Abstract
Methanotrophs are important microbial communities in coastal ecosystems. They reduce CH4 emission in situ, which is influenced by soil conditions. This study aimed to understand the differences in active aerobic methanotrophic communities in mangrove forest soils experiencing different inundation frequency, i.e., in soils from tidal mangroves, distributed at lower elevations, and from dwarf mangroves, distributed at higher elevations. Labeling of pmoA gene of active methanotrophs using DNA-based stable isotope probing (DNA-SIP) revealed that methanotrophic activity was higher in the dwarf mangrove soils than in the tidal mangrove soils, possibly because of the more aerobic soil conditions. Methanotrophs affiliated with the cluster deep-sea-5 belonging to type Ib methanotrophs were the most dominant methanotrophs in the fresh mangrove soils, whereas type II methanotrophs also appeared in the fresh dwarf mangrove soils. Furthermore, Methylobacter and Methylosarcina were the most important active methanotrophs in the dwarf mangrove soils, whereas Methylomonas and Methylosarcina were more active in the tidal mangrove soils. High-throughput sequencing of the 16S ribosomal RNA (rRNA) gene also confirmed similar differences in methanotrophic communities at the different locations. However, several unclassified methanotrophic bacteria were found by 16S rRNA MiSeq sequencing in both fresh and incubated mangrove soils, implying that methanotrophic communities in mangrove forests may significantly differ from the methanotrophic communities documented in previous studies. Overall, this study showed the feasibility of 13CH4 DNA-SIP to study the active methanotrophic communities in mangrove forest soils and revealed differences in the methanotrophic community structure between coastal mangrove forests experiencing different tide frequencies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Coastal marsh ecosystems are believed to be net sinks for carbon sequestration because they accumulate C at a rate of up to 100 times higher than that in terrestrial forest ecosystems [1,2,3]. However, coastal marsh ecosystems are also important sources of greenhouse gases because, compared to other ecosystems, they are characterized by a wide range of anaerobic and biogeochemical conditions along both spatial and temporal gradients. These environmental gradients, as well as the high soil sulfate concentration, may change the dynamics of methane (CH4) and carbon dioxide (CO2) emissions [4]. Because such greenhouse gas emissions are mainly from soil microbial activities, understanding the composition and diversity of active microbial communities in coastal ecosystems, particularly those related to CH4 dynamics, is very important because CH4 is considered nearly 25 times more efficient than CO2 in trapping heat [5].
CH4 emission varies across salinity regimes in tidal wetlands because the sulfate present in salt water affects the competition between sulfate reduction and methanogenesis [6]. Moreover, in strongly tide-influenced wetlands, 52–90% of the CH4 produced in situ is reoxidized to CO2 by methanotrophs before it reaches the atmosphere [7,8,9].
Canonical aerobic methanotrophs include three types. Type I includes the family Methylococcaceae belonging to the γ-Proteobacteria, type II includes the families Methylocystaceae and Beijerinckiaceae belonging to the α-Proteobacteria [10], and type X belongs to the γ-Proteobacteria [11,12,13]. Most methanotrophs, with the exception of Methylocella and Methyloferula, contain the pmoA gene encoding the particulate methane monooxygenase, which catalyzes the first step of methane oxidation [14, 15]. Thus, pmoA has been used as a molecular marker to evaluate the diversity and habitat preferences of cultured and uncultured aerobic methanotrophic bacteria [16].
Methanotrophic activity in coastal ecosystems such as freshwater tidal wetlands and brackish wetland sediments has been discussed in previous reports [8, 17], whereas there have been only few studies on the mangrove ecosystem. In tropical and subtropical areas, mangrove forests are one of the dominant ecosystems in coastal regions, occupying more than 15.2 million hectares globally [3, 18, 19]. Because of the diverse salinity ranges due to different tidal frequency, the CH4 emission was between 0.003 and 7.7 mg C m−2 h−1 [20,21,22]. Gomes et al. [23] have found some methanotrophs, such as Methylomonas, in the rhizosphere of mangrove roots after a 75-day incubation period of mangrove seedlings in mesocosms. However, the active methanotrophs inhabiting mangrove forest soils and their activities are still unclear because the abundance of methanotrophs is relatively low in coastal ecosystems and they are difficult to isolate [12].
DNA-based stable isotope probing (DNA-SIP) with a 13CH4 label has been demonstrated to be a successful tool for the identification of active methanotrophs in rice paddy, grassland soils [24,25,26], geothermal springs, and lake sediments [27,28,29]. In combination with isopycnic centrifugation, it was possible to extract the pmoA gene to quantify the diversity of the active methanotrophs incorporating 13C in the targeted soils [30].
This study was designed to quantify and evaluate the methanotrophs in natural tidal mangrove forests in northern Taiwan, using the DNA-SIP technique, and by quantitative polymerase chain reaction (qPCR) and high-throughput sequencing of the pmoA and 16S ribosomal RNA (rRNA) genes. This study aims to understand how methanotrophic communities vary in mangrove forest soils that experience different frequency of tides and how efficiently they oxidize CH4.
Materials and Methods
Sampling Site
This study was carried out in the Tamsui estuary, Taipei, Taiwan (121° 26′ E, 25° 9′ N), a large portion of which (around 100 ha) is covered with Kandelia obovata (Fig. 1). The average atmospheric temperature in the Tamsui estuary was between 14.7–31.4 °C with an average of 22.4 °C [31]. Tall K. obovata trees, about 2–3.5 m high, are the dominant plants in most of the area (designated as tidal mangrove), where tidal cycles occur every day. At a higher elevation (1–1.5 m above the sea level), located next to the tall mangrove forest, soil is only inundated during monthly high tides, and dwarfed K. obovata plants of about 0.5–1.5 m high are the dominant plant community (designated as dwarf mangrove) [32]. The tidal mangrove soil has an average salinity of 3.65 psu, sulfate (SO4 2−) concentration of 269.6 μg S g−1 soil, and soil oxidation–reduction (redox) potential of 180 mV, and the dwarf mangrove soil has a salinity of 2.88 psu, SO4 2− concentration of 610.5 μg S g−1 soil, and soil redox potential of 370 mV [33], which makes the mangrove forests oligohaline (0.5–5.0 psu) systems. The annual mean ammonium and nitrate concentrations were respectively 1.2 and 0.44 mg N L−1 [34], and the water temperature was 14.7–30.5 °C [31] in the main channel of the Tamsui River.
