Abstract
The effects of various combinations of iron compounds on the contaminant removal performance in constructed wetlands (CWs) were explored under various initial iron concentrations, contaminant concentrations, different hydraulic retention time (HRT), and different temperatures. The Combo 6 (nanoscale zero-valent iron combined with Fe3+) in CW treatments showed the highest pollutant removal performance under the conditions of C2 initial iron dosage concentration (total iron 0.2 mM) and I2 initial contaminant concentration (COD:TN:TP = 60 mg/L:60 mg/L:1 mg/L) in influent after 72-h HRT. These results were directly verified by two different microbial tests (Biolog test and high-throughput pyrosequencing) and microbial community analysis (principal component analysis of community-level physiological profile, biodiversity index, cluster tree, relative abundance at order of taxonomy level). Specific bacteria related to significant improvements in contaminant removal were domesticated by various combinations of iron compounds. Iron dosage was advised as a green, new, and effective option for wastewater treatment.

.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Contaminants in surface water have become a big environmental problem. Contaminants from domestic sewage, agriculture, and industrial wastewater affect the quality of surface water in rivers and lakes. In contaminated surface water, the main pollutants were excessive nitrogen and phosphorus [1]. Ammonia nitrogen can kill fish and other creatures in water and nitrate/nitrite may cause cancers, malformations, and mutations in human body. Phosphate largely affects the water quality in many ecosystems via eutrophication [2]. These pollutants are discharged into surface water and threaten both human beings and the environment. Therefore, it is necessary to remove these contaminants from the environment.
In order to improve the removal performance of pollutants, iron is used in many water treatment processes because iron has variable valence states [3]. Different iron compounds (Fe0, such as iron powder and nanoscale zero-valent iron (nZVI); Fe2+; Fe3+) were used in water treatment for contaminant removal (Table 1). Iron-based ecological wastewater treatment technologies are suitable options for improving the removal performance of contaminants.
Under an oxidation environment, ferrous iron can be easily oxidized into ferric iron. Under a reduced environment, ferric iron is also easily reduced into ferrous iron. Theoretically, zero valence iron and ferric iron can produce ferrous iron:
It is assumed that iron powder or nZVI reacts with Fe3+ to produce Fe2+ under reduction conditions. It is probably an economic way to add iron ion in the treatment system because Fe (0) is easily oxidized into ferrous ion in air.
Constructed wetland (CW) is a kind of water treatment system with significant advantages [14]. CW is a multiple-contaminant removal system involving physical, chemical, and biological processes [15]. Nitrogen removal depends on nitrification and denitrification reactions, and phosphorus removal depends on adsorption and biochemical reactions [16]. In CWs, there are multiple redox zones for contaminant removal [17]. These oxidation–reduction zones promoted iron ions to trigger the contaminant removal in CWs as mentioned above. Iron used in CWs was a suitable option for improving the removal performance of contaminants.
Previous studies focused on the improvements in the removal performance of contaminants driven by iron, but the effects of different iron ions on the removal performance of contaminants were not compared. In water treatment, iron-triggering improvements in the removal performance of contaminants involved about only one type of contaminants, such as heavy metals, nitrogen, phosphorus, or organic matters. In wastewater treatment processes, multiple contaminants were found in water. The iron-intensified treatment performance for multiple contaminants or the synergistic mechanism of different contaminants in the purification process was not reported yet. It is proved that iron affects the microbial community indirectly. However, the structure or evolution of the microbial community in the iron-driven wastewater purification treatment was not reported.
In this study, CW treatment with different iron compounds under different iron dosages, different initial pollution concentrations, different temperatures, and different hydraulic retention time (HRT) for the removal of multiple contaminants were explored. Microbiological experiments were performed to demonstrate the variations of the microbial community.
Materials and Methods
Establishment of Laboratory-Scale Units
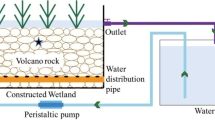
A laboratory-scale vertical subsurface flow CWs (Fig. S1) with an inner size of 0.5 m (diameter) × 1.1 m (height) was built. The filter medium in CWs was quartz sand, and Canna was planted as well. In each CW, two PVC outlets were set up for sampling (0.1 m and 1 m high; one for sampling influent and the other for sampling effluent). Four layers of quartz sand with different diameter ranges as substrates in CWs were set (diameter ranges of quartz sand from top layer to bottom layer: 10~15 mm, 5~8 mm, 1~3 mm, and 10~15 mm). The bottom layer was 0.1 m deep, and the other three layers were 0.3 m deep. During the experimental period, the water level should be kept at 1 cm above the surface of the substrate.
