Abstract
Arbuscular mycorrhizal (AM) fungi play key roles in plant nutrition and plant productivity. AM fungal responses to either plant identity or fertilization have been investigated. However, the interactive effects of different plant species and fertilizer types on these symbiotic fungi remain poorly understood. We evaluated the effects of the factorial combinations of plant identity (grasses Avena sativa and Elymus nutans and legume Vicia sativa) and fertilization (urea and sheep manure) on AM fungi following 2-year monocultures in a sown pasture field study. AM fungal extraradical hyphal density was significantly higher in E. nutans than that in A. sativa and V. sativa in the unfertilized control and was significantly increased by urea and manure in A. sativa and by manure only in E. nutans, but not by either fertilizers in V. sativa. AM fungal spore density was not significantly affected by plant identity or fertilization. Forty-eight operational taxonomic units (OTUs) of AM fungi were obtained through 454 pyrosequencing of 18S rDNA. The OTU richness and Shannon diversity index of AM fungi were significantly higher in E. nutans than those in V. sativa and/or A. sativa, but not significantly affected by any fertilizer in all of the three plant species. AM fungal community composition was significantly structured directly by plant identity only and indirectly by both urea addition and plant identity through soil total nitrogen content. Our findings highlight that plant identity has stronger influence than fertilization on belowground AM fungal community in this converted pastureland from an alpine meadow.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arbuscular mycorrhizal (AM) fungi belong to Glomeromycota and form symbiotic associations with most terrestrial plant species [1]. In AM associations, plants provide photosynthates for the growth and function of AM fungi; as such, plant performance can affect AM fungal community [2]. In return, AM fungi improve plant nutrient uptake and resistance to biotic and abiotic stresses; therefore, these fungi can greatly affect plant productivity, diversity, and ecosystem processes [3–5]. AM associations, which function as vital links between aboveground and belowground communities in ecosystems, are influenced by plant [6] and anthropogenic activities [7, 8].
It is suggested that AM fungi may exhibit low host plant specificity, because relatively low numbers of AM fungal species (ca. 250 species) can colonize high numbers of their plant partners (ca. 200,000 species) [1, 9]. However, there is a growing body of evidence consistently suggesting that AM fungal community in roots is strongly shaped by plant identity [10–12]. For example, the AM fungal community colonizing Trifolium repens differs from the fungal community colonizing Agrostis capillaris [10]. Likewise, the AM fungal community associated with Taraxacum officinale varies from the fungal communities related to Polemonium viscosum and Taraxacum ceratophorum [11]. The AM fungal communities that colonize three leguminous plants, namely, Lotus corniculatus, Ononis repens, and T. repens, are also distinctly different from those that thrive in non-leguminous plants, such as Festuca ovina, Hieracium pilosella, and Plantago lanceolata [13].
In contrast to root-associated AM fungi, soil-dwelling AM fungal extraradical hyphal (ERH) density, spore density, and community composition are inconsistently affected by plant identity [14–21]. For example, the AM fungal ERH density is slightly affected by Calamovilfa longifolia and Deschampsia flexuosa [14]. The AM fungal spore density is also slightly affected by Andropogon gerardii, Sorghastrum nutans, and Schizachyrium scoparium [15]. Likewise, the AM fungal community composition in soil is weakly affected by three crops, namely, pea, lentil, and wheat [16] and by Stipa grandis and Agropyron cristatum, which are dominant plant species in a grassland [17]. Conversely, the AM fungal spore density in Baptisia bracteata is significantly higher than that in Panicum virgatum, Poa pratensis, and Solidago missouriensis [19]. Varela-Cervero et al. [21] found that AM fungi belonging to Diversispora are exclusively detected in soil planted with Genista cinerea and Lavandula latifolia rather than with other species, such as Rosmarinus officinalis, Thymus mastichina, and Thymus zygis. Taken together, although the determinants of plant identity on root-associated AM fungal communities have been increasingly confirmed, the inconsistent dependence of soil-dwelling AM fungi on plant identity has yet to be fully elucidated.
As a key anthropogenic activity in agricultural and natural ecosystems, fertilization elicits various effects on AM fungi [22, 23]. For instance, nitrogen (N) fertilization has negatively [23, 24] and neutrally [23, 25] affected the ERH density and spore density of AM fungi, respectively. Under N addition conditions, the diversity of soil-inhabiting AM fungi in grassland ecosystems can be increased [26], decreased [27, 28], and unaffected [23]. N fertilization has also significantly influenced the AM fungal community compositions in temperate steppe [23, 28] but not in alpine meadow [26] ecosystems. Therefore, the discrepant effects of N fertilization on AM fungi should be confirmed through further investigations. Manure is another important soil input to enhance plant productivity because of the large forage requirement of livestock, as in the Tibetan plateau region, and a huge area previously converted from native meadow to pastureland [29]. Given that manure application has affected soil properties, respiration intensity, and plant community on the Tibetan plateau [29–31], the response of AM fungal community to sheep manure amendment remains poorly understood.
Although the effect of either plant identity or fertilization on AM fungi has been widely explored in various ecosystems, the response of soil-dwelling AM fungi to combined plant identity and fertilization and their interaction has yet to be elucidated. On the basis of a 2-year urea and sheep manure fertilization, we examined the ERH density and spore density of AM fungi in a sown pastureland converted from native meadow by conducting a monoculture experiment with three plants: Avena sativa, Elymus nutans, and Vicia sativa, which are widely used as forage production in the Tibetan alpine meadow region. The AM fungal community composition in soil was further examined by subjecting 18S rDNA to 454 pyrosequencing. Considering previous findings on the effect of plant identity and fertilization on AM fungi, in this study we tested the following two hypotheses: (H1) plant identity likely influences the ERH density, spore density, diversity, and community composition of AM fungi and (H2) fertilization possibly elicits a greater effect on soil AM fungi in grasses (A. sativa and E. nutans) than that in legume (V. sativa) because of the latter N-fixing capacity.
Materials and Methods
Study Site
This study was conducted at the Haibei Alpine Meadow Ecosystem Research Station (HAMERS) of Chinese Academy of Sciences in the Tibetan plateau, China (37° 36′ N, 101° 12′ E; 3250 m above sea level). The HAMERS experiences a typical plateau continental climate, with a mean annual temperature of −1.7 °C and a mean annual precipitation of 580 mm; more than 80 % of precipitation occurs in the plant growing season from May to September [31]. The soil is classified as cambisol [32], and the local plant community is dominated by Kobresia humilis, E. nutans, Stipa aliena, Gentiana straminea, Potentilla nivea, and F. ovina [31].
