Abstract
To study the role of exoelectrogens within the trophic network of deep-sea hydrothermal vents, we performed successive subcultures of a hyperthermophilic community from a hydrothermal chimney sample on a mix of electron donors in a microbial fuel cell system. Electrode (the electron acceptor) was swapped every week to enable fresh development from spent media as inoculum. The MFC at 80 °C yielded maximum current production increasing from 159 to 247 mA m−2 over the subcultures. The experiments demonstrated direct production of electric current from acetate, pyruvate, and H2 and indirect production from yeast extract and peptone through the production of H2 and acetate from fermentation. The microorganisms found in on-electrode communities were mainly affiliated to exoelectrogenic Archaeoglobales and Thermococcales species, whereas in liquid media, the communities were mainly affiliated to fermentative Bacillales and Thermococcales species. The work shows interactions between fermentative microorganisms degrading complex organic matter into fermentation products that are then used by exoelectrogenic microorganisms oxidizing these reduced compounds while respiring on a conductive support. The results confirmed that with carbon cycling, the syntrophic relations between fermentative microorganisms and exoelectrogens could enable some microbes to survive as biofilm in extremely unstable conditions.

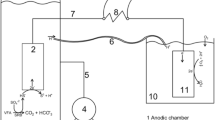
Schematic representation of cross-feeding between fermentative and exoelectrogenic microbes on the surface of the conductive support. B, Bacillus/Geobacillus spp.; Tc, Thermococcales; Gg, Geoglobus spp.; Py, pyruvate; Ac, acetate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The deep-sea hydrothermal vents located along the mid-ocean ridges of the Atlantic, Pacific, and Indian oceans [1, 2] are dynamic habitats characterized by large steep thermal and chemical gradients. Around the hydrothermal chimneys, these gradients provide a wide range of growth conditions for the many extremophilic micro- and macro-organisms composing these specific deep-sea ecosystems [3, 4]. The origin of these ecosystems is thought to be based on (hyper) thermophilic chemolithoautotrophs that use chemical energy from the oxidization of reduced chemical substances to convert inorganic carbon into organic carbon. These organics then serve as primary nutrients for the whole ecosystem [5, 6], enabling the development of heterotrophic microorganisms and macro-organisms through the food web. Studies on the microflora of deep-sea hydrothermal vents have identified hyperthermophilic autotrophs and heterotrophs of both the Bacteria and Archaea domains in the chimney structures [7]. These autotrophic and heterotrophic microorganisms use a wide diversity of metabolisms based on different electron donors (H2, H2S, CH4, Fe0, Fe2+, S0, organic matter) and acceptors (O2, NO3−, SO42−, CO2, Fe3+) found in the environment or produced by other microorganisms [8]. These diverse metabolisms support a complex food web and lend the biocenosis stability in response to critical changes in conditions.
Recent studies on samples from deep-sea hydrothermal vents have revealed a new type of metabolism based on microorganisms called exoelectrogens, which are able to transfer electrons directly from cells to an electrically conductive support like the chimney walls [9,10,11].
A decade of research has shown the possibility of electron transfer through the electrically conductive chimney composed of polymetallic massive sulfide. The electric current was not only produced by chemical reaction between reduced compounds in the hydrothermal fluid and the oxidative conditions surrounding the chimney [12], but could also be generated from biological catalysis by exoelectrogenic microorganisms [10]. Thus, a proportion of the hydrothermal vent chimney’s microbial populations is likely dependent on energetic metabolism based on these extracellular electron transfers (EET) to insoluble extracellular minerals. Most of what we know about EET in microbes comes from studies using bioelectrochemical systems such as microbial fuel cells (MFCs) [13,14,15]. A MFC is a bioelectrochemical device that supports microorganism growth on an anodic electrode, where the microorganisms oxidize substrates used as energy source and transfer electrons to the anode which serves as electron acceptor. The electron flux is then routed toward the cathodic electrode where the reduction of the terminal electron acceptor, usually the oxygen, takes place. In the past few years, exoelectrogenic activity has been reported in almost 100 mesophilic microbial species, mainly affiliated to the bacterial phylum Proteobacteria [16]. Studies have shown electric current production by in situ benthic MFCs on hydrothermal systems, with in one case the dominant presence of mesophilic Bacteriodetes, Firmicutes, and δ-Proteobacteria [17,18,19]. Just recently, three hyperthermophilic archaea, Pyrococcus furiosus, Geoglobus ahangari, and Ferroglobus placidus, were shown to be capable of producing electricity in MFCs [9, 11]. These microorganisms belonging to the orders Archaeoglobales and Thermococcales have been found in hydrothermal chimneys where they could use a conductive support like the pyrite composing the chimney wall [20] as electron acceptor for their metabolism. Another recent study showed the dominant presence of Archaeoglobales and Thermococcales in a hyperthermophilic exoelectrogenic community from a deep hydrothermal vent sample enriched using acetate as electron donor in a microbial electrolysis cell (MEC) system with the anode poised at − 0.110 V vs Ag/AgCl [10]. While only few electrogenic species should be able to grow under these very selective physicochemical conditions, it is reasonable to think the deep-sea hydrothermal ecosystem harbors a broader exoelectrogenic biodiversity.
The importance of exoelectrogens in the biosphere of hydrothermal ecosystems and their roles within the food web are still unclear. There have been attempts to investigate trophic relationships among the different species inhabiting these environments [21, 22], but most studies to date have focused on relations between microflora and macrofauna, and none have integrated exoelectrogenic ability as a potential metabolism and electron sink. Here, we set out to gain further insight into the role of hyperthermophilic exoelectrogens in the hydrothermal biosphere. An exoelectrogenic community sampled from a deep-sea hydrothermal vent was grown using a hyperthermophilic MFC system. The system was configured with a free-potential anode as sole electron acceptor, and a broad diversity of substrates was tested to enrich an exoelectrogenic community. In order to select a community including both exoelectrogens and other closely related species, the community was grown over 1 month on an anodic support that was replaced each week. Current production was monitored in real time, and changes in biodiversity of the on-electrode biofilm and free cells in the liquid media were determined at each electrode change. During the fourth week, when current density was stabilized, the different substrates (acetate, formate, pyruvate, hydrogen, glycogen, starch, yeast extract, peptone, and without substrate) were separately tested alone to determine the current density production attributable to each one. The results obtained here, along with knowledge on the metabolisms of each species retrieved on both electrode and liquid media, argue for interspecies interactions inside the community, prompting us to propose a putative syntrophic model.