System Operation
The division of the experimental period in this study is shown in Fig. 1. Air temperature and relative humidity are 0~35 °C and 45~85% (Air Quality Measure Meter, PRANUS, China) during the whole experimental period. The systems were built in Shanghai Ocean University in the east of Shanghai, China. Initially, the CWs were normally operated with synthetic wastewater for 1 month.
Synthetic wastewater was prepared with tap water, organic matters (two glucose concentrations 30 and 60 mg/L), nitrogen (437.14 mg/L NaNO3 equivalent to 60 mg/L TN), phosphorus (6.032 mg/L KH2PO4 equivalent to 1 mg/L TP), and other micronutrients. According to Environmental Quality Standards for Surface Water of China (GB 3838-2002), synthetic wastewater was prepared in plastic tank and intermittently fed to the systems with different HRT during the experimental period.
During the experimental period, six different iron addition combinations (iron powder, nZVI, Fe2+, Fe3+, iron powder and Fe3+, nZVI and Fe3+) for contaminant removal were arranged and respectively named Combo 1~Combo 6. The control without addition was named Combo 0. Three initial total iron dosages (0.1 mM, 0.2 mM, and 0.3 mM) were arranged in this study and respectively named C1~C3. Iron powder, nZVI, Fe2+ (FeSO4), and Fe3+ (FeCl3) were bought from Sinopharm Chemical Reagent Co., Ltd.
Totally, 114 CW systems were built. In 57 systems, synthetic wastewater was added in influent according to the initial concentrations of contaminants (COD:TN:TP = 30 mg/L:60 mg/L:1 mg/L), and then, six combinations of iron compounds were added according to three initial total iron dosages. One system without iron addition was used as the control. In the other 57 systems, synthetic wastewater was added into influent according to the initial concentrations of organic compounds (COD:TN:TP = 60 mg/L:60 mg/L:1 mg/L), and then, six combinations of iron compounds were added according to three initial total iron dosages. One system without iron addition was used as the control (Table 2).
After the systems were stabilized, the experiments were launched in treatments under different HRT (HRT = 12, 24, 48, and 72 h) and low/high temperature with different initial contaminant concentrations (COD:TN:TP = 60:60:1 and COD:TN:TP = 30:60:1, unit mg/L). In all experiments, treatments were arranged in triplicate.
Water Sampling
Water samples were taken for testing at the beginning and the end of each experimental batch and triplicate samples were collected from each system. Samples were collected in a clean glass bottle and filtered by glass-fiber filters (0.22 μm) for analysis.
Water Analysis
Water temperature, pH, DO, and ORP (HI 9143, HANNA, Italy; HQ40d, HACH, USA) in influent and effluent were analyzed immediately after sampling.
According to the standard methods described by the American Public Health Association (1998), TN (NH4+–N, NO3−–N), TP, COD, Fe3+, Fe2+, and total iron (TI) in water samples were spectrophotometrically analyzed with a spectrophotometer (DR900, HACH, USA; SpectroQuest, UNICOSH, USA).
DNA Extraction and Microbial Test
Quartz sand (20 g from each system) from bottom layers was gathered and mixed from triplicate systems. Microorganisms were collected on the surface of quartz sands by vibrating for 30 min and centrifuging twice. Soil DNA kits were used to extract DNA from microorganisms. DNA samples were stored at − 20 °C until use.
Biolog EcoPlates
Biolog EcoPlates can be used to explore microbial community function based on the community-level physiological profile (CLPP) calculation results. Microorganisms in sample solutions were extracted and then inoculated on the Biolog EcoPlates. Normally, the function of microbial community was explored based on the consumption of each single carbon source by microorganisms in a certain period. There are a total of 31 available carbon sources in Biolog EcoPlates, and triplicates were set for the data analysis. Metabolic fingerprints of microbes were used to indicate the microbial characteristics based on data analysis.
The diluted solution was separately added into each well of Biolog EcoPlate, which was placed at 25 °C in darkness. The absorbance of the wells at 590 nm and 750 nm was measured every 24 h.