Experimental Design and Sampling
The field experiment was established at the HAMERS in accordance with previously described methods [29]. In brief, the study site had been planted with barley for ca. 20 years until the 1980s and with E. nutans pasture in the 1990s. Since then, the study site had been abandoned and allowed to regenerate naturally with grazing in the winter season for 8 years until 2007. An experimental site of 100 m × 100 m was fenced in early May 2007. We conducted a randomized complete block design experiment involving plant monoculture and fertilization with four replicate plots (4 m × 4.5 m in size and with a buffer zone of 2 m in width among plots) for each treatment in 2009. Three main forages in a local region, two grasses (A. sativa and E. nutans), and a legume (V. sativa) were selected and planted in this pasture ecosystem. Fertilization treatments were prepared as follows: urea addition (69 kg N ha−1 year−1), sheep manure addition (1687 kg carbon [C] ha−1, 93 kg N ha−1, and 45 kg phosphorus [P] ha−1) and unfertilized control. The urea and sheep manures have been annually applied in early May and in mid-July since 2009, respectively [29].
In late August 2011, five soil cores (3 cm in diameter; 15 cm in depth) were randomly collected from each plot and then homogenized to form a composite sample. During soil sampling, two manure amendment plots (one for A. sativa and one for V. sativa) were unavailable because of incorrect planting. Therefore, 34 soil samples were collected and analyzed in our study. The soil samples were immediately packed in an ice box and transported to our laboratory. After coarse stones, plant roots, and visible organic debris were removed, the soil samples were sieved through a 2 mm mesh and divided into two portions. One portion of the subsamples was stored at −80 °C until DNA extraction and AM fungal hyphal length measurement. The other subsamples were air dried for AM fungal spore density and soil variable measurement.
Soil Physicochemical Analysis
The following parameters were measured using the corresponding methods: soil pH with a glass electrode at a 1:2.5 (w/v) soil-to-water ratio; soil moisture with gravimetric method at 105 °C for 24 h; soil organic matter (SOM) with K2Cr2O7 oxidation-reduction titration method; soil total N with Kjeldahl method; and soil available P with Murphy–Riley method. Soil NH4 +-N and NO3 −-N were extracted with 2 M KCl solution and determined using a continuous flow analyzer (SAN++, Skalar, Holland).
AM Fungal ERH and Spore Densities
Fungal hyphae were extracted from 4.0 g of frozen soil by using a membrane filter [33] and separated into AM and non-AM fungal hyphae on the basis of their morphological characteristics and staining color [34]. The ERH length of AM fungi was measured via a grid line intersect method by observing 135 view fields for each filter under a microscope (Nikon 80i, Tokyo, Japan) at 200× magnification. AM fungal spores were extracted from 20 g of air-dried soil with distilled water via wet sieving and decanting methods [35] and then counted under the microscope (Nikon 80i, Tokyo, Japan) at 40× magnification.
DNA Extraction, PCR, and Pyrosequencing
DNA was extracted from 0.25 g of frozen soil by using a PowerSoil® DNA isolation kit (MOBIO Laboratories, Inc., Carlsbad, USA) in accordance with the manufacturer’s instructions. The DNA concentration was measured with a TBS 380 fluorescence spectrophotometer (Promega, Madison, WI, USA). A two-step PCR was performed. In the first PCR, a primer set comprising GeoA2 [36] and NS4 [37] was used. The PCR cocktail with a final volume of 25 μL consisted of 2.5 μL of 10× PCR buffer (Mg2+ plus), 2 μL of deoxyribonucleoside triphosphate mixture (each 2.5 mM), 0.5 μL of each primer (10 μM), 1.5 U Taq polymerase (TaKaRa, Dalian, China), and ca. 10 ng of template DNA combined with sterile deionized H2O. PCR was carried out under the following thermocycling conditions: initial setting at 94 °C for 5 min; followed by 30 cycles at 94 °C for 45 s, 54 °C for 1 min, and 72 °C for 1.5 min; and final setting at 72 °C for 10 min. The obtained PCR products were diluted 50 times, and 1 μL of the resulting solution was used as the template for the second PCR amplifications with the primers NS31 [38] and AML2 [39]. A 10-base barcode sequence was tagged with NS31 (Table S1). The thermocycling conditions for the second PCR were similar to those of the first PCR except that the annealing temperature was set at 58 °C. The PCR products were purified with a PCR product gel purification kit (Axygen, Union City, CA, USA) and then quantified with the TBS 380 fluorescence spectrophotometer (Promega, Madison, WI, USA). The purified DNA (50 ng) from each sample was pooled and adjusted to 10 ng μL−1. The pooled product was subjected to pyrosequencing on Roche Genome Sequencer FLX Titanium (454 Life Sciences, Branford, CT, USA). The representative 18S rDNA sequences obtained in this study have been submitted to the European Molecular Biology Laboratory (EMBL) nucleotide sequence database with the accession numbers LN907232–LN907279.
Bioinformatics Analysis
The raw sequences were re-assigned to the samples in Mothur 1.32.2 [40] on the basis of barcodes and then trimmed in accordance with the following parameters to exclude short- and low-quality sequences: minlength = 300, maxambigs = 0, maxhomop = 8, qaverage = 25, and pdiffs = 1. Thus, high-quality sequences were obtained. Potential chimeras in the sampled sequences were detected by using the “chimera.uchime” command in Mothur. Non-chimeric sequences were clustered into operational taxonomic units (OTUs) at a 97 % sequence similarity level in accordance with previous studies [7, 24, 26, 41] after dereplication was conducted and singleton was discarded via UPARSE pipeline in version 8.0.1623_win32 [42]. The representative sequence of each OTU was identified by a basic local alignment search tool (BLAST; [43]) search against the National Center for Biotechnology Information (NCBI) nt database. All non-AM fungal OTUs identified on the basis of the closest BLAST hit not annotated as “glomeromycota” were removed from the dataset. Furthermore, the representative sequences of AM fungal OTUs were blasted against the MaarjAM database [41] and excluded from the dataset when E values > e−100. The number of sequence per sample was normalized to the smallest sample size by using the “sub.sample” command in Mothur to account for the influence of different read numbers on the analysis of AM fungal community. A neighbor-joining tree of the representative OTU sequences obtained in this study and the reference sequences from GenBank were constructed by using the p distance model with 1000 replicates to produce bootstrap values and thus identify the taxonomic position of the obtained AM fungal OTUs.
