In the case of Gram-negative bacteria, a higher antimicrobial and membrane-perturbing activity was formerly demonstrated for the d-isoform H4 (Mangoni et al. 2000). However, working with PE/PG liposomes to mimic the lipid composition of E. coli inner membrane, we have observed that both bombinins H are similarly capable of injuring this type of membrane (Fig. 4 and Table 3), although a slightly stronger membrane-perturbing activity of H4 with respect to H2 is discernable, and this is also true in the case of the peptides’ intercalation into a PE/PG monolayer (Fig. 3). Therefore, the discrepancies found between these two diastereomers when intact bacteria are used might be attributed to differences in their diffusion process through the Gram-negative cell wall before reaching the target cytoplasmic membrane. Note that the outer membrane component of the cell wall of Gram-negative bacteria, the first barrier AMPs are faced with before reaching the plasma membrane, is made by a complex mixture of lipopolysaccharides (LPS), phospholipids, lipoproteins, and proteins.
In addition, our membrane translocation experiments, employing PE/PG trypsin-loaded liposomes, have indicated only a tiny degradation of the all-l H2 in the absence of the trypsin inhibitor SBTI. A possible explanation for this finding is that H2 molecules present in the sample bind the model membrane resembling that of Gram-negative bacteria in a highly aggregated peptide-lipid molecular complex not available for trypsin cleavage, but efficient enough to perturb the membrane causing the leakage of either the low-molecular-weight calcein and of liposome-preincapsulated dextrans of increasing size (Table 3). The aggregation tendency of H2 has been reported before (Mangoni et al. 2006) and discussed above, and both H2 and H4 were shown previously to cause the release of β-galactosidase from E. coli cells in a dose-dependent manner (Mangoni et al. 2000).
Thus, as found for other frog-skin AMPs such as temporins (Rinaldi et al. 2001), it is very likely that bombinins H cause marker leakage from lipid vesicles (and possibly from microbial target cells)—not thanks to a total membrane disruption (detergent-like effect), but rather by perturbing the bilayer organization on a local scale. Concerning H4, a partial peptide degradation by trypsin entrapped into PE/PG liposomes was detected. This might be related to a more flexible conformation of H4, compared to the more structured and aggregated H2 (Bozzi et al. 2008; Mangoni et al. 2006), which would make it easier for H4 to traverse the phospholipids bilayer.
Overall, we can conclude that bombinins H2 and H4 are membrane-active peptides whose different bactericidal activity on Gram-negative and Gram-positive bacteria can be assigned to a different behavior in their binding affinity and permeation of the target cytoplasmic membrane, and/or in their translocation through the cell wall/plasma membrane of the target cell. In general, we can state that the d-amino acid–containing bombinin H4 is more efficient than bombinin H2 at disturbing bacterial membranes.
Bombinin H4 was also earlier indicated to be more active against the protozoan pathogen Leishmania, binding a model of its membrane with a fivefold greater affinity than bombinin H2 (Mangoni et al. 2006).
In the case of Gram-positive bacteria, such as S. aureus, the data reported in this work show a higher ability of bombinin H4 to wreck the structure of phospholipid bilayers of these microorganisms, as supported by the results obtained working with intact cells and model membranes (Figs. 1, 3, and 4).
On the other side, in the case of Gram-negative strains, the higher membrane-perturbing activity of H4 (Mangoni et al. 2000) would reflect its easier partition into the inner cytoplasmic membrane. Furthermore, a partial membrane crossing activity could occur for H4, but not for H2, also in living bacteria. Once having crossed the membrane, H4 might influence the activity of intracellular functions, thus reinforcing the membrane perturbation effect and contributing to killing the microbial cell.
However, we cannot rule out, at this stage, the possibility that additional factors (e.g., interaction with peptidoglycan, LPS layer, and other cell surface components) could induce mechanical stress affecting the cell wall/membrane structure and thus the antimicrobial activity of the two bombinin diastereomers in a different way.
Finally, both bombinins H were also found to preserve their antimicrobial properties in high-ionic strength media resembling physiological environments, which is an important feature for molecules to be used as templates for the development of new drugs.
Importantly, besides getting insight into the molecular mechanism of a pair of natural peptide diastereomers at the level of Gram-positive and Gram-negative bacterial membranes, these studies have expanded our knowledge of the effect of a single l-to-d epimerization on the target cell selectivity of an AMP and its mode of action.