High-Throughput Pyrosequencing
In this experiment, the V4 and V5 regions of 16S rRNA were amplified with the following primers: forward 5′-AY TGGGYDTAAAGNG-3′ and reverse 5′-TACNVGGGTATCTAATCC-3′. DNA ligase was used to connect the extracted DNA fragments from each sample. DNA library (10 nM) was amplified by PCR. An Illumina MiSeq machine (2 × 250 bp reads) was used in the DNA sequencing with DNA library. High-quality sequences from each sample were classified as operational taxonomic units (OTUs), which were taken from the database [18].
Data and Statistical Analysis
Pollutant removal performances and physico-chemical indexes were calculated with measured water flows. The values of triplicate samples of influent and effluent were averaged. The results of triplicate experiments were used to plot standard error bars. The figures in the paper were plotted by Origin Pro 9.0. All experimental data were analyzed in SPSS 20.0.
In Biolog plate wells, the average well color development (AWCD) of each sample was used to indicate the microbial activity [19]. AWCD value at 72 h was assigned to measure carbon source utilization in different samples [20].
The Shannon index (H′), Pielou index (J), and Simpson’s reciprocal index (1/D) were used to evaluate the functional diversity of microorganisms. Simpson index indicates the assemblage from the same species in the community, and the other two indexes indicate the species diversity richness and evenness in the community [21]. The calculation methods of the three diversity indexes were respectively from previous reports [11, 13, 22].
Principal component analysis (PCA) was performed with the Biolog EcoPlate data. In the PCA diagram of all the samples, the distance of each spot indicates the similarity of AWCD in each sample and the higher similarity suggests the shorter distance of each spot [23].
Results and Discussion
General Performance
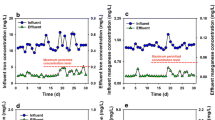
The pH of influent in treatments with/without iron dosage was 6~6.3 and 6.5~7, respectively. The pH of effluent in all treatments was 6.5~6.8. The DO values of influent and effluent in all treatments were 7.83~10.18 mg/L and 1.51~2.31 mg/L, respectively.
The contaminant removal performance was improved obviously at different temperatures (P ≤ 0.05), indicating that the higher temperature promoted the microbial activity in treatments [24, 25]. The higher contaminant removal efficiencies were found after longer HRTs (P ≤ 0.05), demonstrating that denitrification driven by microbes and phosphate adsorption by substrate both depended on longer HRT [26]. Initial concentrations of organic matters in influent significantly affected TN and TP removal efficiencies [27, 28].
The relationships between temperatures, HRTs, initial concentrations of contaminants in influent, different combinations of iron compounds, and different initial total iron dosages were also studied (Tables 3 and 4). Contaminant removal in CW treatment was not only determined by environmental factors, such as temperature, but it was also influenced by system parameters, such as HRT [29]. Therefore, it is necessary to explore the interactions between these environmental factors and operating parameters.
The contaminant removal efficiencies with different combinations of iron compounds were higher than that without iron addition because iron ion promoted the TN and TP removals in treatments [30]. The better removal performances were obtained in Combo 5 and Combo 6, indicating that Fe2+ ion was produced in CWs and promoted the denitrification process.
Initial Concentration of Pollutants in Influent
Water quality affects the removal performance in treatment, so it is necessary to explore the influences of iron ions on the pollutant removal performance in CWs.
Figure 2 shows the relationship between total iron concentration and TN/TP. Higher initial concentration of pollutions in influent corresponded to the better pollution removal performance (Table S1 and S2), and more significantly, positive correlations, indicating that iron ions promoted pollutant removal in the presence of organic matters. If TP removal performance was considered, this positive correlation was more obvious because the R2 value was higher under the high initial pollutant concentration in influent. It may be ascribed to the precipitation between ferric ions and phosphorus [31].
Iron Dosage in Influent
Iron dosage affects iron ions in CWs, and an optimal dosage of iron in influent might contribute to the high performance of the system and low cost.
The linear fitting results between nitrogen/phosphorus concentration and initial total iron dosage are shown in Fig. 3. The better linear fitting result between nitrogen concentration and initial total iron dosage was found in C2, and the better linear fitting result between phosphorus concentration and initial total iron dosage was obtained in C3. Iron addition could complement electronic donors during denitrification by continuously generating Fe2+ in the system [32]. However, an excessive iron dosage was not good for the operation or microbial activity [33]. Considering the nitrogen removal performance, C2 was determined as the optimal iron dosage concentration (Table S1 and S2). Meanwhile, experimental results proved that the high iron dosage promoted phosphorus precipitation [34]. However, considering the operation cost and the problem of system blockage [35], C2 was selected as the optimal iron dosage in the whole treatment process.