Data Analysis
The cumulative number of AM fungal OTUs was calculated using the “specaccum” function in Vegan [44] in an R platform. The frequency of a specific AM fungal OTU is defined as the percentage of the number of samples where OTU was detected to the number of all samples. The abundance of a given AM fungal OTU or family is defined as the number of reads of that OTU or family in a sample. The richness of AM fungal OTUs is defined as the number of OTUs in a sample or in each family. Shannon diversity index calculated from the relative abundance of OTU was considered to estimate the AM fungal diversity for each sample [45]. A two-way analysis of variance (ANOVA) was conducted to evaluate the effect of plant identity, fertilization, and their interaction on the ERH density, spore density, OTU richness, Shannon diversity index, and abundance of AM fungi. Significant differences between treatments were compared through post hoc Tukey’s honest significant difference (HSD) tests at P < 0.05. Data were also examined to determine the normality of distribution and homogeneity of variance before two-way ANOVA was conducted. Among the obtained data, the abundance of OTU10, OTU12, OTU46, OTU50, Archaeosporaceae, and Gigasporaceae and the richness of Archaeosporaceae did not satisfy the homogeneity of variance before and after relevant transformation was carried out. Non-parametric Kruskal–Wallis test was applied to examine the effects of plant identity and fertilization. Significant differences between treatments were then detected through pairwise comparisons at P < 0.05. When AM fungal variables did not significantly differ among the fertilization treatments within each plant species, data were pooled in accordance with plant identity and then subjected to Tukey’s HSD tests (homogeneity of variance) or pairwise comparisons (heterogeneity of variance) at P < 0.05 to reveal the difference among plant species.
Permutational multivariate analysis of variance (PerMANOVA) with distance matrices was conducted using the “adonis” function in Vegan with 999 permutations to evaluate the effect of plant identity, fertilization, and their interaction on AM fungal community composition (Hellinger-transformed OTU read numbers) [44]. Non-metric multidimensional scaling (NMDS) was employed on the basis of Bray–Curtis dissimilarities to visualize the compositional differences in AM fungal communities among the treatments. Furthermore, soil variables were fitted as vectors onto the ordination NMDS plot by using the “envfit” function based on 999 permutations in Vegan [44] to examine the relationship between AM fungal community composition and soil properties at P < 0.05.
A structural equation model (SEM) was used to detect the causal relationships among plant identity, fertilization, soil, and AM fungi by using the AMOS 22.0.0 (Amos Development Corporation, Meadville, PA, USA). Before establishing the SEM, we examined the interrelationships among treatments (plant identity, urea addition, and manure addition), soil variables (pH, moisture, SOM, total N, NH4 +-N, NO3 −-N, and available P), and AM fungal variables (ERH density, spore density, and community composition) by conducting a Mantel test with the “mantel” function in Ecodist [46]. The R values derived from the Mantel tests were considered as input data to create the SEM model. On the basis of fundamental knowledge, we assumed a conceptual model showing that both plant identity and fertilization likely alter soil variables; as a consequence, this alteration affected the AM fungal variables. Maximum likelihood estimation was applied to compare the SEM model with the observation. Model adequacy was determined through χ 2 tests, goodness-of-fit index (GFI), Akaike information criteria (AIC), and root mean square error of approximation (RMSEA). Adequate model fits were indicated by a non-significant χ 2 test (P ≥ 0.05), high GFI (> 0.90), low AIC, and low RMSEA (< 0.05). Statistical analyses were carried out in R 3.0.2 [47].
Results
Soil Properties
Compared with the unfertilized control treatment, soil pH was significantly decreased by manure in E. nutans but not by either fertilizers in A. sativa and V. sativa (Table 1). Soil moisture was not significantly different in the fertilization treatments within each plant species, except for significantly lower soil moisture in V. sativa than that in A. sativa and E. nutans under manure treatment (Table 1). Soil total N was significantly decreased by urea and manure addition in A. sativa but significantly increased by manure in E. nutans and by both fertilizers in V. sativa, compared with the unfertilized control treatment (Table 1). Moreover, soil NH4 +-N was significantly increased in A. sativa with urea and manure additions but not in E. nutans and V. sativa. Compared with the unfertilized control treatment, soil NO3 −-N was significantly increased in the three plant species with urea addition rather than manure addition (Table 1). SOM and available P were not significantly changed by both fertilizers in all of the three plant species (Table 1).
AM Fungal ERH and Spore Densities
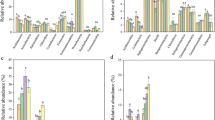
AM fungal ERH density ranged from 0.65 to 1.97, with a mean of 1.19 m g−1 dry soil. Two-way ANOVA revealed that AM fungal ERH density was significantly influenced by plant identity, fertilization, and their interaction (Fig. 1a). For example, the ERH density was significantly higher in E. nutans than that in A. sativa and V. sativa, but no significant difference was observed between A. sativa and V. sativa in the control and manure treatments, respectively (Fig. 1a). In urea addition treatment, the AM fungal ERH density was significantly higher in A. sativa than that in V. sativa, but no significant difference was observed between E. nutans and A. sativa and between E. nutans and V. sativa (Fig. 1a). Compared with the unfertilized control treatment, the AM fungal ERH density was significantly increased by both urea and manure in A. sativa and by manure only in E. nutans, but not by either fertilizer in V. sativa (Fig. 1a). The AM fungal spore density ranged from 26.6 to 39.4, with a mean of 32.5 spores g−1 dry soil. Two-way ANOVA indicated that plant identity and fertilization did not significantly affect the spore density, while a significantly interactive effect was observed (Fig. 1b).