A total of 36 soil core samples (18 from each site) were collected in January 2016 from both dwarf and tidal mangrove soils during low tides on a random walk, using polyvinyl chloride (PVC) tubes (1.5 cm in diameter and 30 cm long). Any visible litter on the soil surface was removed prior to collection. The collected samples were brought to the laboratory and immediately pushed out from the bottom of the PVC tubes, using rubber stoppers. Then, the top 1 cm of the soil samples was sliced off from the bulk soil, using a knife pretreated with 70% ethanol. The sliced-off 1-cm soil samples from the 18 cores from the same site were well mixed in a plastic zipper bag as a composite sample and stored at 4 °C at their initial moisture content until experiments were conducted.
Sub-samples were taken and oven-dried at 105 °C for 72 h to determine the soil moisture content. As supplemental information, total organic C and total N were measured by the combustion method using a Fisons NA1500 elemental analyzer (ThermoQuest Italia, Milan, Italy).
Soil Incubation
Six replicates of samples were re-distributed from the composite sample from each site and incubated within a week after the soil cores were collected from the mangroves. For each replicate, 10 g of soil was weighed from the mixed soil samples, placed into a 146-mL Erlenmeyer flask (125-mL flask with 21 mL of extra headspace above the flask scale), and sealed with a double-sided rubber stopper. Then, the six replicates from each site were separated into two sets. The headspace for one set of samples was replaced with 1.4 mL of pure (99.9%) 12CH4, and that for the other set of samples was replaced with 1.4 mL of pure (99 atom % 13C) 13CH4 (Sigma–Aldrich, St. Louis, MO, USA) to make the headspace contain approximately 1.2% CH4 for all samples.
A 1-mL gas sample was extracted from the headspace of each flask with a syringe to determine the initial CH4 concentration, and 1 mL of nitrogen gas was injected back into the flask to maintain the constant pressure in the headspace. The extracted gas samples were immediately analyzed using a gas chromatograph with a thermal conductivity detector (GC-TCD, 9790II, Fuli Instruments, Zhejiang, China) with five standard CH4 concentrations: 0; 5000; 7500; 10,000; and 15,000 ppmv.
The flasks were incubated in a dark incubator (LM-570RD, Yihder Technology Co., Taiwan) at 20 °C for 15 days, which was comparable to but slightly lower than the field temperature conditions to better capture the change in CH4 concentrations through time. Only the set of samples with injected 12CH4 was periodically tested (every week) during the incubation for the remaining CH4 concentration in the headspace to measure the CH4 oxidation rate while preventing dilution of the 13CH4 concentration in the headspace of the isotope-labeled samples. The incubation was completed when no 12CH4 was detected in the headspace of the flasks.
DNA Extraction and SIP Gradient Fractionation
Total DNA was extracted from 0.8 g of the incubated soil samples, using the PowerSoil® DNA isolation kit (MO BIO Laboratories, Inc., Carlsbad, CA, USA) according to the manufacturer’s instructions. The extracted DNA was stored at − 20 °C until further analysis.
The extracted DNA (6 μg) from each sample was partitioned into 15 density gradient fractions by the density gradient centrifugation and fractionation method described by Lu and Jia [35]. The abundance of the methanotrophic pmoA gene in fractions 2–14 from all 13CH4-labeled samples was then tested by DNA amplification using qPCR [36] with the A189F/mb661r primer pair. The cycling conditions were as follows: 5 min at 95 °C, followed by 40 cycles of 95 °C for 10 s, 55 °C for 30 s, 72 °C for 30 s, and 80 °C for 5 s with acquisition after both last cycling steps. Then, 13C-incubated methanotroph species were identified in fractions with the highest pmoA gene abundance using the Illumina MiSeq platform. The reads were processed and quality-controlled using QIIME [37]. Then, the trimmed sequences were classified using mothur [38] with instructions and reference database provided by Dumont et al. [39]. The abundance of identified methanotrophic sequences in the heavy fractions of each replicate sample at the same site was then pooled for calculation of the relative abundance.
Three replicates of fresh soil total DNA samples at each site were extracted and analyzed for pmoA gene abundance using the same qPCR technique. The sample with the highest pmoA gene abundance was identified for the methanotroph species using the protocol described above.
High-Throughput Sequencing of 16S rRNA Gene and Data Analysis
To access the bacterial community structure in different gradient fractions, the 16S rRNA gene was analyzed in fractions 2–14 of the 12C- and 13C-incubated soil samples at both mangrove sites. Because of the numerous amounts of DNA samples that needed to be processed, only one of the triplicate samples was analyzed for the 16S rRNA genes.
The V4 region of the 16S rRNA gene was amplified with primers 515F and 806R [40]. Both the forward and reverse primers included barcoded sequences and adapters. PCR was performed at 94 °C for 3 min, followed by 25 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s and a final extension at 72 °C for 6 min using the KAPA HiFi HotStart ReadyMix Kit (KAPA Biosystems, MA, USA). The 16S rRNA genes were sequenced using the Illumina MiSeq platform. Sequence analysis was performed using the Ribosomal Database Project (RDP) pipeline (http://pyro.cme.msu.edu). The taxonomic information was analyzed using the naïve Bayesian rRNA classifier [41] in RDP.