Materials and Methods
Sample Collection and Preparation
A hydrothermal chimney sample was collected on the Capelinhos site on the Lucky Strike hydrothermal field (37°17.0′ N, MAR) by the remotely operated underwater vehicle VICTOR 6000 during the MoMARsat cruise in 2014 (doi: https://doi.org/10.17600/14000300) led by IFREMER (France) on board R/V Pourquoi Pas? [23]. The sample (PL583-8) was collected by breaking off a piece of a high-temperature active black smoker using the submersible’s robotic arm and bringing it back to the surface in a decontaminated insulated box. On board, chimney fragments were anaerobically crushed in an anaerobic chamber under H2:N2 atmosphere (2.5:97.5)(La Calhene, France), placed in flasks under anaerobic conditions (anoxic seawater at pH 7 with 0.5 mg L−1 of Na2S and N2:H2:CO2 (90:5:5) gas atmosphere), and stored at 4 °C. Prior to the experiment, pieces of hydrothermal chimney were removed from the sulfidic seawater flask, crushed with a sterile mortar and pestle in an anaerobic chamber (Coy Laboratories, Grass Lake, Mich.), and distributed into anaerobic tubes for use in the various experiments.
Exoelectrogenic Enrichment in a Bioelectrochemical System
A bioelectrochemical system (BES) was filled with 1.5 L of sterile mineral medium as previously described [10, 24], and set at 80 °C (monitored by an internal temperature probe). To ensure anaerobic condition, the culture medium was continually sparged with a 50-mL/min flow of N2. The pH was maintained at 6.5 ± 0.1 by the addition of sodium hydroxide (NaOH 0.5 mM) or hydrochloric acid (HCl 0.5 mM). The stirring, driven by two axial impellers, was set to 300 rpm. All the parameters, as pH and ORP (Mettler Toledo InPro 3253, Switzerland), temperature (Prosensor pt. 100, France), stirring speed, gaseous H2, N2, CH4, O2, and CO2 concentration and NaOH and HCl consumption, were measured and managed by the BatchPro software (Decobecq Automatismes, France)The liquid media were supplemented with 10-mM acetate, 10-mM formate, 10-mM pyruvate, 0.5-g L−1 yeast extract, 0.5-g L−1 peptone, 0.5-g L−1 starch, and 0.1-g L−1 glycogen as substrate. These compounds, added in excess, can be found in hydrothermal systems through Fischer–Tropsch process reactions, produced by microorganisms for energy storage or released during cell degradation. The working electrode (WE) composed of 20 cm2 of carbon cloth (PaxiTech SAS, France) served as a potential electron acceptor for exoelectrogen growth. An anion exchange membrane (Membrane International Inc., Ringwood, NJ) separated the two chambers. The counter electrode (CE) was a 20-cm2 carbon cloth coated with platinum. The system was inoculated with 5 g of crushed chimney in anaerobic conditions. Current production was monitored using SP-240 potentiostats and EC-Lab software (BioLogic, France) via the constant load discharge method, with titanium wire connected to the WE and CE and to a 3M Ag/AgCl reference electrode (Mettler Toledo InPro 3253, Switzerland). Current density and WE and CE potential were measured every 10 s. The resistance imposed between the WE and CE was set at 700 Ω, based on the maximum power density obtained on the polarization curve (see Supplementary Fig. 1), and the system’s internal resistance of 675 Ω. Gas production during experiment was monitored every 3 min as previously described [24], using a micro-GC equipped with a catharometric detector (MS5A, SRA Instrument, France) and a CARBOCAP CO2 probe (Vaisala GMT 221, Finland) with a sensibility threshold at 0.001%. The WE was swapped three times for a new sterile one, once maximum current density had stabilized for a few days, adding 1250 mL of fresh mineral medium supplemented with substrates (see Supplementary Material & Methods). A volume of 250 mL of spent media from the previous experiment with the environmental inoculum (crushed chimney) was left in the bottom of the reactor before starting each subculture. After the first week, an open-circuit control was performed in a similar way as for week 2 enrichment but with the electrodes disconnected from the potentiostat. Tests on substrates were carried out succesively by emptying the spent medium and filling with fresh medium supplemented with a single substrate in the same concentrations as described above. Tests on H2 were carried out at 50 mL min−1 of H2 flux sparged in the agitated bioreactor, which is far higher than observed by fermentation in order to overcome the lack of information on the solubility of our H2 gas supply compared with the solubility of H2 produced by fermentation in close vicinity to the electrode. An abiotic control with H2 was performed in rich liquid media, but no current production was observed. A spent media control was performed in sterile BES filled with 0.2-μm filtered culture media from the end of first culture. A killed inoculum control was performed in same conditions as the enrichment experiment but inoculated with chimney sample sterilized at 121 °C during 20 min. Cyclic voltammetries were carried out at initial time and prior to collecting the WE at 20 mV s−1 between − 0.5 and 0.5 V vs the Ag/AgCl reference electrode. Due to the limited inoculum quantity and accessibility and its quick degradation when opened, even in anaerobic conditions, the experiment could not be replicated.
Biodiversity Analysis
Taxonomic affiliation was performed according to [25]). DNA was extracted from 1 g of the crushed chimney and at the end of each week of culture from scrapings of half of the WE and from centrifuged pellets of 50 mL of spent media. DNA extraction was carried out using the PowerSoil DNA Isolation Kit (MoBio laboratories, Inc., Carlsbad, CA). The V4 region of the 16S rRNA gene was amplified using the universal primers 515F (5′-GTG CCA GCM GCC GCG GTA A-3′) and 806R (5′-GGA CTA CNN GGG TAT CTA AT-3′) [26] with Taq-&Load PCR MasterMix (Promega, Madison, WI). PCR reactions were carried out using a C1000 Thermal Cycler (BioRad) in the following conditions: initial denaturation at 94 °C for 3 min followed by 30 denaturation cycles at 94 °C for 30 s, primer annealing at 50 °C for 30 s and extension at 72 °C for 90 s, followed by a final extension step at 72 °C for 5 min. The amplified gene regions were sequenced on an Illumina MiSeq 2500 platform (GeT-PlaGe, France) to generate paired-end 150-bp reads. Reads were merged using FLASH software (V1.2.7, http://ccb.jhu.edu/software/FLASH/). Taxonomic affiliation was done using the QIIME software package (v 1.9.1, http://qiime.org/index.html). Chimera were removed from the merged sequence using the UCHIME algorithm (http://www.drive5.com/usearch/manual/uchime_algo.html). The filtered sequences were then clustered into OTUs using the RDP method with a minimum bootstrap confidence of 0.8, and OTUs were affiliated using the Silva database as reference (http://www.arb-silva.de). To analyze alpha diversity, the OTU tables were rarefied to a sampling depth of 13,190 sequences per library, and two metrics were calculated: the richness component represented by number of OTUs observed, and the evenness index (Pielou’s index) which measures distribution of individuals within species independently of species richness. Rarefaction curves (see Supplementary Fig. 2) for each enrichment approached an asymptote, suggesting that the sampling depths were sufficient to capture overall microbial diversities in the studied samples. Week 4 biodiversity was not studied due to the bias of further analysis on substrates which would have specifically influenced the exoelectrogenic community, so we cannot compare week 4 biodiversity to weeks 1–3. The raw sequences for all samples are registered in the European Nucleotide Archive (accession number: PRJEB25397).