Abbreviations
- AMP:
-
Antimicrobial peptide
- CD:
-
Circular dichroism
- CFU:
-
Colony-forming units
- CL:
-
Cardiolipin
- CZE:
-
Capillary zone electrophoresis
- FITC-D4/D20/D70:
-
Fluorescein isothiocyanate-dextrans of 4, 20, and 70 kDa average molecular mass, respectively
- LPS:
-
Lipopolysaccharide
- MHB:
-
Mueller-Hinton broth
- MIC:
-
Minimum inhibitory concentration
- PBS:
-
Phosphate-buffered saline
- PE:
-
l-α-phosphatidylethanolamine
- PG:
-
l-α-phosphatidyl-dl-glycerol
- SBTI:
-
Soybean trypsin inhibitor
- SDS:
-
Sodium-dodecyl sulphate
- SPB:
-
Sodium phosphate buffer, pH 7.4 with 0.1 mM EDTA
References
Allen TM (1981) A study of phospholipid interactions between high-density lipoproteins and small unilamellar vesicles. Biochim Biophys Acta 640:385–397
Bechinger B, Lohner K (2006) Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim Biophys Acta 1758:1529–1539
Beisswenger C, Bals R (2005) Functions of antimicrobial peptides in host defense and immunity. Curr Protein Pept Sci 6:255–264
Boman HG (2000) Innate immunity and the normal microflora. Immunol Rev 173:5–16
Boman HG (2003) Antibacterial peptides: basic facts and emerging concepts. J Intern Med 254:197–215
Bozzi A, Mangoni ML, Rinaldi AC, Mignogna G, Aschi M (2008) Folding propensity and biological activity of peptides: the effect of a single stereochemical isomerization on the conformational properties of bombinins in aqueous solution. Biopolymers 89:769–778
Brockman H (1999) Lipid monolayers: why use half a membrane to characterize protein-membrane interactions? Curr Opin Struct Biol 9:438–443
Buczek O, Yoshikami D, Bulaj G, Jimenez EC, Olivera BM (2005) Post-translational amino acid isomerization: a functionally important D-amino acid in an excitatory peptide. J Biol Chem 280:4247–4253
CLSI (2006) Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard, 7th edn. CLSI document M7–A7, vol. 26, no.2. Clinical and Laboratory Standards Institute, Wayne
Dempsey CE, Hawrani A, Howe RA, Walsh TR (2010) Amphipathic antimicrobial peptides—from biophysics to therapeutics? Protein Pept Lett 17:1334–1344
Den Hertog AL, Wong Fong Sang HW, Kraayenhof R, Bolscher JG, Van’t Hof W, Veerman EC, Nieuw Amerongen AV (2004) Interactions of histatin 5 and histatin 5-derived peptides with liposome membranes: surface effects, translocation and permeabilization. Biochem J 379:665–672
Easton DM, Nijnik A, Mayer ML, Hancock RE (2009) Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol 27:582–590
Epand RM, Epand RF (2009) Lipid domains in bacterial membranes and the action of antimicrobial agents. Biochim Biophys Acta 1788:289–294
Erspamer V, Melchiorri P, Falconieri-Erspamer G, Negri L, Corsi R, Severini C, Barra D, Simmaco M, Kreil G (1989) Deltorphins: a family of naturally occurring peptides with high affinity and selectivity for delta opioid binding sites. Proc Natl Acad Sci USA 86:5188–5192
Ganz T (2003) Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol 3:710–720
Gibson BW, Tang DZ, Mandrell R, Kelly M, Spindel ER (1991) Bombinin-like peptides with antimicrobial activity from skin secretions of the Asian toad, Bombina orientalis. J Biol Chem 266:23103–23111
Giuliani A, Rinaldi AC (2010) Antimicrobial peptides. Methods and protocols. Methods in molecular biology, vol 618. Humana Press, New York
Hancock RE, Diamond G (2000) The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol 8:402–410
Jilek A, Mollay C, Tippelt C, Grassi J, Mignogna G, Mullegger J, Sander V, Fehrer C, Barra D, Kreil G (2005) Biosynthesis of a D-amino acid in peptide linkage by an enzyme from frog skin secretions. Proc Natl Acad Sci USA 102:4235–4239
Kamatani Y, Minakata H, Kenny PT, Iwashita T, Watanabe K, Funase K, Sun XP, Yongsiri A, Kim KH, Novales-Li P et al (1989) Achatin-I, an endogenous neuroexcitatory tetrapeptide from Achatina fulica Ferussac containing a D-amino acid residue. Biochem Biophys Res Commun 160:1015–1020
Kreil G (1994) Conversion of L- to D-amino acids: a posttranslational reaction. Science 266:996–997
Kreil G (1997) D-amino acids in animal peptides. Annu Rev Biochem 66:337–345
Kuwada M, Teramoto T, Kumagaye KY, Nakajima K, Watanabe T, Kawai T, Kawakami Y, Niidome T, Sawada K, Nishizawa Y et al (1994) Omega-agatoxin-TK containing d-serine at position 46, but not synthetic omega-[L-Ser46]agatoxin-TK, exerts blockade of P-type calcium channels in cerebellar Purkinje neurons. Mol Pharmacol 46:587–593
Li XZ, Poole K, Nikaido H (2003) Contributions of MexAB-OprM and an EmrE homolog to intrinsic resistance of Pseudomonas aeruginosa to aminoglycosides and dyes. Antimicrob Agents Chemother 47:27–33
MacDonald RC, MacDonald RI, Menco BP, Takeshita K, Subbarao NK, Hu LR (1991) Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim Biophys Acta 1061:297–303