Different Iron Compounds in Influent
Contaminant removal in CWs depended on the microbial activity in denitrification, indicating that organic matters were related to nitrogen/phosphorus removal. Adding different iron compounds in influent would result in different contents of iron ions (ferrous or ferric ions) in CWs. Ferrous ions could work as donor suppliers in the denitrification process when the carbon source concentration was limited. Therefore, the relationship between contaminant concentration and iron concentration with different iron compounds in influent should be explored.
The linear fitting results between nitrogen concentration and initial total iron dosage in Combo 1~Combo 6 after 72-h HRT are given in Fig. 4. Better linear fitting results were found in the combinations Combo 5 and Combo 6, indicating that nitrogen removal was directly related to iron content in Combo 3, Combo 5, and Combo 6 [36]. It was also proved that iron ions, especially Fe2+, promoted the denitrification process when the concentrations of organic matters were low in the treatment. In Combo 5 and Combo 6, Fe2+ was produced in multiple oxidation–reduction zones in CWs. The content of Fe2+ was stored and continuously consumed during the experimental period. Due to nZVI added, iron ions in the system worked stably [37].
The linear fitting results between phosphorus concentration and initial total iron concentration in Combo 1~Combo 6 after 72-h HRT are also given in Fig. 5. The better linear fitting results of phosphorus and iron were found in Combo 4, Combo 5, and Combo 6 because the precipitation reactions between contaminants and Fe3+ happened [38]. Changeable valences of iron ions in Combo 5 and Combo 6 promoted the iron ion recycling, which continuously provided Fe2+ for denitrification driven by microbial activity and Fe3+ for precipitation of phosphorus.
Microbial Analysis
In this study, the influences of different combinations of iron compounds and different total initial iron dosages on CW treatment for contaminant removal were investigated. The improved performance under the optimal HRT, temperature, and initial influent concentration was discussed above. The effects of different combinations of iron compounds under the optimal initial total iron dosages (C2) and I2 contaminant concentration in influent at high temperature after 72-h HRT on the microbial community in treatments were discussed below. We investigated the variations of the microbial community with two different methods (Biolog test and high-throughput pyrosequencing).
In this study, a total of 922,098 sequence reads for bacteria were extracted by pyrosequencing 16S rDNA fragments from bacterial samples. The high-quality sequences were described as OTU mentioned in “High-Throughput Pyrosequencing.” OTU classification and identification results were given in Table S3 for further analysis. All the sequences were classified. The classification results demonstrated that the bacteria in treatments grew well and were adapted to the treatment system stably.
According to cluster analysis results, microbial communities in the treatments with Combo 1~Combo 6 were significantly different from those in the treatment with Combo 0. Based on biodiversity indexes of high-throughput pyrosequencing test (Table 5), the above results were validated. Among the treatments with different combinations of iron compounds, Combo 5 and Combo 6 belonged to the similar bacteria group. In the classification tree of OTUs (Fig. 6), various bacteria were marked as different letters (presented the different bacteria) and colors and the sizes of nodes with marked colors indicated the bacterial richness (the larger the node was, the higher the bacterial richness was) [39]. These results indicated that the dominant bacteria in treatment with Combo 1~Combo 6 were ACTINOBACTERIA and PROTEOBACTERIA, which were widely used in wastewater treatment process and allowed contaminant removal smoothly [40].
According to the AWCD values obtained in Biolog test (Fig. 7a), more carbon-consuming bacteria were found in the treatment with Combo 5 and Combo 6, indicating that the bacterial activities in the treatment with Combo 5 and Combo 6 were obviously higher than that in the treatments with Combo 0~Combo 4. According to the 72-h biodiversity indexes of Biolog test, similar results were found. The value of the diversity index of Combo 5 and Combo 6 was higher than that of other combinations. According to PCA of the CLPP results by Biolog test (Fig. 7b), two components (PCA1, 88.2%; PCA2, 47.1%) were extracted and the position of Combo 6 was close to positions of six different carbon sources. The results indicated that the carbon source utilization by bacteria in the treatment with Combo 6 was the highest. It was directly proved that the improved contaminant removal performance was triggered by the bacteria activity [41]. Bacteria in CW treatment generally utilized the carbon source of Carbon 1 [42]. However, due to the iron addition in the treatment with Combo 6, carbon source utilization behaviors of bacteria were changed and they consumed the carbon source of Carbon 2~Carbon 5, which were composed of various contaminants, such as nitrogen and phosphorus contaminants. As a result, the bacteria in the treatment with Combo 6 were acclimatized to consume nitrogen and phosphorus.