Quantitative PCR of Archaeal and Bacterial 16S rRNA Gene Copies
Bacterial and archaeal quantification was carried out by qPCR with SsoAdvanced™ Sybr Green Supermix on a CFX96 Real-Time System (C1000 Thermal Cycler, Bio-Rad Laboratories, CA) with the primers GML5F (5′-GCC TAC GGG AGG CAG CAG-3′) and Univ516 (5′-GTD TTA CCG CGG CKG CTG RCA-3′) specific to Bacteria [27] and Arc931F (5′-AGG AAT TGG CGG GGG AGC A-3′) and m1100R (5′-BTG GGT CTC GCT CGT TRC C-3′) for Archaea [28]. The PCR program was composed of a 10-s denaturation step at 94 °C, a hybridization step of 10 s at 55 °C (Bacteria) or 62 °C (Archaea), and a 10-s elongation step at 72 °C, with melting curves generated at the end of each reaction to ensure product specificity. A standard curve from 102 to 1010 16S rRNA gene copies was obtained by diluting pGEM-T plasmids harboring hyperthermophilic bacterial or archaeal rRNA gene fragments obtained from the microbial community of interest. Results were expressed in number 16S rRNA gene copies per gram of crushed chimney per milliliter of liquid media or per square centimeter of electrode surface.
Microscopy Observation with Fluorescent In Situ Hybridization
Prior to microscopic observation, the WE and the liquid media from the end of the week 2 experiment were fixed with 2% paraformaldehyde and kept at 4 °C. The liquid media (100 mL) were then filtrated on a 0.2-μm polycarbonate Whatman Nuclepore membrane. To highlight the presence of microorganisms on the electrode surface, Syto9 was used as total fluorescent nucleic acid stain. FISH probes specific to Bacteria (EUB338-FITC), Archaea (ARCH917-CY3), Thermococcales (Tcoc164-FITC), and Archeoglobales (Arglo32-FITC) [29] were used separately on different areas of the electrode. Total cell staining was performed by immersing a patch of electrode in 10 μM of Syto9 for 15 min then rinsing it several times in sterile water. For FISH observation, samples of WE or cells on filter were incubated with fluorescent probes in an equilibrated humidity chamber at 48 °C for 2–6 h and then washed with washing buffer at 42 °C for 15 min. Samples were then dried in 80% ethanol and mounted on glass slides with antifadent AF1 (Citifluor, Hatfield, PA) added with 4′-6′-diamidino-2-phenylindole (DAPI) (Sigma Aldrich) as counterstain at a final concentration of 2 μg mL−1. Electrode samples were observed under a confocal LSM780 microscope (ZEISS, Germany) equipped with a × 10 EC PLAN-NEOFLUAR objective. Blue and green fluorescence emissions were acquired by laser excitation at 358 and 488 nm, respectively. Image stacks (at 0.5–1 μm steps) were acquired with GaAsP photomultiplier tube detectors. Epifluorescence micrographs were processed using Zen software (ZEISS, Germany) and Fiji software (http://imagej.nih.gov/;http://fiji.sc/Fiji). Liquid samples on filters were observed on an Olympus BX61 microscope with a Hamamatsu digital camera c11440, and processed with an Olympus cellSens™ software.
Results
Exoelectrogenic Community Enrichments and Current Density Production
To allow the growth of a hyperthermophilic exoelectrogenic biofilm (HEB) with a maximum of biodiversity and trophic relations, a MFC was filled with synthetic seawater supplemented with a mix of different carbon and energy sources (acetate, formate, pyruvate, yeast extract, peptone, glycogen, and starch). Current density production was measured using the constant load discharge (CLD) method with a fixed external resistance (700 Ω) in the circuit between anode and cathode. Once current stabilization was reached after a few days, the anode was replaced by a sterile one to allow a new development of exoelectrogenic community. Four successive enrichments were done during 4 weeks in order to stabilize the exoelectrogenic community over the subcultures.
The current density production results are reported in Fig. 1 and Table 1. Over the subcultures, current densities increased from 159 to 247 mA m−2. This was correlated to an increase of electrical power density (Pmax from 49 to 110 mW m−2) while lag time before current production decreased from 52 h (week 1) to 5 h (week 4) (Table 1). These current density and maximum power density values are in good agreement with another study on (hyper) thermophilic MFCs operated at temperature between 55 to 95 °C in similar carbon/energy-source conditions, which found current density ranging from 100 to 350 mA m−2 and maximum power density ranging from 37 to 165 mW m−2 [30, 31]. The increase of maximum current density coupled to the decrease of lag phase before current production over each subculture suggests a specialization of the community colonizing the electrode used as an electron acceptor. The increase in current was concomitant with a decrease in H2 and CO2 production down to 0.08%(v/v) for both in the MFC gas outlet (100 mL min−1), which is typical of fermentative metabolism occurring in the liquid media. In contrast, the abiotic, spent media, and inoculum-killed controls produced a weak current (30–40 mA m−2) during the first few hours which rapidly decreased to nearly 5–10 mA m−2 with no gas production, whereas the open-circuit control showed higher production of H2 and CO2 up to 0.2% (v/v) in the gas outlet (see Supplementary Fig. 3).