Maget-Dana R (1999) The monolayer technique: a potent tool for studying the interfacial properties of antimicrobial and membrane-lytic peptides and their interactions with lipid membranes. Biochim Biophys Acta 1462:109–140
Maisetta G, Batoni G, Esin S, Florio W, Bottai D, Favilli F, Campa M (2006) In vitro bactericidal activity of human beta-defensin 3 against multidrug-resistant nosocomial strains. Antimicrob Agents Chemother 50:806–809
Mangoni ML, Grovale N, Giorgi A, Mignogna G, Simmaco M, Barra D (2000) Structure-function relationships in bombinins H, antimicrobial peptides from Bombina skin secretions. Peptides 21:1673–1679
Mangoni ML, Papo N, Saugar JM, Barra D, Shai Y, Simmaco M, Rivas L (2006) Effect of natural L- to D-amino acid conversion on the organization, membrane binding, and biological function of the antimicrobial peptides bombinins H. Biochemistry 45:4266–4276
Mangoni ML, Marcellini L, Simmaco M (2007) Biological characterization and modes of action of temporins and bombinins H, multiple forms of short and mildly cationic anti-microbial peptides from amphibian skin. J Pept Sci 13:603–613
Mangoni ML, Maisetta G, Di Luca M, Gaddi LM, Esin S, Florio W, Brancatisano FL, Barra D, Campa M, Batoni G (2008) Comparative analysis of the bactericidal activities of amphibian peptide analogues against multidrug-resistant nosocomial bacterial strains. Antimicrob Agents Chemother 52:85–91
Marcellini L, Borro M, Gentile G, Rinaldi AC, Stella L, Aimola P, Barra D, Mangoni ML (2009) Esculentin-1b(1–18)–a membrane-active antimicrobial peptide that synergizes with antibiotics and modifies the expression level of a limited number of proteins in Escherichia coli. FEBS J 276:5647–5664
Montecucchi PC, de Castiglione R, Piani S, Gozzini L, Erspamer V (1981) Amino acid composition and sequence of dermorphin, a novel opiate-like peptide from the skin of Phyllomedusa sauvagei. Int J Pept Protein Res 17:275–283
Mor A, Amiche M, Nicolas P (1992) Enter a new post-translational modification: D-amino acids in gene-encoded peptides. Trends Biochem Sci 17:481–485
Rinaldi AC (2002) Antimicrobial peptides from amphibian skin: an expanding scenario. Curr Opin Chem Biol 6:799–804
Rinaldi AC, Di Giulio A, Liberi M, Gualtieri G, Oratore A, Bozzi A, Schininà ME, Simmaco M (2001) Effects of temporins on molecular dynamics and membrane permeabilization in lipid vesicles. J Pept Res 58:213–220
Rinaldi AC, Mangoni ML, Rufo A, Luzi C, Barra D, Zhao H, Kinnunen PK, Bozzi A, Di Giulio A, Simmaco M (2002) Temporin L: antimicrobial, haemolytic and cytotoxic activities, and effects on membrane permeabilization in lipid vesicles. Biochem J 368:91–100
Selsted ME, Ouellette AJ (2005) Mammalian defensins in the antimicrobial immune response. Nat Immunol 6:551–557
Shai Y (1999) Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta 1462:55–70
Simmaco M, Barra D, Chiarini F, Noviello L, Melchiorri P, Kreil G, Richter K (1991) A family of bombinin-related peptides from the skin of Bombina variegata. Eur J Biochem 199:217–222
Simmaco M, Mignogna G, Barra D (1998) Antimicrobial peptides from amphibian skin: what do they tell us? Biopolymers 47:435–450
Simmaco M, Kreil G, Barra D (2009) Bombinins, antimicrobial peptides from Bombina species. Biochim Biophys Acta 1788:1551–1555
Stewart JC (1980) Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem 104:10–14
Szoka F Jr, Papahadjopoulos D (1978) Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc Natl Acad Sci USA 75:4194–4198
Torres AM, Menz I, Alewood PF, Bansal P, Lahnstein J, Gallagher CH, Kuchel PW (2002) D-amino acid residue in the C-type natriuretic peptide from the venom of the mammal, Ornithorhynchus anatinus, the Australian platypus. FEBS Lett 524:172–176
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395
Zhao H, Kinnunen PK (2002) Binding of the antimicrobial peptide temporin L to liposomes assessed by Trp fluorescence. J Biol Chem 277:25170–25177
Acknowledgments
This work was supported by grants from Università di Roma La Sapienza, MIUR (Italian Ministry of Education, University and Research; PRIN 2008), and Istituto di Biologia e Patologia Molecolari of the Italian National Research Council (CNR).
Author information
Authors and Affiliations
Corresponding author
Additional information
Membrane-active peptides: 455th WE-Heraeus-Seminar and AMP 2010 Workshop.
Rights and permissions
About this article
Cite this article
Coccia, C., Rinaldi, A.C., Luca, V. et al. Membrane interaction and antibacterial properties of two mildly cationic peptide diastereomers, bombinins H2 and H4, isolated from Bombina skin. Eur Biophys J 40, 577–588 (2011). https://doi.org/10.1007/s00249-011-0681-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-011-0681-8