The bacterial community structure in the fresh soil was also analyzed using the V4 region of the 16S rRNA gene for comparison. Similar to the analysis of the pmoA gene in fresh mangrove soils, three replicates of total DNA samples from fresh soil at each site were extracted, and the V4 region of the 16S rRNA gene in the sample with the highest pmoA gene abundance was amplified and sequenced using the Illumina MiSeq platform.
Statistical Analysis
Two-way factorial analysis was performed to determine whether the abundance of the pmoA gene was spatially or temporally different. Student’s paired t test was applied to further test the means of dependent variables when the two-way factorial analysis revealed a difference. Because the abundance of the pmoA gene was non-linearly distributed, log transformation of the raw data was performed prior to the statistical analysis.
All statistical analyses were performed using JMP 11.0 (SAS Inc., Cary, NC, USA). P < 0.05 was considered significant for all tests.
Results
The moisture contents in the top layer (0–1 cm) of the dwarf and tidal mangrove soils were 23.5 and 30%, respectively. The total organic C content was higher in the tidal mangrove soils (35.4 mg C g−1 soil) than in the dwarf mangrove soils (8.4 mg C g−1 soil). Similarly, the total soil N content was also higher in the tidal mangrove forest soils (2.4 mg N g−1 soil), whereas it was negligible in the dwarf mangrove forest soils (0.1 mg N g−1 soil).
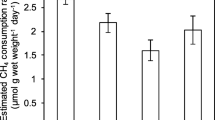
The results showed that the replicates of both dwarf and tidal mangrove soils had similar initial CH4 concentrations (12,100 ppmv). The CH4 concentration quickly decreased in the dwarf mangrove samples, and CH4 was completely depleted from these samples on day 8, whereas it was not depleted from the tidal mangrove samples until day 15. On average, the dwarf mangrove and tidal mangrove soils utilized the headspace CH4 at a rate of 15.13 and 9.63 μg g−1 day−1, respectively (Fig. 2).
qPCR analysis of the fresh and incubated soil samples showed that the pmoA gene abundance significantly increased after incubation (P = 0.003), and the abundance of the pmoA gene was higher in tidal mangrove soils (fresh: 5.7 × 107 copies g−1 soil; incubated: 2.4 × 108 copies g−1 soil) than in dwarf mangrove soils (un-incubated: 1.7 × 107 copies g−1 soil; incubated: 8.5 × 107 copies g−1 soil) (P = 0.02). However, no interaction was found between the sites and the treatments (i.e., fresh and incubated soils).
qPCR analysis of the DNA extracted and fractioned from the incubated soil samples showed that the pmoA gene was present in both dwarf and tidal mangrove forest soils. About 60–100% of the pmoA gene was successfully separated by ultracentrifugation and distributed in heavy fractions (layers 6–9) of the 13C-labeled dwarf and tidal mangrove soil samples. On the contrary, 70–100% of the pmoA gene was in light fractions (layers 11–13) of the non-isotope-labeled (incubated with 12CH4) dwarf and tidal mangrove soil samples (Fig. 3).
Relative abundance of the pmoA gene and 16S rRNA gene in fractionated DNA samples of dwarf and tidal mangrove soils. Solid symbols represent the 13CH4-labeled gene. Open symbols represent the 12CH4-labeled gene. Only one representative result is presented from the 3 replicate samples. The ratio of the maximum quantities of pmoA genes was calculated from the copies of the pmoA genes in one fraction layer divided by the number of pmoA copies in the layer with the highest quantity of pmoA genes × 100%
For the pmoA analysis using the MiSeq platform, 22,460 (dwarf: 10,098 sequences; tidal: 12,362 sequences) and 564,497 (dwarf: 260,888 sequences; tidal: 303,609 sequences) pmoA gene sequences were retrieved, respectively, from the fresh total soil DNA and 13C-labeled DNA peak layer from both mangrove sites, and 30 and 26 OTUs were respectively identified at the level of 93% identity [42] in the fresh soil samples and the isotope-labeled DNA peak layer soil samples.
In the fresh mangrove forest samples, the majority of the sequences (11,717 sequences) belonged to type Ib methanotrophs, 7490 sequences of the pmoA gene belonged to type Ia methanotrophs, and 2241 sequences of the pmoA gene belonged to type II methanotrophs (Fig. 4). Methanotrophs affiliated with the cluster deep-sea-5 appeared to be the most dominant methanotroph genus among all methanotrophic genes in the mangrove forests and were more dominant in the tidal mangrove soils than in the dwarf mangrove soils (64.4 and 29.8%). In contrast, more Methylobacter and Methylocystis were found in the dwarf mangrove soils than in the tidal mangrove soils.
In the incubated samples, the majority of the sequences (476,320 sequences) belonged to type Ia methanotrophs, 48,029 sequences of the pmoA gene belonged to type Ib methanotrophs, and only 34 sequences of the pmoA gene belonged to type II methanotrophs. Methylosarcina was the most dominant methanotrophs in both mangrove forests and comprised approximately 50% of the relative abundance among all methanotrophs. Methylobacter appeared to have higher abundance in the dwarf mangrove soils, whereas Methylomonas had higher abundance in the tidal mangrove soils.
The total microbial composition analyzed from the 16S rRNA gene sequence using MiSeq sequencing of the fresh soil samples (dwarf: 248,775 sequences; tidal: 300,400 sequences) is shown in the supplementary file (Table S1 and Fig. S2). Among the methanotrophs identified from 16S rRNA, a large portion (dwarf: 58.3%; tidal: 63.5%) of unclassified methanotrophs was present in both dwarf and tidal mangrove soils by 16S rRNA MiSeq sequencing (Fig. 5). Among the identified methanotrophs, both a type II methanotroph, i.e., Methylocystis, and type Ia methanotrophs, i.e., Methylobacterium and Methylobacter, were found in the dwarf mangrove soils. However, the type Ia Methylosarcina, Methylobacter, and Methylomonas were mostly found in the tidal mangrove soils.