To determine the potential of the oxidation and reduction reactions by the exoelectrogenic biofilm, cyclic voltammetry measurements were performed at the end of each subculture when current density had stabilized. Figure 2 shows the increase in catalytic and capacitive currents after each subculture corresponding to a change in electrochemical properties of the electrode surface that may indicate the development of a HEB. The catalytic current was higher and more distinct than for abiotic controls, suggesting that the current was not caused by the complex medium. The week 4 voltammogram of the MFC anode showed two catalytic oxidation waves: (i) the first started at − 0.5 V and increased sharply around 1.8 mA at a midpoint potential of about − 0.360 V; (ii) the second started at − 0.18 V at a midpoint potential of − 0.014 V. These oxidation waves increased over the weeks of culture and correspond to the oxidation of different compounds that may be catalyzed by the electroactive biofilm. Similar midpoint potentials at − 0.350 V vs. Ag/AgCl electrode have been observed in previous investigations of thermophilic exoelectrogenic enrichments on acetate [11, 32]. A pattern difference was observed in a previous study in MEC inoculated with hydrothermal chimney (Pillot et al., 2018) showing less capacitive current and lower maximum current peaks at − 0.300 V. This difference is probably due to the use of only acetate as electron donor, the method of enrichment used with electrode poised at higher potential than observed in this study, and the absence of renewal of the conductive support for exoelectrogenic community selection.
Evolution in Microbial Diversity During the First 3 Weeks of Enrichment
To identify the evolution of microbial diversity on the electrode and in liquid media over the first 3 weeks of enrichment, a 16S rRNA gene survey was performed by the Illumina MiSeq sequencing the hypervariable V4 region (Fig. 3) of the chimney sample and after each stabilization of current density (at about day 7 for each). During the first 3 weeks of enrichment, biodiversity became poorer with a loss of richness from 177 OTUs in the inoculum to 80 in week 3, correlated to a decrease in evenness index (from 0.695 to 0.299, respectively). This decrease of biodiversity and dominance of a few species can be explained by our selective enrichment conditions with high temperature, substrate specificity, anode electron acceptor, and quick renewal of electrically conductive support.
Dominant taxonomic affiliation at order-level and biodiversity indices of microbial communities from a crushed chimney sample, as plotted on the anode and liquid media over the weeks of culture and in the non-polarized electrode control. OTUs representing less than 1% of total sequences of the samples are pooled as “rare OTUs”. Biodiversity indexes of species richness and evenness represent the number of observed OTUs and Pielou’s evenness (J) index, respectively
Based on average abundance analysis, the taxonomic composition of the environmental sample (Fig. 3) was dominated by Bacteria (99.6% of microbial biodiversity), where Proteobacteria (77%) was the major phylum with 50% γ-proteobacteria, mainly represented by Alteromonadales, 11% α-proteobacteria, 7% ε-proteobacteria, 5% unclassified proteobacteria, 2% δ-proteobacteria, and 2% β-proteobacteria. The remaining bacterial diversity was spread across the Deinococcus–Thermus (8%), Aquificae (6%) and Bacteroidetes (5%) phyla, and 4% shared in other phyla. The Archaeal diversity (0.4%) was composed of Thermoproteales, Thermococcales, and Archaeoglobales.
The evolution of archaeal diversity on the successive enrichment cultures over the 3 weeks was dominated by the Euryarchaeota phyla (mainly the orders Archaeoglobaceae and Thermococcaceae), which rose from 0.4% in the initial sample to > 70% in week 3, whether on the electrode or in liquid media. Interestingly, at genus level, dominant archaeal OTUs on the electrode were closely related to uncultured Thermococcus species (22% (week 1), 32% (week 2), and 56% (week 3)) and Geoglobus spp. (0.2% (week 1), 22% (week 2), and 18% (week 3)) affiliated to Geoglobus ahangari (99% identity on ~ 300 bp 16S rDNA fragments). Moreover, planktonic microbial diversity was mainly enriched in uncultured Thermococcus species (4.9% (week 1), 46.5% (week 2), and 72.2% (week 3)) rather than Geoglobus spp. (< 0.7% during the 3 weeks).
Bacterial diversity over the first 3 weeks on the electrode and in liquid media was mainly composed of species belonging to the order Bacillales (representing up to 94% of biodiversity in liquid media during week 1). The remaining OTUs, mainly found on electrodes, were surprisingly distributed among the mainly mesophilic orders Burkholderiales, Pseudomonadales, Xanthomonadales, and Sphingomonadales, which were not expected in these hyperthermophilic conditions. However, most of these phylogenetic orders harbor some thermophilic or hyperthermophilic species [33, 34] and could potentially be exoelectrogens. Note the gradual decrease in proportion of these bacteria on the electrode, which fell to less than 15% of cumulative total by week 3, indicating lower competitiveness in our conditions compared with Thermococcus, Geoglobus, and Bacillales species.
In order to identify members of the community requiring the electrode as electron acceptor for their growth, a comparative enrichment without electron acceptor was performed in parallel with week 2 enrichment. We carried out an open-circuit control with week 1 final culture medium as inoculum and with the electrode as inert support without the possibility of electron exchange with the counter electrode. This control enrichment showed the following major phyla (on the electrode and liquid media, respectively): Proteobacteria (21% and 0.18%), Firmicutes (52% and 77%), and Euryarchaeota (15% and 22%). Interestingly, based on average abundance analysis, microbial biodiversity was richer on the electrode than in the liquid media (see the richness and evenness indexes in Fig. 3). The major members of the Proteobacteria phylum, Burkholderiales (10%), Enterobacteriales (7%), and Sphingomonadales (3%) orders, thus prefer to grow into biofilm than planktonic cells. At order level, Bacillales (40%), Clostridiales (9%), and Lactobacillales (3%) were the remaining major bacterial orders growing on the electrode. However, only the archaeal order Thermococcales (15%) was present in this enrichment control, whether on the electrode or in liquid media. No OTUs of the order Archaeoglobales were observed. Microbial diversity in the liquid media was mainly composed of Bacillales (73%), and Thermococcales (22%), which are known to be fermentative microorganisms.
Microscopic Observation of Exoelectrogenic Biofilm
The HEB obtained during the second week was observed under a confocal microscope with DNA staining using (i) Syto9 as fluorescent nucleic acid stain and (ii) FISH probes specific to Bacteria, Archaea, Thermococcales, and Archeoglobales on separate observations. Figure 4 presents the micrographs obtained on the Syto9 abiotic control (Fig. 4a) with only reflectance from the carbon fibers (blue color) and the week 2 anode (Fig. 4b) with total cell staining (green color) covering the anode’s carbon fibers. A hyperthermophilic biofilm was observable on the electrode surface, with extracellular polymeric substances (EPS) most likely bridging the spaces between carbon fibers. The Achaea-specific FISH probe (Fig. 4c) revealed two morphologies of biofilm distribution, with a diffuse signal all over the carbon fibers and concentrated clusters distributed on the fiber surfaces. FISH probes specific to the main archaeal taxonomic orders found in 16S barcoding analyses attributed the diffuse presence all over the carbon fibers to Archeoglobales (green color, Fig. 4d) and concentrated clusters to Thermococcales (green color, Fig. 4e). The Bacteria-specific FISH probe did not produce enough signal to visibly identify bacerial distribution on the electrode. However, some cells were visible on carbon fibers when the × 63 (NA1, 4PLAN APO) objective was used. Note that the important Bacillales proportion found on the electrode (Fig. 3) during week 2 was skewed by the higher 16S gene copy number present in species belonging to Bacillaceae (10 copies on average; rrnDB, Stoddard et al., 2014) compared with Archaea (1 copy on average). On the other hand, microscopic observation of DAPI-stained filtrated liquid media (Fig. 4f) showed the presence of long-chained bacilli cells and some irregularly shaped coccoid species, corresponding most probably to Bacillus sp. and Thermococcus sp., respectively.