Microbial community analysis of different iron dosages with C2 and I2 pollutant concentrations in influent after 72-h HRT in the whole experimental period. a Average well color development (AWCD) results by Biolog test. b PCA results by Biolog test. c Phylum and abundance distribution by high-throughput pyrosequencing. Different types of carbon sources are expressed as the following: carbon 1-glucose and its ramification, carbon 2-amino acids, carbon 3-polymer, carbon 4-phenolic acids, carbon 5-amines, and carbon 6-carboxylic acids
According to the relative abundance at the taxonomy level by high-throughput pyrosequencing (Fig. 7c), FIRMICUTES was rich in the treatment without iron dosage. The bacteria might over-assimilate the energy in the treatment [43]. This behavior possibly led to a waste of energy and increased the operation cost of the system. BACTEROIDETES can utilize carbon sources as electronic donor [44]. In the treatment with Combo 5 and Combo 6, the abundance of this bacteria was significantly lower than that in the treatment with Combo 0~Combo 4. Higher contaminant removal efficiencies indicated that iron ion (such as Fe2+) worked as electronic donors for bacteria to remove nitrogen in treatments. Additionally, PLANCTOMYCETES was considered as a beneficial strain for pollutant removal and nitrogen cycling [45] and VERRUCOMICROBIA was reported to be highly adapted to extreme environments [46, 47]. Both PLANCTOMYCETES and VERRUCOMICROBIA were rich in the treatment with Combo 5 and Combo 6, indicating that the combinations of iron compounds in Combo 5 and Combo 6 promoted the optimization of the microbial community composition for wastewater treatment.
In this study, the Biolog EcoPlate test was performed to investigate microbial metabolism in CWs and the high-throughput sequencing of 16S rRNA was used to reveal the microbial diversity and community structure [20]. The microbial data were extracted with the two methods from the samples in CWs, and more detailed information was found by the high-throughput sequencing of 16S rRNA, which was an advisable analysis tool for environmental microbes.
Conclusions
In this study, different combinations of iron compounds were applied in CWs to improve contaminant removal performances. The combination with Fe (0) and Fe (III) in CW treatments had the higher contaminant removal efficiencies, and the longer efficacy was obtained with Combo 6 (nZVI combined with Fe3+). According to microbial analysis results, Combo 6 of iron compounds in the treatment was the optimal combination. As a result, the optimal conditions for contaminant removal were determined as follows: Combo 6 (nZVI combined with Fe3+), C2 initial iron dosage concentration (total iron 0.2 mM), and I2 initial contaminant concentration (COD:TN:TP = 60 mg/L:60 mg/L:1 mg/L) in influent after 72-h HRT. The effects of iron dosage in open-scale treatments and different carbon sources in influent will be further explored in the future.
References
Xu P, Zeng GM, Huang DL, Feng CL, Hu S, Zhao MH, Lai C, Wei Z, Huang C, Xie GX, Liu ZF (2012) Use of iron oxide nanomaterials in wastewater treatment: a review. Sci Total Environ 424:1–10. https://doi.org/10.1016/j.scitotenv.2012.02.023
Kumar P, Prot T, Korving L, Keesman KJ, Dugulan L, Van Loosdrecht MCM, Witkamp GJ (2017) Effect of pore size distribution on iron oxide coated granular activated carbons for phosphate adsorption - importance of mesopores. Chem Eng J 326:231–239. https://doi.org/10.1016/j.cej.2017.05.147
Ma XC, Zhou WG, Fu ZQ, Cheng YL, Min M, Liu YH, Zhang YK, Chen P, Ruan R (2014) Effect of wastewater-borne bacteria on algal growth and nutrients removal in wastewater-based algae cultivation system. Bioresour Technol 167:8–13. https://doi.org/10.1016/j.biortech.2014.05.087