Results of 16S rRNA gene analysis from the isotope-labeled DNA peak layers (dwarf fraction 7: 207,308 sequences; tidal fraction 6: 223,021 sequences) also identified a large portion of unclassified methanotrophs in both dwarf and tidal mangrove soils, and their sequences were most similar to those of Methylomarinum commonly found in marine sediments (Fig. 6) [43]. In contrast, approximately 30% of the methanotrophs was composed of Methylosarcina and Methylomonas in the tidal mangrove soils.
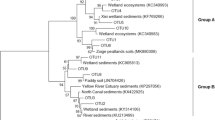
Phylogeny of pmoA and 16S rRNA lineages detected in dwarf (DF) and tidal (TL) mangrove soils. The neighbor-joining tree was constructed by using the MEGA 7.0 software package. Representatives of the OTUs detected in the two genes are shown in bold italic font. The pmoA sequences of type II methane-oxidizing bacteria were used as the outgroup. The scale bar represents 10% sequence divergence for the pmoA gene and 2% sequence divergence for the 16S rRNA gene. The values following the OTU numbers are the percentage of reads for the pmoA genes and the 16S rRNA gene in peak fractions from dwarf (DF) and tidal (TL) mangrove soil samples
Discussion
Activity of Methanotrophs and Feasibility of Using 13CH4 DNA-SIP
Methanotrophs present in the dwarf mangrove soils showed a stronger CH4 oxidation potential than those present in the tidal mangrove soils. This may be due to the difference in the frequency of tides between the two sampling locations, resulting in different redox potentials (dwarf: 370 mV; tidal: 180 mV) of the soils [33]. Since the methanotrophs identified in the present study were aerobic, it is reasonable to assume that the CH4 oxidation potential is higher in the dwarf site than in the tidal site. The absolute abundance of methanotrophs was higher at tidal mangrove than at dwarf mangrove based on the results of the qPCR for the pmoA genes in both the fresh and incubated soil samples. However, the methanotrophic activity was higher in the dwarf mangrove soils since CH4 was depleted within 8 days in the incubated dwarf mangrove soil samples. The reason for the contradiction between the abundance of methanotrophs and their activity is unclear. However, the diffusion rates of CH4 and O2 are both more than 10,000 times higher in air [44] than in water [45].
Although the soil moisture from the collected samples was similar at the two sites, possibly because they were collected during the low tide, the different soil redox potentials implied that the soil retained a higher water content for a longer period of time in the tidal mangrove than in dwarf mangrove. Therefore, less CH4 and O2 could be available for methanotrophs in the tidal mangrove soils. Moreover, the soil total organic C and N contents were higher at the tidal mangrove than at the dwarf mangrove. Because the soil organic matter has a high water-holding capacity [46, 47], it may also help to keep water in the soil for a long period and prevent gas diffusion. The physiochemical difference between the two mangrove forest soils may further affect the methanotrophic composition in situ, resulting in the significantly different CH4 oxidizing activities observed at the two sites. This may provide a possible explanation for the low methanotrophic activity in the tidal mangrove soils.
When comparing the abundance of pmoA genes in the fresh mangrove forest soils to that in other ecosystems, the number of pmoA copies was similar to that of other coastal ecosystems [48] but was slightly lower than that of other freshwater wetlands in East Asia [49, 50]. This may be due to the presence of SO4 2− in coastal soils that reduced the CH4 efflux [4] and indirectly inhibited the growth of methanotrophs [12].
The patterns of abundance of the 13C- and 12C-labeled pmoA gene in different gravity fractions were similar to those detected in previous studies that used the same technique [30], indicating that active methanotrophic genes were successfully labeled with 13CH4. Therefore, the methanotrophs identified by this technique could be regarded as bacteria that participated in CH4 oxidation during the 15-day soil incubation period.
Methanotrophs Identified from the pmoA gene
Henckel et al. [51] indicated that type I methanotrophs are more common at a relatively low soil water content because they prefer a high-oxygen environment compared to type II methanotrophs. In the present study, we collected the mangrove soil samples from the top layer (0–1 cm) during low tides when the soil should be mostly aerobic. This may have resulted in mostly type I methanotrophs in the fresh soils at both sites. Although the two mangrove sites experienced different tidal frequencies, type I methanotrophs were dominant in both sites. A possible reason may be that the soil redox at both sites was still in a relatively oxidative condition [52, 53], which provided a favorable environment for the growth of type I methanotrophs at both sites even though the compositions were different at the two sites. However, as previously mentioned, the different compositions of type I methanotrophs at the two sites still affected their overall CH4-oxidizing potential.
Research has also shown that type I methanotrophs are usually found in soils with a limited CH4 supply because they grow better than type II methanotrophs in a low-CH4 environment [12]. On the contrary, type II methanotrophs such as Methylocystis are usually found in high-CH4 systems [54]. Coastal ecosystems typically release less CH4 than freshwater ecosystems because of the presence of SO4 2− in soil [4, 6, 55,56,57]. In mangrove forests, CH4 emission is 0.003–7.7 mg C m−2 h−1 [20,21,22], which is lower than that in other freshwater ecosystems such as rice paddies (4.3–16.4 mg C m−2 h−1) [58, 59] and wet ponds (0.75–40.5 mg C m−2 h−1) [60]. This may be another reason why mostly type I methanotrophs were predominant in the fresh mangrove soils.
In addition, previous research showed that the type I methanotrophic population was stimulated in incubated soil using the same DNA-SIP technique [61,62,63], possibly because the communities could rapidly react to the added CH4 concentration [64, 65]. It is therefore possible that type I methanotrophs were activated through the nature of the experimental design even though type II methanotrophs inhabited the soils.