Confocal microscopic observation with Syto9 of abiotic control electrode after 1 week of culture (a) and biofilm on the end of the second week of culture (b). FISH observation with Archaea (red) (c), Archeoglobales (green) (d), and Thermococcales (green) (e) probes. Three-dimensional top projection of 1.2 × 1.2 mm patch of anode at × 100 magnification. Epifluorescent observation of liquid media of the second week of culture (f) at × 1000 magnification with DAPI
Current Density Production with a Single Substrate
During the fourth week, current production was measured on each separate substrate in fresh media in order to identify the metabolic interactions and substrate specificities involved in the exoelectrogenic process. Figure 5 reports current density and gas production for each substrate, highlighting the immediate current density production with 10-mM acetate (80 mA m−2), 10 mM of pyruvate (108 mA m−2), and a flow rate of 50 mL min−1 of H2 (165 mA m−2) compared with abiotic controls in the same conditions. The current density on H2 is certainly higher than in normal fermentative condition, due to high rate of H2 sparged in the bioreactor to overcome potential diffusion limitation. Interestingly, there was a gradual increase of current with a mix of 0.5-g L−1 yeast extract and 0.5-g L−1 peptone (up to 83 mA m−2), correlated with the weak production of CO2, H2, and acetate. This gradual increase suggests an indirect use of fermentation products by the on-electrode HEB. No current was obtained on formate and polysaccharides.
Discussion
Hyperthermophilic Exoelectrogenic Enrichments in MFC
Despite the discovery, more than 8 years ago, of the electrically conductive property of the chimney wall of hydrothermal vents [12], there has been relatively little investigation yet of microorganisms able to use this property for their metabolism, nor of their role in the trophic chain of the biosphere. We previously demonstrated the presence of hyperthermophilic exoelectrogenic communities from a hydrothermal vent using an enrichment method with acetate as sole energy source and electrode as electron acceptor in an MEC (Pillot et al., 2018). To gain further insight into hyperthermophilic exoelectrogenic diversity and its trophic interactions in the deep-sea hydrothermal ecosystem, a MFC was inoculated with chimney sample with a rich diversity of electron donors. Given the current production in the MFC inoculated with chimney sample (Fig. 1), the increase of capacitive and catalytic currents on cyclic voltammograms (Fig. 2), the microscopic observation of a biofilm on the electrode surface (Fig. 4), and the increase of bacterial and archaeal 16S rRNA gene copies on the electrode (see Supplementary Fig. 4), it could be concluded that a HEB was enriched in our system. The quick electrode change each week allowed to select the exoelectrogenic biodiversity colonizing the electrode and other tightly related microorganisms (Fig. 3), increasing the current production and decreasing the lag time before current production. This selection phenomenon is commonly observed with the enrichment of exoelectrogenic communities from environmental samples [35, 36].
Microbial Composition of the Biofilm Community
Thermococcales, Archaeoglobales, and Bacillales are the three dominant orders retrieved on the electrode surface and in liquid media over each week of enrichment (Fig. 3). Interestingly, all three orders had already been retrieved as the main representatives of exoelectrogenic biofilm diversity in a previous study in more selective conditions, inoculated with sample from another hydrothermal field [10]. Thus, the fact that little more biodiversity was enriched in far more different substrate and electrode potential conditions seems to indicate that species of these three orders are potentially (i) the sole hyperthermophilic exoelectrogenic species in those environments, (ii) the sole in obligatory close relation with exoelectrogenic species, or (iii) the most competitive in such conditions.
Thermococcales are hyperthermophilic archaea known to mainly grow by fermentation of peptides or carbohydrates. The final fermentation products are mainly acetate, hydrogen, and CO2. They are recognized as ubiquitous to hydrothermal vents and easily come to dominate these environments in fermentative conditions [37]. Here, despite their colonization as clusters on the carbon fibers of the electrode (Fig. 4e), we were unable to determine the exact role of Thermococcales in electric current production. However, some Thermococcales species, like strains of Pyrococcus furiosus, have been shown to produce electric current in MFCs [9] or to reduce insoluble iron oxide through EET [38, 39].
The Archeoglobales present all over the carbon fibers of the anode (Fig. 4d) are mainly affiliated to Geoglobus spp. (98.6% similarity with Geoglobus ahangari). Geoglobus species are hyperthermophilic archaea known to grow lithoautotrophically on H2 and CO2 or heterotrophically on volatile fatty acids and peptides as electron donors and carbon source, and to obligatorily use soluble or insoluble Fe (III) as electron acceptor [40, 41]. Recent studies have shown that Geoglobus ahangari has exoelectrogenic capacity on anodes when oxidizing acetate, whereas it did not produce current with H2 [11]. Moreover, a Geoglobus species was found in the hyperthermophilic community on an MEC inoculated with hydrothermal chimney sample [10]. This is in good agreement with specific enrichment of Geoglobus spp. on the electrode over time and the absence of Geoglobus spp. enrichment in the open-circuit control. Indeed, these results were fully coherent with the fact that Geoglobus spp. needs the electrode as electron acceptor.
The Bacillales enriched in our experiment, mainly in liquid media (Fig. 3, Supplementary Fig. 2, Fig. 4), were affiliated to Bacillus and Geobacillus species. Bacillus are Gram-positive sporulating rod-shaped bacteria frequently found in marine sediments or shallow hydrothermal vents. They are known to degrade a wide range of substrates, including peptide and polysaccharides. All Bacillus spp. grow aerobically but some have the ability to undergo fermentation or anaerobic respiration on nitrate. Most Bacillus spp. are mesophilic, with optimum temperature between 15 to 55 °C, but former thermophilic Bacillus were reclassified as Geobacillus in 2001 and can grow from 55 to 80 °C [42]. Moreover, microscopic observation of high amounts of bacilli chains in liquid media (Fig. 4f), typical of Bacillus spp., tends to confirm the enrichment of hyperthermophilic members of Bacillales. We suppose this strain can either perform fermentation or potentially use the electrode as alternative electron acceptor. Indeed, different species of Bacillus and Geobacillus have already been shown to produce electricity in MFCs, on polysaccharides [43], on glucose [44, 45], or on acetate [30]. However, whatever the species, an electron shuttle had to be added or endogenously produced by the bacteria to generate some amount of current. In our study, as no current was observed on the control experiment on filtered spent media with sterile electrode, we can reject the hypothesis of mediated electron transfer in our experiments.