Extraradical hyphal (ERH) density (a) and spore density (b) of arbuscular mycorrhizal (AM) fungi in different fertilization treatments of three plant species. Two-way ANOVA showed the effect of plant identity (PI), fertilization (F), and their interactions (PI × F) on AM fungal variables (ns, P ≥ 0.05; **P < 0.01; ***P < 0.001). Data are means ± SE. Shared letters above bars (lowercase) and lines (uppercase) denote no significant difference among nine treatments and three plant species, respectively, as indicated by Tukey’s HSD test at P < 0.05
454 Pyrosequencing Analysis and Identification of AM Fungi
A total of 82,080 reads were retained from 132,209 raw reads after a denoising step was completed. Subsequently, 5627 potential chimeras were excluded from the dataset. Thus, 76,453 non-chimeric reads were obtained and clustered into 162 OTUs at 97 % sequence similarity. Of these OTUs, 52 (45,471 reads) belonged to AM fungi, 39 (23,846 reads) to metazoa, 36 (2099 reads) to non-AM fungi, 14 (127 reads) to plants, 4 (149 reads) to alveolata, 3 (24 reads) to stramenopiles, 2 (5 reads) to centroheliozoa, 2 (5 reads) to cercozoa, 1 (2 reads) to amoebozoa, and 9 (95 reads) to unknown taxonomic eukaryotes. The AM fungal read numbers were further normalized to 612 (612 to 2311 in all of the soil samples). As a result, a normalized dataset containing 48 AM fungal OTUs (20,804 reads) was obtained. AM fungal OTUs ranged from 18 to 35, with a mean of 28.0 ± 1.7 in the nine treatments (Fig. 2a). Of these 48 AM fungal OTUs, 37 OTUs (99.7 % of total reads) were detected in at least three samples (frequency ≥ 8.8 %) and the 11 other OTUs (0.3 % of total reads) were found in ≤ 2 samples (Fig. 2b). In addition, the 15 most abundant OTUs (reads ≥ 100) accounted for 96.0 % of the total AM fungal reads and the 33 remaining OTUs accounted for 4.0 % (Fig. 2c). Of these 48 AM fungal OTUs, the following classifications were observed (Fig. 3): 3 in Acaulosporaceae (3 Acaulospora OTUs); 3 in Ambisporaceae (3 Ambispora OTUs); 4 in Archaeosporaceae (4 Archaeospora OTUs); 9 in Claroideoglomeraceae (9 Claroideoglomus OTUs); 4 in Diversisporaceae (4 Diversispora OTUs); 1 in Entrophosporaceae (1 Entrophospora OTU); 4 in Gigasporaceae (4 Scutellospora OTUs); 18 in Glomeraceae (1 Funneliformis OTU, 10 Glomus OTUs, 3 Rhizophagus OTUs, 2 Septoglomus OTUs, and 2 genus-unassigned OTUs); 1 in Pacisporaceae (1 Pacispora OTU); and 1 in Paraglomeraceae (1 Paraglomus OTU).
Neighbor-joining tree was constructed based on ca. 400 bp fragment of the 18S rDNA sequences of the 48 OTUs (bold) obtained in this study and the reference sequences downloaded from GenBank (the corresponding accession numbers are indicated in parentheses). Bootstrap values were calculated on the basis of 1000 data resampling (> 50 % of the values are shown). Paraglomeraceae was used as an outgroup. Scale bar represents 1 % sequence divergence
AM Fungal Community
Plant identity rather than fertilization significantly affected the abundance of OTU10, OTU12, OTU25, OTU39, OTU46, and OTU50 (Fig. S1). Likewise, plant identity significantly influenced the abundance of Archaeosporaceae, Claroideoglomeraceae, and Gigasporaceae (Figs.S2a, b, d). By contrast, plant identity and fertilization elicited a significant effect on the abundance of Diversisporaceae (Fig. S2c). For instance, the abundances of OTU10, OTU12, OTU39, OTU50, Archaeosporaceae, and Gigasporaceae were significantly higher in E. nutans than those in A. sativa and/or V. sativa (Fig. S1a, b, d, f; Fig. S2a, d). The abundances of OTU25 and Claroideoglomeraceae were significantly lower in V. sativa than those in A. sativa and E. nutans (Fig. S1c; Fig. S2b). By comparison, the abundance of Diversisporaceae was significantly higher in V. sativa than that in E. nutans (Fig. S2c). The abundance of OTU46 was significantly higher in E. nutans than that in A. sativa under the control treatment, whereas no significant difference was observed among the three plant species under either urea or manure treatment (Fig. S1e).
Plant identity, not fertilization, significantly affected the OTU richness and Shannon diversity index of AM fungi (Fig. 4). For example, OTU richness was significantly higher in E. nutans than that in V. sativa. By contrast, OTU richness did not significantly differ between A. sativa and E. nutans and between A. sativa and V. sativa (Fig. 4a). Shannon diversity index was significantly higher in E. nutans than that in A. sativa and V. sativa, but no significant difference between A. sativa and V. sativa was observed (Fig. 4b). Plant identity, not fertilization, significantly influenced the OTU richness of Archaeosporaceae, Diversisporaceae, and Glomeraceae (Figs. S2e, f, g). For example, the OTU richness of Archaeosporaceae and Glomeraceae was significantly higher in E. nutans than that in A. sativa and/or V. sativa (Figs. S2e, g). By comparison, the OTU richness of Diversisporaceae was significantly lower in E. nutans than that in V. sativa (Figs. S2f).
The OTU richness (a) and Shannon diversity index (b) of arbuscular mycorrhizal (AM) fungi in different fertilization treatments of three plant species. Two-way ANOVA revealed the effect of plant identity (PI), fertilization (F), and their interactions (PI × F) on AM fungal variables (ns, P ≥ 0.05; *P < 0.05; **P < 0.01). Data are means ± SE. Shared letters above bars (lowercase) and lines (uppercase) denote no significant difference among nine treatments and three plant species, respectively, as shown by Tukey’s HSD test at P < 0.05
PerMANOVA analysis revealed that the AM fungal community composition was significantly affected by plant identity (R 2 = 0.258, P = 0.001) but not by fertilization (R 2 = 0.054, P = 0.320) or their interaction (R 2 = 0.082, P = 0.710). Similarly, NMDS indicated that the AM fungal community composition was significantly separated by three plant species, but not by urea or manure fertilization (Fig. 5). In addition, the AM fungal community composition was significantly related to soil moisture (R 2 = 0.381, P = 0.001), SOM (R 2 = 0.321, P = 0.004), and soil total N (R 2 = 0.222, P = 0.022) (Fig. 5).