In the fresh dwarf mangrove soil, nearly 20% of the methanotrophs were categorized as type II Methylocystis. Several studies have shown that type II methanotrophs can perform N2 fixation to provide a nutrient source [12, 66]. As the soil at the dwarf mangrove contained low soil TN, this may favor the growth of some type II methanotrophic bacteria. In addition, methanotrophs belonging to deep-sea-5 cluster were more abundant in the fresh tidal mangrove soils than in the dwarf mangrove soils. Although the exact reason for the higher abundance of the deep-sea-5 cluster was not clear, it may involve the more frequent tides at the tidal site that somehow provided environmental conditions more similar to those of marine sediments, as the deep-sea-5 cluster was mostly found in the marine ecosystems [16].
Among the methanotrophs identified using the pmoA gene after incubation, type Ia methanotrophs were the most dominant CH4-oxidizing bacteria in the dwarf and tidal mangrove soils. In particular, Methylosarcina expanded its population to nearly 50% of the relative abundance and became the predominant methanotroph in both mangrove soils after incubation. Research has shown that Methylosarcina can inhabit a wide pH (4.0–9.0) and temperature (4–37 °C) range [67]. The exact reason for the fast growth of Methylosarcina is unclear, but it could be due to our selection of experimental conditions suitable for its growth.
Research has shown that Methylobacter that has high adaptation to various salinity conditions is commonly found in freshwater to brackish water environments. Some species in this genus can even grow in water containing more than 3% NaCl [68]. Although the salinity in the dwarf and tidal mangrove soils was similar at the time when the soil samples were collected [33], coastal soils were found to have a higher salinity at higher elevations in summer because of less tidal inundation and high evapotranspiration during this time, which leads to the accumulation of salt in the surface soil [4]. Therefore, the higher relative abundance of Methylobacter in the dwarf mangrove soil than in the tidal mangrove soil may imply that it tolerates high salinity levels, especially in summer when salinity of soil-pore water could be potentially high.
Previous research has indicated that most species belonging to Methylomonas grow at pH 6.5–7.0 [68, 69], whereas the optimal pH for Methylobacter is pH 7.0 [68]. Although the optimal pH for the two methanotrophs is not widely different, the soil pH values in the dwarf mangrove soils (pH = 6.34) were slightly lower than those in the tidal mangrove soils (pH = 7.55) [33]. Therefore, this pH difference might have contributed to the larger proportion of the Methylomonas community in the tidal mangrove soils (fresh: 1.8%; incubated: 18.7%) compared to the dwarf soils (fresh: 0.0%; incubated: 3.5%).
The different compositions of methanotrophic communities in the fresh dwarf and tidal mangrove soils suggest the influence of the tide frequencies on the methanotrophic composition in mangrove soils.
Methanotrophs Detected in Mangrove Soils by 16S rRNA Gene Sequencing
The results of the 16S rRNA gene sequencing showed that the majority of methanotrophs in both dwarf and tidal mangrove soils were unclassified (OTUs 5–7) and similar to Methylomarinum, which is commonly found in marine sediments. However, after aligning the pmoA primers and the sequences of the methanotrophs identified in the present study, we found that all the identified methanotrophs contained the same conserved genes, and their respective sequence regions basically matched the sequences of the pmoA primers used in this study. This result showed that the methanotrophs of OTUs 5–7 were different from Methylomarinum species because we could not find the same OTUs (OTUs 5–7) after extracting the pmoA genes from the mangrove forest soils. Therefore, the exact composition of the OTU 5–7 methanotrophs is still unknown, possibly because of the short fragments of the 16S rRNA gene that were sequenced. However, the results clearly indicate that methanotrophs living in estuarine mangroves may be widely different from those inhabiting freshwater systems. Other than the unclassified methanotrophs, the composition of the rest of the methanotrophs identified using the 16S rRNA gene was similar to that determined using the pmoA genes at both sites. This implies that both methods can be successfully applied in tidal mangrove forests.
In addition, as previously mentioned, the dwarf mangrove soils showed high activity of methanotrophic bacteria but low abundance of pmoA genes. The methanotrophic communities in dwarf and tidal mangrove soils identified from the pmoA genes showed similar composition. Additionally, the unclassified methanotroph in dwarf mangrove soils largely increased in relative abundance during the incubation. This implies that the high methane-oxidizing potential of dwarf mangrove soils may be mainly attributed to this methanotroph, which has yet to be classified.
Several studies have found that anaerobic oxidation of CH4 may be an important pathway in coastal freshwater and brackish wetlands because microbes use sulfate as an alternative electron acceptor when oxygen is depleted [17, 70, 71]. Since we used aerobic incubation to label aerobic methanotrophs in the present study, we may consider incubation of subsurface mangrove soils under anaerobic conditions in further studies to evaluate the importance of anaerobic oxidation of CH4 in tidal mangroves as the present study may only identify aerobic methanotrophs in the mangrove forest soils.
Conclusions
Our study indicated that shallow mangrove soils mostly contained active type I methanotrophs. The methanotrophic activity was higher in dwarf mangrove soils, which are only inundated during monthly high tides and have a higher soil redox potential than that of tidal mangrove soils. The compositions of methanotrophs in both sites were similar when using the 13CH4 DNA-SIP and pmoA genes, with methanotrophs belonging to the deep-sea-5 cluster and Methylosarcina being the most abundant methanotrophs in the fresh dwarf and tidal mangrove soils, whereas Methylosarcina appeared to be the most active methanotroph after the incubation.
The results of the high-throughput sequencing analysis of the 16S rRNA gene indicated that a considerable number of methanotrophs were unclassified, implying that methanotrophic communities in estuarine mangroves may differ from those in freshwater ecosystems. Moreover, the increment of the unclassified methanotroph in the dwarf mangrove soils may be critical for methane oxidation in the mangrove forest. Overall, this study showed that the 13CH4 DNA-SIP technique enabled successful identification of active methanotrophic communities and also demonstrated that environmental fluctuations in mangrove ecosystems could result in differences in methanotrophic activity.