The biodiversity obtained in the open-circuit control inoculated with week 1 spent medium demonstrated that the connected electrode plays a specific role in microbial composition. Indeed, Geoglobus sp. was totally extinguished on the electrode and liquid media yet was enriched on the connected electrode in week 2. This could be directly correlated with the fact that they require an electron acceptor for growth. Thermococcales and Bacillales, both fermentative microbes, were still equally represented on the electrode as in the liquid media of the open-circuit control. Finally, the conservation of more biodiversity (higher evenness index, Fig. 3) on solid support than in liquid media can be related to the protective role of biofilm colonizing conductive or inert supports [46].
Otherwise, the results show similar amount of current density between our experiments and in the literature while the on-electrode biodiversity was different. This suggests that the maximum of current production is not necessarily community-dependant but more related to the total surface area colonized by exoelectrogens.
Trophic Relation Between Fermentative and Exoelectrogenic Microorganisms
In order to identify the role of each electron donor in current production and in the trophic chain of the community, each one was tested separately on the mature biofilm of week 4 enrichment. These tests showed no current production on polysaccharides while most Bacillales, and a few Thermococcus sp., have the ability to hydrolyze starch and ferment glucose. This could be explained by the renewal of the liquid media, removing planktonic fermentatives such as Bacillales and Thermococcus, or by their lack of the enzymatic machinery to hydrolyze polysaccharides, as reported in some Thermococus sp. [47, 48].
However, we confirm the presence of direct respiratory metabolism on the electrode from acetate, pyruvate, and H2. Geoglobus spp., present on the electrode, would be the only known species able to produce current from these substrates. Indeed, Geoglobus spp. are known to metabolize these substrates and grow using iron oxide particles as electron acceptor [41]. However, a previous study found that Geoglobus ahangari was unable to produce current on H2 [11]. Sequencing of the ~ 300-bp hypervariable region V4-V5 of the gene coding for 16S rRNA failed to precisely affiliate the OTUs obtained with the species Geoglobus ahangari. Thus, we are potentially in the presence of several new exoelectrogenic species belonging to the Geoglobus genus. Furthermore, the presence of a microbial community in our experiment can allow microbial interactions, and thereby increase the range of metabolic capacity. However, this is the first time that direct biotic current production has been observed on H2 in a hyperthermophilic MFC. Immediate current production in presence of a fermentation product such as pyruvate, acetate, and hydrogen suggests a community already adapted to the direct oxidation of this substrate on the electrode. In contrast, we observed a gradual and slow increase of current production with a complex substrate such as peptone and yeast extract. This suggests that a first fermentation step is necessary in order to release small molecules readily oxidizable on the anode. This hypothesis is supported by the observation of higher production of CO2 and hydrogen released in the open-circuit control when no final electrode acceptor was available (see Supplementary Fig. 4). These trophic relations were also observed in co-cultures of different species of Thermococcus—producing H2 and CO2 from fermentable compounds—with G. ahangari—consuming H2 and CO2 to produce fermentable compounds (unpublished data). Note that indirect current production in a thermophilic MFC was already observed from the autotrophic community conversion of syngas (composed of H2 and CO2) to acetate which is then used as electron donor by exoelectrogenic biofilm to produce current [49].
Taken together, these results support the hypothesis of trophic interactions between fermentative and exoelectrogenic microbes. In the environment, this syntrophic relationship would allow carbon cycling inside hydrothermal vent biofilms. Indeed, the surrounding organic compound released by living and dead organisms (substituted by peptone and yeast extract in our experiment) would be metabolized by fermentative microorganisms like Thermococcus spp. and Bacillus spp. (present as clusters in our biofilm in Fig. 4e or freely dispersed in the seawater) producing hydrogen, carbon dioxide, and organic acids. Indeed, some Thermococcus species can use microbial-origin extrapolymeric substances (dextran, pullulan, peptides, etc.) for fermentative growth [50]. These fermentation products may then be used as electron donor (acetate, H2) and carbon source (CO2) for exoelectrogenic microorganisms, such as Geoglobus species (covering the carbon fibers in Fig. 4d), which would breath on the conductive pyrite composing the chimney wall and contribute to the abiotic electric current previously observed [20]. Finally, these (autotrophic) exoelectrogens may potentially release organic compounds like EPS in the biofilm that can be later reused by fermentative microbes. This potential cross-feeding phenomenon could allow the survival, in oligotrophic and extremely unstable conditions, of these microbes grouped as biofilm on the hydrothermal chimney surface by concentrating and recycling the organic matter inside the biofilm.