Non-metric multidimensional scaling (NMDS) of the community composition of arbuscular mycorrhizal fungi among nine treatments (stress = 0.122). Small dots represent the ordination data of 34 samples belonging to nine treatments, and large dots correspond to the weighted average of NMDS scores and their standard errors within each treatment (mean ± SE). Significant variables of soil moisture (R 2 = 0.381, P = 0.001), soil organic matter (SOM, R 2 = 0.321, P = 0.004), and total nitrogen (TN, R 2 = 0.222, P = 0.022) are fitted onto the NMDS graph based on the results of “envfit” function analysis
Relationships Among Plant Identity, Fertilization, Soil, and AM Fungal Variables
Mantel tests showed that the AM fungal ERH density was significantly related to plant identity, manure addition, SOM, and soil moisture (Table S2). The AM fungal community composition was significantly related to plant identity and soil total N (Table S2). Significant relationships were found between plant identity, soil moisture, soil total N, or urea addition (Table S2). Subsequently, the final SEM model with Mantel r values (relation coefficients) as input adequately fitted the data describing the interaction pathways among soil parameters, ERH density, and community composition in response to plant identity and fertilization (χ 2 = 6.542, df = 6, P = 0.365, GFI = 0.997, AIC = 66.542, RMSEA = 0.013; Fig. 6). The final SEM model explained 12.3 and 12.4 % of the variation in the ERH density and community composition of AM fungi, respectively (Fig. 6a). The AM fungal ERH density was significantly influenced directly by plant identity (λ = 0.152), manure addition (0.242), and SOM (0.145) (Fig. 6a). The AM fungal community composition was significantly affected directly by plant identity (0.206) and indirectly by urea addition (0.059) and plant identity (0.040) via soil total N (0.246) (Fig. 6a, b).
a Structural equation model (SEM) showing the causal relationships among plant identity, fertilization, soil, arbuscular mycorrhizal (AM) fungal extraradical hyphal (ERH) density, and community composition. The final model fits the data well: maximum likelihood, χ 2 = 6.542, df = 6, P = 0.365, goodness-of-fit index = 0.997, Akaike information criteria = 66.542, and root mean square error of approximation = 0.013. Solid lines and dashed lines indicate significant and non-significant pathways, respectively. The width of the solid lines indicates the strength of the causal effect. R 2 represents the proportion of variance explained for each variable. b Direct, indirect, and total effect coefficients (λ) of plant identity, urea, and manure additions on soil and AM fungal variables in this SEM. SOM soil organic matter, TN total nitrogen, AP available phosphorus
Discussion
Effects of Plant Identity and Fertilization on AM Fungal ERH Density and Spore Density
The AM fungal ERH density was significantly affected by plant identity in this study, and this finding partly supported H1. Radić et al. [48] also found that the AM fungal ERH density is significantly different among P. lanceolata, Tanacetum cinerariifolium, and Vitis vinifera in a pot experiment. This phenomenon indicated that different plants provide photosynthetic products differentially for their fungal partners [49]. As a result, the hyphal growth of AM fungi varies. We also found that urea and/or manure fertilization significantly increased the AM fungal ERH densities in A. sativa and E. nutans but not in V. sativa. This finding supported H2. Fertilization positively affects the AM fungal ERH densities in A. sativa and E. nutans possibly because fertilization increases soil nutrient availability for plant growth; in return, plants provide more photosynthetic resources for fungal partners and simultaneously promote the ERH growth of AM fungi. However, fertilization unlikely affects the AM fungal ERH density in V. sativa probably because leguminous plants exhibit N-fixing capacity, which slightly depends on AM fungi for their N absorption. As a consequence, AM fungal hyphae are not increased by fertilization. In addition, we observed that urea application significantly increased the AM fungal ERH density in A. sativa rather than that in E. nutans. These two grasses may exhibit different uptake capacities for soil N because the accumulated soil NH4 +-N in A. sativa was distinctly higher than that in E. nutans under urea fertilization (Table 1). Therefore, A. sativa depended more on AM fungal hyphae for N uptake than E. nutans did. We also found an interactive effect of plant identity and fertilization on AM fungal ERH density. This finding suggested that the effect of fertilization likely relied on plant species. Furthermore, the AM fungal ERH density was much lower in our study area (a mean of 1.19 m g−1 dry soil) than that in an undisturbed natural grassland in the same site (3.55 m g−1 dry soil; [26]). This phenomenon could occur because AM fungal hyphal growth is highly disturbed by land use changes, such as monoculture and tillage in the current study compared with natural conditions [26].
We found that neither plant identity nor fertilization significantly affected AM fungal spore density. However, this finding did not partly confirm H1 and H2. Similarly, the spore density of AM fungi is unaffected by plant species (A. gerardii, S. nutans, and S. scoparium) in a serpentine grassland [15] and unaffected by N addition in two temperate grassland ecosystems [23, 25]. Nevertheless, the spore density of AM fungi is significantly affected by host plant species, such as Crotalaria grahamiana, Allium porrum, Zea mays, and Helianthus annuus, in an agricultural ecosystem in Switzerland [20] and by Caragana korshinskii, Artemisia sphaerocephala, and Salix psammophila in a farming-pastoral zone in China [50]. This discrepancy could be obtained because plants and ecosystem heterogeneity, especially soil factors, such as moisture, organic carbon, and available N, critically influence AM fungal sporulation [26, 51, 52]. Furthermore, AM fungal spore densities are decreased by N fertilization in grassland in Minnesota, USA [53] and in similar alpine meadows [24, 26]. We manipulated a sown pasture experiment by using three plant species monocultures, and our procedures completely differed from those focusing on natural plant communities [24, 26]. Therefore, the outcome of N (urea used in our study) application is inconsistent. Plant identity and fertilization also interactively affected AM fungal spore density, suggesting that the potential effects of fertilization relied on plant species. Overall, the response of the spore density of AM fungi to plant identity and fertilization may depend on multiple factors, such as soil context, plant species, ecosystem type, and land use pattern [14, 53–55].
Effects of Plant Identity and Fertilization on Diversity and Community Composition of AM Fungi
Plant identity significantly affected the diversity and community composition of soil AM fungi in this study, as expected partly (H1). Plant identity also influences the richness, genetic diversity, and community composition of AM fungi in various ecosystems [e.g., [12, 13, 19, 56, 57]. This phenomenon occurred because different plant species may allocate various qualitative and quantitative carbon resources to their AM fungal partners [49, 58] or supply various root exudates; as a consequence, distinct rhizospheric aspects in terms of physical, chemical, or biological conditions are observed [59]. Thus, the diversity and community composition of AM fungi are altered [60, 61].