References
Chmura GL, Anisfeld SC, Cahoon DR, Lynch JC (2003) Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles 17:1111. https://doi.org/10.1029/2002gb001917
Kennedy H, Bjork M (2009) Seagrass meadows. In: Laffoley D, Grimsditch G (eds) The management of natural coastal carbon sinks, vol 53. IUCN, Gland, pp. 23–30
Pidgeon E (2009) Carbon sequestration by coastal marine habitats: important missing sinks. In: Laffoley D, Grimsditch G (eds) The management of natural coastal carbon sinks, vol 53. IUCN, Gland, pp. 47–51
Shiau Y, Burchell MR, Krauss KW, Birgand F, Broome SW (2016) Greenhouse gas emissions from a created brackish marsh in eastern North Carolina. Wetlands 36:1009–1024. https://doi.org/10.1007/s13157-016-0815-y
IPCC (2013) Climate change 2013: the physical science basis. http://www.ipcc.ch/report/ar5/wg1/
Bridgham SD, Megonigal JP, Keller JK, Bliss NB, Trettin C (2006) The carbon balance of North American wetlands. Wetlands 26:889–916
Le Mer J, Roger P (2001) Production, oxidation, emission and consumption of methane by soils: a review. Eur. J. Soil Biol. 37:25–50. https://doi.org/10.1016/s1164-5563(01)01067-6
Megonigal JP, Schlesinger WH (2002) Methane-limited methanotrophy in tidal freshwater swamps. Glob. Biogeochem. Cycles 16:35-1–35-10. https://doi.org/10.1029/2001gb001594
Roslev P, King GM (1996) Regulation of methane oxidation in a freshwater wetland by water table changes and anoxia. FEMS Microbiol. Ecol. 19:105–115. https://doi.org/10.1111/j.1574-6941.1996.tb00203.x
Bowman JP, Sly LI, Nichols PD, Hayward AC (1993) Revised taxonomy of the methanotrophs - description of Methylobacter gen-nov, emendation of Methylococcus, validation of Methylosinus and Methylocystis species, and a proposal that the family Methylococcaceae includes only the group-I methanotrophs. Int. J. Syst. Bacteriol. 43:735–753
Gagliano AL, D'Alessandro W, Tagliavia M, Parello F, Quatrini P (2014) Methanotrophic activity and diversity of methanotrophs in volcanic geothermal soils at Pantelleria (Italy). Biogeosciences 11:5865–5875. https://doi.org/10.5194/bg-11-5865-2014
Hanson RS, Hanson TE (1996) Methanotrophic bacteria. Microbiol. Rev. 60:439–471
Wise MG, McArthur JV, Shimkets LJ (1999) Methanotroph diversity in landfill soil: isolation of novel type I and type II methanotrophs whose presence was suggested by culture-independent 16S ribosomal DNA analysis. Appl. Environ. Microbiol. 65:4887–4897
Hakemian AS, Rosenzweig AC (2007) The biochemistry of methane oxidation. Annu. Rev. Biochem. 76:223–241
McDonald IR, Bodrossy L, Chen Y, Murrell JC (2008) Molecular ecology techniques for the study of aerobic methanotrophs. Appl. Environ. Microbiol. 74:1305–1315. https://doi.org/10.1128/aem.02233-07
Knief C (2015) Diversity and habitat preferences of cultivated and uncultivated aerobic methanotrophic bacteria evaluated based on pmoA as molecular marker. Front. Microbiol. 6:1346. https://doi.org/10.3389/fmicb.2015.01346
Segarra KEA, Comerford C, Slaughter J, Joye SB (2013) Impact of electron acceptor availability on the anaerobic oxidation of methane in coastal freshwater and brackish wetland sediments. Geochim. Cosmochim. Acta 115:15–30. https://doi.org/10.1016/j.gca.2013.03.029
Adam P (2011) The energetics of mangrove forests. Austral Ecology 36:E18–E19. https://doi.org/10.1111/j.1442-9993.2010.02190.x
Duke NC, Ball MC, Ellison JC (1998) Factors influencing biodiversity and distributional gradients in mangroves. Glob. Ecol. Biogeogr. Lett. 7:27–47. https://doi.org/10.2307/2997695
Alongi DM, Pfitzner J, Trott LA, Tirendi F, Dixon P, Klumpp DW (2005) Rapid sediment accumulation and microbial mineralization in forests of the mangrove Kandelia candel in the Jiulongjiang estuary, China. Estuar. Coast. Shelf Sci. 63:605–618. https://doi.org/10.1016/j.ecss.2005.01.004
Chauhan R, Datta A, Ramanathan AL, Adhya TK (2015) Factors influencing spatio-temporal variation of methane and nitrous oxide emission from a tropical mangrove of eastern coast of India. Atmos. Environ. 107:95–106. https://doi.org/10.1016/j.atmosenv.2015.02.006
Wang H, Liao G, D’Souza M, Yu X, Yang J, Yang X, Zheng T (2015) Temporal and spatial variations of greenhouse gas fluxes from a tidal mangrove wetland in Southeast China. Environ. Sci. Pollut. Res. 23:1873–1885. https://doi.org/10.1007/s11356-015-5440-4
Gomes NCM, Cleary DFR, Pinto FN, Egas C, Almeida A, Cunha A, Mendonca-Hagler LCS, Smalla K (2010) Taking root: enduring effect of rhizosphere bacterial colonization in mangroves. PLoS One 5. https://doi.org/10.1371/journal.pone.0014065
Daebeler A, Bodelier PLE, Yan Z, Hefting MM, Jia Z, Laanbroek HJ (2014) Interactions between Thaumarchaea, Nitrospira and methanotrophs modulate autotrophic nitrification in volcanic grassland soil. The ISME Journal 8:2397–2410. https://doi.org/10.1038/ismej.2014.81