Skoog A, Arias-Esquivel VA (2009) The effect of induced anoxia and re-oxygenation on benthic fluxes of organic carbon, phosphate, iron, and manganese. Sci Total Environ 407:6085–6092. https://doi.org/10.1016/j.scitotenv.2009.08.030
Zhou LJ, Zhuang WQ, Wang X, Yu K, Yang SF, Xia SQ (2017) Potential effects of loading nano zero valent iron discharged on membrane fouling in an anoxic/oxic membrane bioreactor. Water Res 111:140–146. https://doi.org/10.1016/j.watres.2017.01.007
Choi H, Al-Abed SR, Agarwal S, Dionysiou D (2008) Synthesis of reactive nano-Fe/Pd bimetallic system-impregnated activated carbon for the simultaneous adsorption and dechlorination of PCBs. Chem Mater 20(11):3649–3655. https://doi.org/10.1021/cm8003613
O’Carroll D, Sleep B, Krol M, Boparai H, Kocur C (2013) Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv Water Resour 51:104–122. https://doi.org/10.1016/j.advwatres.2012.02.005
Kumar MA, Choe JK, Lee WJ, Yoon SH (2017) Synthesis of benzaldoxime from benzaldehyde using nanoscale zero-valent iron and dissolved nitrate or nitrite. Environ Nanotechnol Monit Manage 8:97–102. https://doi.org/10.1016/j.enmm.2017.06.003
Lu Q, Jeen SW, Gui L, Gillham RW (2017) Nitrate reduction and its effects on trichloroethylene degradation by granular iron. Water Res 112:48–57. https://doi.org/10.1016/j.watres.2017.01.031
Jordan TE, Cornwell JC, Boynton WR, Anderson JT (2008) Changes in phosphorus biogeo-chemistry along an estuarine salinity gradient: the iron conveyer belt. Limnol Oceanogr 53:172–184. https://doi.org/10.4319/lo.2008.53.1.0172
Song XS, Wang SY, Wang YH, Zhao ZM, Yan DH (2016) Addition of Fe2+ increase nitrate removal in vertical subsurface flow constructed wetlands. Ecol Eng 91:487–494. https://doi.org/10.1016/j.ecoleng.2016.03.013
Rodriguez-Narvaez OM, Peralta-Hernandez JM, Goonetilleke A, Bandala ER (2017) Treatment technologies for emerging contaminants in water: a review. Chem Eng J 323:261–380. https://doi.org/10.1016/j.cej.2017.04.106
Zhao ZM, Song XS, Wang W, Xiao YP, Gong ZJ, Wang YH, Zhao YF, Chen Y, Mei MY (2016) Influences of iron and calcium carbonate on wastewater treatment performances of algae-based reactors. Bioresour Technol 216:1–11. https://doi.org/10.1016/j.biortech.2016.05.043
Vymazal J (2007) Removal of nutrients in various types of constructed wetlands. Sci Total Environ 380:48–65. https://doi.org/10.1016/j.scitotenv.2006.09.014
Vymazal J (2010) Constructed wetlands for wastewater treatment: five decades of experience. Environ Sci Technol 45(1):61–69. https://doi.org/10.1021/es101403q
Zhao ZM, Song XS, Zhao YF, Xiao YP, Wang YH, Wang JF, Yan DH (2017) Effects of iron and calcium carbonate on the variation and cycling of carbon source in integrated wastewater treatments. Bioresour Technol 225:262–271. https://doi.org/10.1016/j.biortech.2016.11.074
Zhao ZM, Song XS, Xiao YP, Zhao YF, Gong ZJ, Lin FD, Ding Y, Wang W, Qin TL (2016) Influences of seasons, N/P ratios and chemical compounds on phosphorus removal performance in algal pond combined with constructed wetlands. Sci Total Environ 573:906–914. https://doi.org/10.1016/j.scitotenv.2016.08.148
Zhao ZM, Song XS, Zhang YJ, Zhao YF, Wang BD, Wang YH (2017) Effects of iron and calcium carbonate on contaminant removal efficiencies and microbial communities in integrated wastewater treatment systems. Chemosphere 189:10–20. https://doi.org/10.1016/j.chemosphere.2017.09.020
Choi KH, Dobbs FC (1999) Comparison of two kinds of Biolog microplates (GN and ECO) in their ability to distinguish among aquatic microbial communities. J Microbiol Methods 36:203–213. https://doi.org/10.1016/S0167-7012(99)00034-2
Pan F, Xu AH, Xia DS, Yu Y, Chen G, Meyer M, Zhao DY, Huang CH, Wu QW, Fu J (2015) Effects of octahedral molecular sieve on treatment performance, microbial metabolism, and microbial community in expanded granular sludge bed reactor. Water Res 87:127–136. https://doi.org/10.1016/j.watres.2015.09.022