In this study, some AM fungi of Archaeosporaceae (OTU50), Gigasporaceae (OTU12 and OTU39), and Glomeraceae (OTU10) were abundant in E. nutans. By contrast, Diversisporaceae seemed to prefer V. sativa. In a previous study, seven groups (Glomus numbered as G14, G16, G19, G22, G23, G25, and G26) of Glomeraceae can be found in Anagallis arvensis; conversely, Glomus G20 likely exists more frequently in Carduus tenuiflorus and Hieracium vulgare [62]. Some of Diversispora-related members are exclusively detected in soil-dwelling fractions of G. cinerea and L. latifolia; likewise, Glomus-related members are found in the root and hyphal compartments of G. cinerea and T. zygis [21]. Combining previous findings and our results from the sown pasture, we suggested that some AM fungi may exhibit host plant preference.
Fertilization changes soil properties, such as nutrient availability [e.g., [28, 63], which can influence soil microbes directly and indirectly by supplying more carbon resources via increased plant growth and plant residue returns [64]. As such, fertilization may affect the diversity and community composition of soil AM fungi. For example, N or manure addition elicits directly significant effects on the diversity and community composition of AM fungi in different ecosystems [23, 65]. However, we found that fertilization did not directly affect AM fungal diversity and community composition in grasses A. sativa and E. nutans and legume V. sativa (Fig. 5). This finding did not confirm H2. These contradictory results were obtained possibly because fertilization was conducted for 2 years, which was shorter than that in previous studies (5–21 years) [23, 65]. Furthermore, soil total N in the background (average 5.18 g kg−1, Table 1) in our study site was much higher than that described in previous studies (0.40–1.72 g kg−1 [23, 65]). Therefore, AM fungi were slightly sensitive to fertilization in the present study. We also found that the AM fungal community composition was significantly affected by soil total N as a result of urea addition. Similar findings were reported in the same site [26] and in a temperate steppe in northern China [17]. Indeed, the community composition of AM fungi can be structured on the basis of soil variables, such as pH, moisture, texture, N content, and C:N in various ecosystems [54, 63, 66–68].
Conclusions
The response of AM fungal community to combined plant identity and fertilization was investigated in a sown pasture on the Tibetan plateau. The AM fungal ERH density, but not spore density, was significantly affected by both plant identity and fertilization. The effect of urea and sheep manure on the ERH density of AM fungi was dependent on plant identity. The AM fungal diversity was significantly influenced by plant identity rather than fertilization. The community composition of AM fungi was significantly structured directly by plant identity and indirectly by urea addition and plant identity via soil total N content. Our results suggested that, compared with fertilization, plant identity exerted stronger effect on soil AM fungal community in this converted pastureland from an alpine meadow.
References
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, London
Johnson D, Vandenkoornhuyse PJ, Leake JR, Gilbert L, Booth RE, Grime JP, Young JPW, Read DJ (2004) Plant communities affect arbuscular mycorrhizal fungal diversity and community composition in grassland microcosms. New Phytol 161:503–515. doi:10.1046/j.1469-8137.2003.00938.x
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72. doi:10.1038/23932
van der Heijden MGA, Verkade S, de Bruin SJ (2008) Mycorrhizal fungi reduce the negative effects of nitrogen enrichment on plant community structure in dune grassland. Glob Chang Biol 14:2626–2635. doi:10.1111/j.1365-2486.2008.01691.x
Rillig MC (2004) Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol Lett 7:740–754. doi:10.1111/j.1461-0248.2004.00620.x
Klironomos J, Zobel M, Tibbett M et al (2011) Forces that structure plant communities: quantifying the importance of the mycorrhizal symbiosis. New Phytol 189:366–370. doi:10.1111/j.1469-8137.2010.03550.x
Lumini E, Orgiazzi A, Borriello R, Bonfante P, Bianciotto V (2010) Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach. Environ Microbiol 12:2165–2179. doi:10.1111/j.1462-2920.2009.02099.x
Vályi K, Rillig MC, Hempel S (2015) Land-use intensity and host plant identity interactively shape communities of arbuscular mycorrhizal fungi in roots of grassland plants. New Phytol 205:1577–1586. doi:10.1111/nph.13236
Hart MM, Aleklett K, Chagnon PL et al (2015) Navigating the labyrinth: a guide to sequence-based, community ecology of arbuscular mycorrhizal fungi. New Phytol 207:235–247. doi:10.1111/nph.13340
Vandenkoornhuyse P, Husband R, Daniell TJ, Watson IJ, Duck JM, Fitter AH, Young JPW (2002) Arbuscular mycorrhizal community composition associated with two plant species in a grassland ecosystem. Mol Ecol 11:1555–1564. doi:10.1046/j.1365-294X.2002.01538.x
Becklin KM, Hertweck KL, Jumpponen A (2012) Host identity impacts rhizosphere fungal communities associated with three alpine plant species. Microb Ecol 63:682–693. doi:10.1007/s00248-011-9968-7
Li XL, Gai JP, Cai XB, Li XL, Christie P, Zhang FS, Zhang JL (2014) Molecular diversity of arbuscular mycorrhizal fungi associated with two co-occurring perennial plant species on a Tibetan altitudinal gradient. Mycorrhiza 24:95–107. doi:10.1007/s00572-013-0518-7
Scheublin TR, Ridgway KP, Young JPW, van der Heijden MGA (2004) Nonlegumes, legumes, and root nodules harbor different arbuscular mycorrhizal fungal communities. Appl Environ Microbiol 70:6240–6246. doi:10.1128/aem.70.10.6240-6246.2004