Jia ZJ, Cai ZC, Xu H, Li XP (2001) Effect of rice plants on CH4 production, transport, oxidation and emission in rice paddy soil. Plant Soil 230:211–221. https://doi.org/10.1023/a:1010366631538
Zheng Y, Huang R, Wang BZ, Bodelier PLE, Jia ZJ (2014) Competitive interactions between methane- and ammonia-oxidizing bacteria modulate carbon and nitrogen cycling in paddy soil. Biogeosciences 11:3353–3368. https://doi.org/10.5194/bg-11-3353-2014
He R, Wooller MJ, Pohlman JW, Catranis C, Quensen J, Tiedje JM, Leigh MB (2012) Identification of functionally active aerobic methanotrophs in sediments from an arctic lake using stable isotope probing. Environ. Microbiol. 14:1403–1419. https://doi.org/10.1111/j.1462-2920.2012.02725.x
Ho A, Angel R, Veraart AJ, Daebeler A, Jia Z, Kim SY, Kerckhof F-M, Boon N, Bodelier PLE (2016) Biotic interactions in microbial communities as modulators of biogeochemical processes: methanotrophy as a model system. Front. Microbiol. 7. https://doi.org/10.3389/fmicb.2016.01285
Sharp CE, Martínez-Lorenzo A, Brady AL, Grasby SE, Dunfield PF (2014) Methanotrophic bacteria in warm geothermal spring sediments identified using stable-isotope probing. FEMS Microbiol. Ecol. 90:92–102. https://doi.org/10.1111/1574-6941.12375
Cai Y, Zheng Y, Bodelier PLE, Conrad R, Jia Z (2016) Conventional methanotrophs are responsible for atmospheric methane oxidation in paddy soils. Nat. Commun. 7. https://doi.org/10.1038/ncomms11728
WRA (2005) Investigation of current status in Tanshui River system, Final Report. Water Resources Agency, Taiwan,
Chiu CY, Chou CH (1991) The distribution and influence of heavy-metals in mangrove forests of the Tamsui estuary in Taiwan. Soil Science and Plant Nutrition 37:659–669
Shiau Y, Dham V, Tian G, Chiu CY (2016) Factors influencing removal of sewage nitrogen through denitrification in mangrove soils. Wetlands 36:621–630. https://doi.org/10.1007/s13157-016-0770-7
Shiau Y-J, Lin M-F, Tan C-C, Tian G, Chiu C-Y (2017) Assessing N2 fixation in estuarine mangrove soils. Estuar. Coast. Shelf Sci. 189:84–89. https://doi.org/10.1016/j.ecss.2017.03.005
Lu L, Jia ZJ (2013) Urease gene-containing Archaea dominate autotrophic ammonia oxidation in two acid soils. Environ. Microbiol. 15:1795–1809. https://doi.org/10.1111/1462-2920.12071
Daebeler A, Abell GCJ, Bodelier PLE, Bodrossy L, Frampton DMF, Hefting MM, Laanbroek HJ (2012) Archaeal dominated ammonia-oxidizing communities in Icelandic grassland soils are moderately affected by long-term N fertilization and geothermal heating. Front. Microbiol. 3:352. https://doi.org/10.3389/fmicb.2012.00352
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Kozich J, Westcott S, Baxter N, Highlander S, Schloss P (2013) Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79:5112–5120
Dumont MG, Luke C, Deng Y, Frenzel P (2014) Classification of pmoA amplicon pyrosequences using BLAST and the lowest common ancestor method in MEGAN. Front. Microbiol. 5:34. https://doi.org/10.3389/fmicb.2014.00034
Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME Journal 6:1621–1624. https://doi.org/10.1038/ismej.2012.8
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73:5261–5267. https://doi.org/10.1128/aem.00062-07
Degelmann DM, Borken W, Drake HL, Kolb S (2010) Different atmospheric methane-oxidizing communities in European beech and Norway Spruce soils. Appl. Environ. Microbiol. 76:3228–3235. https://doi.org/10.1128/aem.02730-09
Hirayama H, Fuse H, Abe M, Miyazaki M, Nakamura T, Nunoura T, Furushima Y, Yamamoto H, Takai K (2013) Methylomarinum vadi gen. nov., sp nov., a methanotroph isolated from two distinct marine environments. Int. J. Syst. Evol. Microbiol. 63:1073–1082. https://doi.org/10.1099/ijs.0.040568-0
Cussler EL (1997) Diffusion: Mass transfer in fluid systems. Cambridge University Press, Cambridge
Cowie M, Watts H (1970) Diffusion of methane and chloromethanes in air. Can. J. Chem. 49:74–77
Hudson BD (1994) Soil organic-matter and available water capacity. J. Soil Water Conserv. 49:189–194
Manns HR, Berg AA (2014) Importance of soil organic carbon on surface soil water content variability among agricultural fields. J. Hydrol. 516:297–303. https://doi.org/10.1016/j.jhydrol.2013.11.018
Zhou Z, Chen J, Cao H, Han P, Gu J-D (2015) Analysis of methane-producing and metabolizing archaeal and bacterial communities in sediments of the northern South China Sea and coastal Mai Po Nature Reserve revealed by PCR amplification of mcrA and pmoA genes. Front. Microbiol. 5:789. https://doi.org/10.3389/fmicb.2014.00789
Yun J, Yu Z, Li K, Zhang H (2013) Diversity, abundance and vertical distribution of methane-oxidizing bacteria (methanotrophs) in the sediments of the Xianghai wetland, Songnen Plain, northeast China. J. Soils Sediments 13:242–252. https://doi.org/10.1007/s11368-012-0610-1
Yun J, Zhang H, Deng Y, Wang Y (2015) Aerobic methanotroph diversity in Sanjiang wetland, northeast China. Microb. Ecol. 69:567–576. https://doi.org/10.1007/s00248-014-0506-2