Allen B, Kon M, Bar-Yam Y (2009) A new phylogenetic diversity measure generalizing the Shannon index and its application to phyllostomid bats. Am Nat 174(2):236–243. https://doi.org/10.1086/600101
Jiang L, Han GM, Lan Y, Liu SN, Gao JP, Yang X, Meng J, Chen WF (2017) Corn cob biochar increases soil culturable bacterial abundance without enhancing their capacities in utilizing carbon sources in Biolog Eco-plates. J Integr Agric 16(3):713–724. https://doi.org/10.1016/S2095-3119(16)61338-2
Rutgers M, Wouterse M, Drost SM, Breure AM, Mulder C, Stone D, Creamer RE, Winding A, Bloem J (2016) Monitoring soil bacteria with community-level physiological profiles using Biolog TM ECO-plates in the Netherlands and Europe. Appl Soil Ecol 97:23–35. https://doi.org/10.1016/j.apsoil.2015.06.007
Chang J, Wu SQ, Dai Y, Liang W, Wu ZB (2012) Treatment efficiency of integrated vertical-flow constructed wetland plots for domestic wastewater. Ecol Eng 44:152–159. https://doi.org/10.1016/j.ecoleng.2012.03.019
Akratos CS, Tsihrintzis VA (2007) Effect of temperature, HRT, vegetation and porous media on removal efficiency of pilot-scale horizontal subsurface flow constructed wetlands. Ecol Eng 29(2):173–191. https://doi.org/10.1016/j.ecoleng.2006.06.013
Mateus DMR, Vaz MN, Pinho HJO (2012) Fragmented limestone wastes as a constructed wetland substrate for phosphorus removal. Ecol Eng 41:65–69. https://doi.org/10.1016/j.ecoleng.2012.01.014
Ding Y, Song XS, Wang YH, Yan DH (2013) Effect of supplying a carbon extracting solution on denitrification in horizontal subsurface flow constructed wetlands. Korean J Chem Eng 30(2):379–384. https://doi.org/10.1007/s11814-012-0139-4
Moussavi G, Jafari SJ, Yaghmaeian K (2015) Enhanced biological denitrification in the cyclic rotating bed reactor with catechol as carbon source. Bioresour Technol 189:266–272. https://doi.org/10.1016/j.biortech.2015.04.019
Stefanakis AI, Tsihrintzis VA (2012) Effects of loading, resting period, temperature, porous media, vegetation and aeration on performance of pilot-scale vertical flow constructed wetlands. Chem Eng J 181-182:416–430. https://doi.org/10.1016/j.cej.2011.11.108
Picardal F (2012) Abiotic and microbial interactions during anaerobic transformations of Fe (II) and NOx-. Front Microbiol 3:1–7. https://doi.org/10.3389/fmicb.2012.00112
Zhao ZM, Song XS, Wang YH, Wang DY, Wang SY, He Y, Ding Y, Wang W, Yan DH, Wang JF (2016c) Effects of algal ponds on vertical flow constructed wetlands under different sewage application techniques. Ecol Eng 93:120–128. https://doi.org/10.1016/j.ecoleng.2016.05.033
Shrestha J, Rich J, Ehrenfeld JG, Jaffe PR (2009) Oxidation of ammonium to nitrite under iron-reducing conditions in wetland soils: laboratory, field demonstrations, and push-pull rate determination. Soil Sci 174:156–164. https://doi.org/10.1097/SS.0b013e3181988fbf
Straub KL, Benz M, Schink B, Widdel F (1996) Anaerobic: nitrate-dependent microbial oxidation of ferrous iron. Appl Environ Microbiol 62:1458–1460. https://doi.org/10.1016/0921-8777(95)00057-7
Rozan TF, Taillefert M, Trouwborst RE, Glazer BT, Ma S, Herszage J, Lexia MV, Kent SP, George WL (2002) Iron-sulfur-phosphorus cycling in the sediments of a shallow coastal bay: implications for sediment nutrient release and benthic microalga blooms. Limnol Oceanogr 47:1346–1354. https://doi.org/10.4319/lo.2002.47.5.1346
Bruch I, Fritsche J, Bänninger D, Alewell U, Sendelov M, Hürlimann H, Hasselbach R, Alewell C (2011) Improving the treatment efficiency of constructed wetlands with zeolite-containing filter sands. Bioresour Technol 102:937–941. https://doi.org/10.1016/j.biortech.2010.09.0