Sikes BA, Maherali H, Klironomos JN (2014) Mycorrhizal fungal growth responds to soil characteristics, but not host plant identity, during a primary lacustrine dune succession. Mycorrhiza 24:219–226. doi:10.1007/s00572-013-0531-x
Ji BM, Bentivenga SP, Casper BB (2012) Comparisons of AM fungal spore communities with the same hosts but different soil chemistries over local and geographic scales. Oecologia 168:187–197. doi:10.1007/s00442-011-2067-0
Bainard LD, Bainard JD, Hamel C, Gan Y (2014) Spatial and temporal structuring of arbuscular mycorrhizal communities is differentially influenced by abiotic factors and host crop in a semi-arid prairie agroecosystem. FEMS Microbiol Ecol 88:333–344. doi:10.1111/1574-6941.12300
Li XL, Zhu TY, Peng F, Chen Q, Lin S, Christie P, Zhang JL (2015) Inner Mongolian steppe arbuscular mycorrhizal fungal communities respond more strongly to water availability than to nitrogen fertilization. Environ Microbiol 17:3051–3068. doi:10.1111/1462-2920.12931
Johnson NC, Tilman D, Wedin D (1992) Plant and soil controls on mycorrhizal fungal communities. Ecology 73:2034–2042. doi:10.2307/1941453
Eom AH, Hartnett DC, Wilson GWT (2000) Host plant species effects on arbuscular mycorrhizal fungal communities in tallgrass prairie. Oecologia 122:435–444. doi:10.1007/s004420050050
Mathimaran N, Ruh R, Vullioud P, Frossard E, Jansa J (2005) Glomus intraradices dominates arbuscular mycorrhizal communities in a heavy textured agricultural soil. Mycorrhiza 16:61–66. doi:10.1007/s00572-005-0014-9
Varela-Cervero S, Vasar M, Davison J, Barea JM, Öpik M, Azcón-Aguilar C (2015) The composition of arbuscular mycorrhizal fungal communities differs among the roots, spores and extraradical mycelia associated with five Mediterranean plant species. Environ Microbiol 17:2882–2895. doi:10.1111/1462-2920.12810
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355. doi:10.1111/j.1469-8137.2004.01159.x
Kim YC, Gao C, Zheng Y, He XH, Yang W, Chen L, Wan SQ, Guo LD (2015) Arbuscular mycorrhizal fungal community response to warming and nitrogen addition in a semiarid steppe ecosystem. Mycorrhiza 25:267–276. doi:10.1007/s00572-014-0608-1
Liu YJ, Shi GX, Mao L et al (2012) Direct and indirect influences of 8 yr of nitrogen and phosphorus fertilization on Glomeromycota in an alpine meadow ecosystem. New Phytol 194:523–535. doi:10.1111/j.1469-8137.2012.04050.x
Eom AH, Hartnett DC, Wilson GWT, Figge DAH (1999) The effect of fire, mowing and fertilizer amendment on arbuscular mycorrhizas in tallgrass prairie. Am Midl Nat 142:55–70. doi:10.1674/0003-0031(1999)142[0055:teofma]2.0.co;2
Zheng Y, Kim YC, Tian XF, Chen L, Yang W, Gao C, Song MH, Xu XL, Guo LD (2014) Differential responses of arbuscular mycorrhizal fungi to nitrogen addition in a near pristine Tibetan alpine meadow. FEMS Microbiol Ecol 89:594–605. doi:10.1111/1574-6941.12361
Egerton-Warburton LM, Allen EB (2000) Shifts in arbuscular mycorrhizal communities along an anthropogenic nitrogen deposition gradient. Ecol Appl 10:484–496. doi:10.2307/2641108
Chen YL, Zhang X, Ye JS, Han HY, Wan SQ, Chen BD (2014) Six-year fertilization modifies the biodiversity of arbuscular mycorrhizal fungi in a temperate steppe in Inner Mongolia. Soil Biol Biochem 69:371–381. doi:10.1016/j.soilbio.2013.11.020
Zhang ZH, Duan JC, Wang SP et al (2012) Effects of land use and management on ecosystem respiration in alpine meadow on the Tibetan plateau. Soil Tillage Res 124:161–169. doi:10.1016/j.still.2012.05.012
Zhang D, Zhou ZH, Zhang B, Du SH, Liu GC (2012) The effects of agricultural management on selected soil properties of the arable soils in Tibet, China. Catena 93:1–8. doi:10.1016/j.catena.2012.01.004
Zhang ZH, Duan JC, Wang SP et al (2013) Effects of seeding ratios and nitrogen fertilizer on ecosystem respiration of common vetch and oat on the Tibetan plateau. Plant Soil 362:287–299. doi:10.1007/s11104-012-1279-3
IUSS WGW (2007) World Reference Base for Soil Resources 2006, first update 2007. World Soil Resources Reports No. 103, FAO, Rome
Rillig MC, Field CB, Allen MF (1999) Soil biota responses to long-term atmospheric CO2 enrichment in two California annual grasslands. Oecologia 119:572–577. doi:10.1007/s004420050821
Miller RM, Reinhardt DR, Jastrow JD (1995) External hyphal production of vesicular-arbuscular mycorrhizal fungi in pasture and tallgrass prairie communities. Oecologia 103:17–23. doi:10.1007/bf00328420
Daniels BA, Skipper HD (1982) Methods for the recovery and quantitative estimation of propagules from soil. In: Schenck NC (ed) Methods and principles of Mycorrhizal research. American Phytopathological Society, Minn, pp 29–37
Schwarzott D, Schüßler A (2001) A simple and reliable method for SSU rRNA gene DNA extraction, amplification, and cloning from single AM fungal spores. Mycorrhiza 10:203–207. doi:10.1007/pl00009996
White TJ, Bruns TD, Lee SB, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, San Diego
Simon L, Lalonde M, Bruns TD (1992) Specific amplification of 18S fungal ribosomal genes from vesicular-arbuscular endomycorrhizal fungi colonizing roots. Appl Environ Microbiol 58:291–295
Lee J, Lee S, Young JPW (2008) Improved PCR primers for the detection and identification of arbuscular mycorrhizal fungi. FEMS Microbiol Ecol 65:339–349. doi:10.1111/j.1574-6941.2008.00531.x
Schloss PD, Westcott SL, Ryabin T et al (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi:10.1128/aem.01541-09
Öpik M, Vanatoa A, Vanatoa E, Moora M, Davison J, Kalwij JM, Reier Ü, Zobel M (2010) The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol 188:223–241. doi:10.1111/j.1469-8137.2010.03334.x
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998. doi:10.1038/Nmeth.2604
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. doi:10.1006/jmbi.1990.9999