Henckel T, Jackel U, Conrad R (2001) Vertical distribution of the methanotrophic community after drainage of rice field soil. FEMS Microbiol. Ecol. 34:279–291. https://doi.org/10.1111/j.1574-6941.2001.tb00778.x
Lyle M (1983) The brown-green color transition in marine sediments: a marker of the Fe(III)-Fe(II) redox boundary. Limnol. Oceanogr. 28:1026–1033. https://doi.org/10.4319/lo.1983.28.5.1026
Chester R (1990) Marine geochemistry. Unwin Hyman Ltd., London,
Graham DW, Chaudhary JA, Hanson RS, Arnold RG (1993) Factors affecting competition between type-I and type-II methanotrophs in 2-organism, continuous-flow reactors. Microb. Ecol. 25:1–17
Bartlett KB, Bartlett DS, Harriss RC, Sebacher DI (1987) Methane emissions along a salt-marsh salinity gradient. Biogeochemistry 4:183–202
Krauss KW, Whitbeck JL (2012) Soil greenhouse gas fluxes during wetland forest retreat along the Lower Savannah River, Georgia (USA). Wetlands 32:73–81
Poffenbarger HJ, Needelman BA, Megonigal JP (2011) Salinity influence on methane emissions from tidal marshes. Wetlands 31:831–842
Liu H, Wu X, Li Z, Wang Q, Liu D, Liu G (2017) Responses of soil methanogens, methanotrophs, and methane fluxes to land-use conversion and fertilization in a hilly red soil region of southern China. Environ. Sci. Pollut. Res. 24:8731–8743. https://doi.org/10.1007/s11356-017-8628-y
Wagner-Riddle C, Thurtell GW, Kidd GE, Edwards GC, Simpson IJ (1996) Micrometeorological measurements of trace gas fluxes from agricultural and natural ecosystems. Infrared Phys. Technol. 37:51–58. https://doi.org/10.1016/1350-4495(95)00111-5
Stadmark J, Leonardson L (2005) Emissions of greenhouse gases from ponds constructed for nitrogen removal. Ecol. Eng. 25:542–551
Ho A, Luke C, Cao Z, Frenzel P (2011) Ageing well: methane oxidation and methane oxidizing bacteria along a chronosequence of 2000 years. Environ. Microbiol. Rep. 3:738–743. https://doi.org/10.1111/j.1758-2229.2011.00292.x
Krause S, Lüke C, Frenzel P (2012) Methane source strength and energy flow shape methanotrophic communities in oxygen-methane counter-gradients. Environ. Microbiol. Rep. 4:203–208. https://doi.org/10.1111/j.1758-2229.2011.00322.x
Zheng Y, Jia Z (2016) The application of biomarker genes for DNA/RNA-stable isotope probing of active methanotrophs responsible for aerobic methane oxidation in six paddy soils. Acta Pedol. Sin. 53:490–501. 10.11766/trxb201509020090
Ho A, Kerckhof F-M, Luke C, Reim A, Krause S, Boon N, Bodelier PLE (2013) Conceptualizing functional traits and ecological characteristics of methane-oxidizing bacteria as life strategies. Environ. Microbiol. Rep. 5:335–345. https://doi.org/10.1111/j.1758-2229.2012.00370.x
Shrestha M, Shrestha PM, Frenzel P, Conrad R (2010) Effect of nitrogen fertilization on methane oxidation, abundance, community structure, and gene expression of methanotrophs in the rice rhizosphere. The ISME Journal 4:1545–1556. https://doi.org/10.1038/ismej.2010.89
Steenbergh AK, Meima MM, Kamst M, Bodelier PLE (2010) Biphasic kinetics of a methanotrophic community is a combination of growth and increased activity per cell. FEMS Microbiol. Ecol. 71:12–22. https://doi.org/10.1111/j.1574-6941.2009.00782.x
Kalyuzhnaya MG (2016) Methylosarcina. In: Whitman WB (ed) Bergey's Manual of Systematics of Archaea and Bacteria. John Wiley & Sons, Inc., New Jersey. https://doi.org/10.1002/9781118960608.gbm01184.pub2
Bowman JP (2015) Methylobacter. In: Whitman WB (ed) Bergey’s Manual of Systematics of Archaea and Bacteria. John Wiley & Sons, Inc., New Jersey. https://doi.org/10.1002/9781118960608.gbm01179.pub2
Bowman JP (2015) Methylomonas. In: Whitman, WB (ed) Bergey’s Manual of Systematics of Archaea and Bacteria. John Wiley & Sons, Inc., New Jersey. https://doi.org/10.1002/9781118960608.gbm01183.pub2
Caldwell SL, Laidler JR, Brewer EA, Eberly JO, Sandborgh SC, Colwell FS (2008) Anaerobic oxidation of methane: mechanisms, bioenergetics, and theecology of associated microorganisms. Environ. Sci. Technol. 42:6791–6799
Knittel K, Boetius A (2009) Anaerobic oxidation of methane: progress with an unknown process. Annu. Rev. Microbiol. 63:311–334
Acknowledgements
We thank Ms. Yu-Shiuan Huang from the Biodiversity Research Center, Academia Sinica, Taipei, Taiwan, and Ms. Dongmei Wang from the Nanjing Soil Research Institute, Chinese Academy of Science, Nanjing, PRC, for the laboratory work.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Data Availability
All of the pmoA and 16S rRNA gene sequences obtained from Miseq sequencing have been deposited in NCBI with accession codes SRP111433.
Rights and permissions
About this article
Cite this article
Shiau, YJ., Cai, Y., Lin, YT. et al. Community Structure of Active Aerobic Methanotrophs in Red Mangrove (Kandelia obovata) Soils Under Different Frequency of Tides. Microb Ecol 75, 761–770 (2018). https://doi.org/10.1007/s00248-017-1080-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-017-1080-1