Bongoua-Devisme AJ, Mustin C, Berthelin J (2012) Responses of iron-reducing bacteria to salinity and organic matter amendment in paddy soils of Thailand. Pedosphere 22:375–393. https://doi.org/10.1016/S1002-0160(12)60024-1
Li XQ, Zhang WX (2006) Iron nanoparticles: the core-shell structure and unique properties for Ni (II) sequestration. Langmuir 22:4638–4642. https://doi.org/10.1021/la060057k
Li L, Stanforth R (2000) Distinguishing adsorption and surface precipitation of phosphate on goethite (a-FeOOH). J Colloid Interface Sci 230:12–21. https://doi.org/10.1006/jcis.2000.7072
Asnicar F, Weingart G, Tickle TL, Huttenhower C, Segata N (2015) Compact graphical representation of phylogenetic data and metadata with GraPhlAn. Peer J 3:e1029. https://doi.org/10.7717/peerj.1029
Vandewalle JL, Goetz GW, Huse SM, Morrison HG, Sogin ML, Hoffmann RG, Yan K, Mclellan SL (2012) Acinetobacter, Aeromonas and Trichococcus populations dominate the microbial community within urban sewer infrastructure. Environ Microbiol 14(9):2538–2552. https://doi.org/10.1111/j.1462-2920.2012.02757.x
Mohanakrishnan J, Gutierrez O, Sharma KR, Guisasola A, Werner U, Meyer RL, Keller J, Yuan Z (2009) Impact of nitrate addition on biofilm properties and activities in rising main sewers. Water Res 43:4225–4237. https://doi.org/10.1016/j.watres.2009.06.021
Kong X, Wang C, Ji M (2013) Analysis of microbial metabolic characteristics in mesophilic and thermophilic biofilters using Biolog plate technique. Chem Eng J 230:415–421. https://doi.org/10.1016/j.cej.2013.06.073
Park SJ, Kim J, Lee JS, Rhee SK, Kim H (2014) Characterization of the fecal microbiome in different swine groups by high-through put sequencing. Anaerobe 28:157–162. https://doi.org/10.1016/j.anaerobe.2014.06.002
Desai C, Parikh RY, Vaishnav T, Shouche YS, Madamwar D (2009) Tracking the influence of long-term chromium pollution on soil bacterial community structures by comparative analyses of 16S rRNA gene phylotypes. Res Microbiol 160:1–9. https://doi.org/10.1016/j.resmic.2008.10.003
Sinninghe Damsté JS, Rijpstra WIC, Geenevasen JAJ, Strous M, Jetten MSM (2005) Structural identification of ladderane and other membrane lipids of planctomycetes capable of anaerobic ammonium oxidation (anammox). FEBS J 272:4270–4283. https://doi.org/10.1111/j.1742-4658.2005.04842.x
Dunfield PF, Yuryev A, Senin P, Angela VS, Matthew BS, Hou SB, Ly B, Saw JH, Zhou ZM, Ren Y, Wang JM, Mountain BW, Crowe MA, Weatherby TM, Bodelier PLE, Liesack W, Feng L, Wang L, Alam M (2007) Methane oxidation by an extremely acidophilic bacterium of the phylum Verrucomicrobia. Nature 450(7171):879–882. https://doi.org/10.1038/nature06411
Yousuf B, Keshri J, Mishra A, Jha B (2012) Application of targeted metagenomics to explore abundance and diversity of CO2-fixing bacterial community using cbbL gene from the rhizosphere of Arachis hypogaea. Gene 506:18–24. https://doi.org/10.1016/j.gene.2012.06.083
Author information
Authors and Affiliations
Corresponding author
Additional information
Highlights
• Ferrous ions produced by nZVI and ferric ions realized the longer and higher performance in CWs.
• Produced ferrous ions rearranged the microbial abundance and diversity in CWs.
• The direct microbiological evidence of iron-driven contaminant removal was given.
• Produced iron ions promoted TP removal through precipitation and optimizing microbial conditions.
Electronic Supplementary Material
ESM 1
(DOCX 2000 kb)
Rights and permissions
About this article
Cite this article
Zhao, Z., Zhang, X., Cheng, M. et al. Influences of Iron Compounds on Microbial Diversity and Improvements in Organic C, N, and P Removal Performances in Constructed Wetlands. Microb Ecol 78, 792–803 (2019). https://doi.org/10.1007/s00248-019-01379-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-019-01379-7