References
Dick GJ, Anantharaman K, Baker BJ, Li M, Reed DC, Sheik CS (2013) The microbiology of deep-sea hydrothermal vent plumes: ecological and biogeographic linkages to seafloor and water column habitats. Front. Microbiol. 4. https://doi.org/10.3389/fmicb.2013.00124
Konn C, Charlou JL, Holm NG, Mousis O (2015) The production of methane, hydrogen, and organic compounds in ultramafic-hosted hydrothermal vents of the Mid-Atlantic Ridge. Astrobiology 15:381–399. https://doi.org/10.1089/ast.2014.1198
Kelley DS, Baross JA, Delaney JR (2002) Volcanoes, fluids, and life at mid-ocean ridge spreading centers. Annu. Rev. Earth Planet. Sci. 30:385–491. https://doi.org/10.1146/annurev.earth.30.091201.141331
Kristall B, Kelley DS, Hannington MD, Delaney JR (2006) Growth history of a diffusely venting sulfide structure from the Juan de Fuca Ridge: a petrological and geochemical study. Geochem. Geophys. Geosyst. 7. https://doi.org/10.1029/2005GC001166
Reysenbach A-L, Longnecker K, Kirshtein J (2000) Novel bacterial and archaeal lineages from an in situ growth chamber deployed at a Mid-Atlantic Ridge hydrothermal vent. Appl. Environ. Microbiol. 66:3798–3806
Takai K, Nakagawa S, Reysenbach A-L, Hoek J (2006) Microbial ecology of Mid-Ocean ridges and back-arc basins. In: Christie DM, Fisher CR, Lee S-M, Givens S (eds) Back-arc spreading systems: geological, biological, chemical, and physical interactions. American Geophysical Union, pp 185–213
Nakagawa S, Takai K (2008) Deep-sea vent chemoautotrophs: diversity, biochemistry and ecological significance. FEMS Microbiol. Ecol. 65:1–14. https://doi.org/10.1111/j.1574-6941.2008.00502.x
Frank KL (2013) Linking metabolic rates with the diversity and functional capacity of endolithic microbial communities within hydrothermal vent structures. Doctoral dissertation, Harvard University
Sekar N, Wu C-H, Adams MWW, Ramasamy RP (2017) Electricity generation by Pyrococcus furiosus in microbial fuel cells operated at 90°C. Biotechnol. Bioeng. 114:1419–1427. https://doi.org/10.1002/bit.26271
Pillot G, Frouin E, Pasero E, Godfroy A, Combet-Blanc Y, Davidson S, Liebgott PP (2018) Specific enrichment of hyperthermophilic electroactive Archaea from deep-sea hydrothermal vent on electrically conductive support. Bioresour. Technol. 259:304–311. https://doi.org/10.1016/j.biortech.2018.03.053
Yilmazel YD, Zhu X, Kim K-Y, Holmes DE, Logan BE (2018) Electrical current generation in microbial electrolysis cells by hyperthermophilic archaea Ferroglobus placidus and Geoglobus ahangari. Bioelectrochemistry 119:142–149. https://doi.org/10.1016/j.bioelechem.2017.09.012
Nakamura R, Takashima T, Kato S, Takai K, Yamamoto M, Hashimoto K (2010) Electrical current generation across a black smoker chimney. Angew. Chem. Int. Ed. 49:7692–7694. https://doi.org/10.1002/anie.201003311
Allen RM, Bennetto HP (1993) Microbial fuel-cells. Appl. Biochem. Biotechnol. 39–40:27–40. https://doi.org/10.1007/BF02918975
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40:5181–5192
Schröder U, Harnisch F, Angenent LT (2015) Microbial electrochemistry and technology: terminology and classification. Energy Env Sci 8:513–519. https://doi.org/10.1039/C4EE03359K
Koch C, Harnisch F (2016) Is there a specific ecological niche for electroactive microorganisms? ChemElectroChem 3:1282–1295. https://doi.org/10.1002/celc.201600079
Nielsen ME, Reimers CE, Stecher HA (2007) Enhanced power from chambered benthic microbial fuel cells. Environ Sci Technol 41:7895–7900. https://doi.org/10.1021/es071740b
Gong Y, Radachowsky SE, Wolf M, Nielsen ME, Girguis PR, Reimers CE (2011) Benthic microbial fuel cell as direct power source for an acoustic modem and seawater oxygen/temperature sensor system. Environ Sci Technol 45:5047–5053. https://doi.org/10.1021/es104383q
Girguis PR, Holden JF (2012) On the potential for bioenergy and biofuels from hydrothermal vent microbes. Oceanography 25:213–217
Yamamoto M, Nakamura R, Kasaya T, Kumagai H, Suzuki K, Takai K (2017) Spontaneous and widespread electricity generation in natural deep-sea hydrothermal fields. Angew. Chem. Int. Ed. 56:5725–5728. https://doi.org/10.1002/anie.201701768
Desbruyères D, Almeida A, Biscoito M, Comtet T, Khripounoff A, le Bris N, Sarradin PM, Segonzac M (2000) A review of the distribution of hydrothermal vent communities along the northern Mid-Atlantic Ridge: dispersal vs. environmental controls. Hydrobiologia 440:201–216. https://doi.org/10.1023/A:1004175211848
Portail M, Olu K, Dubois SF, Escobar-Briones E, Gelinas Y, Menot L, Sarrazin J (2016) Food-web complexity in Guaymas Basin hydrothermal vents and cold seeps. PLoS One 11:e0162263. https://doi.org/10.1371/journal.pone.0162263
Sarradin P-M, Cannat M (2014) MOMARSAT2014 cruise, Pourquoi pas ? R/V
Boileau C, Auria R, Davidson S, Casalot L, Christen P, Liebgott PP, Combet-Blanc Y (2016) Hydrogen production by the hyperthermophilic bacterium Thermotoga maritima part I: effects of sulfured nutriments, with thiosulfate as model, on hydrogen production and growth. Biotechnol Biofuels 9(269):269. https://doi.org/10.1186/s13068-016-0678-8
Zhang L, Kang M, Xu J, Xu J, Shuai Y, Zhou X, Yang Z, Ma K (2016) Bacterial and archaeal communities in the deep-sea sediments of inactive hydrothermal vents in the Southwest India Ridge. Sci. Rep. 6. https://doi.org/10.1038/srep25982
Bates ST, Berg-Lyons D, Caporaso JG, Walters WA, Knight R, Fierer N (2011) Examining the global distribution of dominant archaeal populations in soil. ISME J 5:908–917. https://doi.org/10.1038/ismej.2010.171
Michotey V, Guasco S, Boeuf D, Morezzi N, Durieux B, Charpy L, Bonin P (2012) Spatio-temporal diversity of free-living and particle-attached prokaryotes in the tropical lagoon of Ahe atoll (Tuamotu Archipelago) and its surrounding oceanic waters. Mar. Pollut. Bull. 65:525–537. https://doi.org/10.1016/j.marpolbul.2012.01.009
Einen J, Thorseth IH, Øvreås L (2008) Enumeration of Archaea and Bacteria in seafloor basalt using real-time quantitative PCR and fluorescence microscopy. FEMS Microbiol. Lett. 282:182–187. https://doi.org/10.1111/j.1574-6968.2008.01119.x
Rusch A, Amend JP (2004) Order-specific 16S rRNA-targeted oligonucleotide probes for (hyper)thermophilic Archaea and Bacteria. Extremophiles 8:357–366. https://doi.org/10.1007/s00792-004-0396-1
Wrighton KC, Agbo P, Warnecke F, Weber KA, Brodie EL, DeSantis TZ, Hugenholtz P, Andersen GL, Coates JD (2008) A novel ecological role of the Firmicutes identified in thermophilic microbial fuel cells. ISME J 2:1146–1156. https://doi.org/10.1038/ismej.2008.48
Fu Q, Fukushima N, Maeda H, Sato K, Kobayashi H (2015) Bioelectrochemical analysis of a hyperthermophilic microbial fuel cell generating electricity at temperatures above 80 °C. Biosci. Biotechnol. Biochem. 79:1200–1206. https://doi.org/10.1080/09168451.2015.1015952
Shehab NA, Ortiz-Medina JF, Katuri KP, Hari AR, Amy G, Logan BE, Saikaly PE (2017) Enrichment of extremophilic exoelectrogens in microbial electrolysis cells using Red Sea brine pools as inocula. Bioresour. Technol. 239:82–86. https://doi.org/10.1016/j.biortech.2017.04.122
Sutrisno A, Ueda M, Abe Y, Nakazawa M, Miyatake K (2004) A chitinase with high activity toward partially N-acetylated chitosan from a new, moderately thermophilic, chitin-degrading bacterium, Ralstonia sp. A-471. Appl. Microbiol. Biotechnol. 63:398–406. https://doi.org/10.1007/s00253-003-1351-2
Wagner ID, Wiegel J (2008) Diversity of thermophilic anaerobes. Ann. N. Y. Acad. Sci. 1125:1–43. https://doi.org/10.1196/annals.1419.029
Dai K, Wen J-L, Zhang F, Ma XW, Cui XY, Zhang Q, Zhao TJ, Zeng RJ (2017) Electricity production and microbial characterization of thermophilic microbial fuel cells. Bioresour. Technol. 243:512–519. https://doi.org/10.1016/j.biortech.2017.06.167
Xu F, Cao F, Kong Q, Zhou LL, Yuan Q, Zhu YJ, Wang Q, du YD, Wang ZD (2018) Electricity production and evolution of microbial community in the constructed wetland-microbial fuel cell. Chem. Eng. J. 339:479–486. https://doi.org/10.1016/j.cej.2018.02.003
Bertoldo C, Antranikian G (2006) The order Thermococcales. In: Dworkin M, Falkow S, Rosenberg E et al (eds) The prokaryotes. Springer, New York, pp 69–81
Slobodkin AI, Jeanthon C, L’Haridon S et al (1999) Dissimilatory reduction of Fe(III) by thermophilic Bacteria and Archaea in deep subsurface petroleum reservoirs of Western Siberia. Curr. Microbiol. 39:99–102. https://doi.org/10.1007/s002849900426
Slobodkin A, Campbell B, Cary SC, Bonch-Osmolovskaya E, Jeanthon C (2001) Evidence for the presence of thermophilic Fe(III)-reducing microorganisms in deep-sea hydrothermal vents at 13°N (East Pacific Rise). FEMS Microbiol. Ecol. 36:235–243. https://doi.org/10.1111/j.1574-6941.2001.tb00844.x
Kashefi K, Holmes DE, Reysenbach A-L, Lovley DR (2002) Use of Fe(III) as an electron acceptor to recover previously uncultured hyperthermophiles: isolation and characterization of Geothermobacterium ferrireducens gen. nov., sp. nov. Appl. Environ. Microbiol. 68:1735–1742. https://doi.org/10.1128/AEM.68.4.1735-1742.2002
Manzella MP, Reguera G, Kashefi K (2013) Extracellular electron transfer to Fe(III) oxides by the hyperthermophilic archaeon Geoglobus ahangari via a direct contact mechanism. Appl. Environ. Microbiol. 79:4694–4700. https://doi.org/10.1128/AEM.01566-13
Nazina TN, Tourova TP, Poltaraus AB et al (2001) Taxonomic study of aerobic thermophilic bacilli: descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilus, G. th. Int. J. Syst. Evol. Microbiol. 51:433–446. https://doi.org/10.1099/00207713-51-2-433
Choi Y-J, Jung E-K, Park H-J et al (2004) Construction of microbial fuel cells using thermophilic microorganisms, Bacillus licheniformis and Bacillus thermoglucosidasius. Bull. Kor. Chem. Soc. 25:813–818. https://doi.org/10.5012/bkcs.2004.25.6.813
Nimje VR, Chen C-Y, Chen C-C, Jean JS, Reddy AS, Fan CW, Pan KY, Liu HT, Chen JL (2009) Stable and high energy generation by a strain of Bacillus subtilis in a microbial fuel cell. J. Power Sources 190:258–263. https://doi.org/10.1016/j.jpowsour.2009.01.019
Borah D, More S, Yadav RNS (2013) Construction of double chambered microbial fuel cell (MFC) using household materials and Bacillus megaterium isolate from tea garden soil. J. Microbiol. Biotechnol. Food Sci. 3:84–86
Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM (1995) Microbial biofilms. Annu. Rev. Microbiol. 49:711–745
Jolivet E, L’Haridon S, Corre E et al (2003) Thermococcus gammatolerans sp. nov., a hyperthermophilic archaeon from a deep-sea hydrothermal vent that resists ionizing radiation. Int. J. Syst. Evol. Microbiol. 53:847–851. https://doi.org/10.1099/ijs.0.02503-0
Atomi H, Fukui T, Kanai T, Morikawa M, Imanaka T (2004) Description of Thermococcus kodakaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea 1:263–267
Hussain A, Guiot SR, Mehta P, Raghavan V, Tartakovsky B (2011) Electricity generation from carbon monoxide and syngas in a microbial fuel cell. Appl. Microbiol. Biotechnol. 90:827–836. https://doi.org/10.1007/s00253-011-3188-4
Legin E, Copinet A, Duchiron F (1998) Production of thermostable amylolytic enzymes by Thermococcus hydrothermalis. Biotechnol. Lett. 20:363–367. https://doi.org/10.1023/A:1005375213196
Acknowledgments
The authors thank Erwan Roussel (LM2E, IFREMER Brest) for helpful suggestions, Céline Rommevaux and Françoise Lesongeur for sampling during the MOMARSAT 2014 cruise, the MIM platform (MIO, France) for providing access to their confocal microscopy facility, and the GeT-PlaGe platform (GenoToul, France) for DNA sequencing.
Funding
This work received financial support from the CNRS national interdisciplinary research program (PEPS-ExoMod 2016). The project leading to this publication has received funding from European FEDER program under project 1166-39417.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pillot, G., Davidson, S., Auria, R. et al. Production of Current by Syntrophy Between Exoelectrogenic and Fermentative Hyperthermophilic Microorganisms in Heterotrophic Biofilm from a Deep-Sea Hydrothermal Chimney. Microb Ecol 79, 38–49 (2020). https://doi.org/10.1007/s00248-019-01381-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-019-01381-z