Oksanen J, Blanchet FG, Kindt R, et al. (2013) Vegan: community ecology package. R package version 2.0-10. URL http://CRAN.R-project.org/package=vegan
Fierer N, Lauber CL, Ramirez KS, Zaneveld J, Bradford MA, Knight R (2012) Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J 6:1007–1017. doi:10.1038/ismej.2011.159
Goslee SC, Urban DL (2007) The ecodist package for dissimilarity-based analysis of ecological data. J Stat Softw 22:1–19. doi:10.18637/jss.v022.i07
R Core Team (2013) R: a language and environment for statistical computing. URL http://www.R-project.org/
Radić T, Hančević K, Likar M, Protega I, Jug-Dujaković M, Bogdanović I (2012) Neighbouring weeds influence the formation of arbuscular mycorrhiza in grapevine. Symbiosis 56:111–120. doi:10.1007/s13199-012-0165-3
Hamilton EW, Frank DA (2001) Can plants stimulate soil microbes and their own nutrient supply? Evidence from a grazing tolerant grass. Ecology 82:2397–2402. doi:10.2307/2679923
Chen Z, He XL, Guo HJ, Yao XQ, Chen C (2012) Diversity of arbuscular mycorrhizal fungi in the rhizosphere of three host plants in the farming-pastoral zone, north China. Symbiosis 57:149–160. doi:10.1007/s13199-012-0186-y
Anderson RC, Liberta AE, Dickman LA (1984) Interaction of vascular plants and vesicular-arbuscular mycorrhizal fungi across a soil moisture-nutrient gradient. Oecologia 64:111–117. doi:10.1007/Bf00377552
Yang HS, Yuan YG, Zhang Q, Tang JJ, Liu Y, Chen X (2011) Changes in soil organic carbon, total nitrogen, and abundance of arbuscular mycorrhizal fungi along a large-scale aridity gradient. Catena 87:70–77. doi:10.1016/j.catena.2011.05.009
Antoninka A, Reich PB, Johnson NC (2011) Seven years of carbon dioxide enrichment, nitrogen fertilization and plant diversity influence arbuscular mycorrhizal fungi in a grassland ecosystem. New Phytol 192:200–214. doi:10.1111/j.1469-8137.2011.03776.x
van Diepen LTA, Lilleskov EA, Pregitzer KS (2011) Simulated nitrogen deposition affects community structure of arbuscular mycorrhizal fungi in northern hardwood forests. Mol Ecol 20:799–811. doi:10.1111/j.1365-294X.2010.04969.x
Bahram M, Peay KG, Tedersoo L (2015) Local-scale biogeography and spatiotemporal variability in communities of mycorrhizal fungi. New Phytol 205:1454–1463. doi:10.1111/nph.13206
Bainard LD, Koch AM, Gordon AM, Newmaster SG, Thevathasan NV, Klironomos JN (2011) Influence of trees on the spatial structure of arbuscular mycorrhizal communities in a temperate tree-based intercropping system. Agric Ecosyst Environ 144:13–20. doi:10.1016/j.agee.2011.07.014
Martínez-García LB, Armas C, Miranda JD, Padilla FM, Pugnaire FI (2011) Shrubs influence arbuscular mycorrhizal fungi communities in a semi-arid environment. Soil Biol Biochem 43:682–689. doi:10.1016/j.soilbio.2010.12.006
Aguilar-Trigueros CA, Powell JR, Anderson IC, Antonovics J, Rillig MC (2014) Ecological understanding of root-infecting fungi using trait-based approaches. Trends Plant Sci 19:432–438. doi:10.1016/j.tplants.2014.02.006
Ehrenfeld JG, Ravit B, Elgersma K (2005) Feedback in the plant-soil system. Annu Rev Environ Resour 30:75–115. doi:10.1146/annurev.energy.30.050504.144212
De Deyn GB, Quirk H, Bardgett RD (2011) Plant species richness, identity and productivity differentially influence key groups of microbes in grassland soils of contrasting fertility. Biol Lett 7:75–78. doi:10.1098/rsbl.2010.0575
Bever JD, Dickie IA, Facelli E et al (2010) Rooting theories of plant community ecology in microbial interactions. Trends Ecol Evol 25:468–478. doi:10.1016/j.tree.2010.05.004
Torrecillas E, Alguacil MM, Roldan A (2012) Host preferences of arbuscular mycorrhizal fungi colonizing annual herbaceous plant species in semiarid Mediterranean prairies. Appl Environ Microbiol 78:6180–6186. doi:10.1128/Aem.01287-12
Toljander JF, Santos-González JC, Tehler A, Finlay RD (2008) Community analysis of arbuscular mycorrhizal fungi and bacteria in the maize mycorrhizosphere in a long-term fertilization trial. FEMS Microbiol Ecol 65:323–338. doi:10.1111/j.1574-6941.2008.00512.x
Bünemann EK, Schwenke GD, van Zwieten L (2006) Impact of agricultural inputs on soil organisms—a review. Aust J Soil Res 44:379–406. doi:10.1071/sr05125
Lin XG, Feng YZ, Zhang HY, Chen RR, Wang JH, Zhang JB, Chu HY (2012) Long-term balanced fertilization decreases arbuscular mycorrhizal fungal diversity in an arable soil in north China revealed by 454 pyrosequencing. Environ Sci Technol 46:5764–5771. doi:10.1021/es3001695
Dumbrell AJ, Nelson M, Helgason T, Dytham C, Fitter AH (2010) Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J 4:337–345. doi:10.1038/ismej.2009.122
Jansa J, Erb A, Oberholzer H-R, Šmilauer P, Egli S (2014) Soil and geography are more important determinants of indigenous arbuscular mycorrhizal communities than management practices in Swiss agricultural soils. Mol Ecol 23:2118–2135. doi:10.1111/mec.12706
Davison J, Moora M, Öpik M et al (2015) Global assessment of arbuscular mycorrhizal fungus diversity reveals very low endemism. Science 349:970–973. doi:10.1126/science.aab1161
Acknowledgments
The authors would like to appreciate Dong-Qing Chen and Wei Yang for the assistance with soil sampling and Xiao-Ling Zhang for the support on bioinformatics analysis. We thank the editor and anonymous referees for valuable suggestions and constructive comments on earlier versions of the manuscript. This study was financially supported by the National Natural Science Foundation of China (nos. 31470228, 41001149, 31070434) and Strategic Priority Research Program (B) of the Chinese Academy of Sciences (no. XDB05010200).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1
Abundance of arbuscular mycorrhizal fungal OTUs in different treatments. Non-parametric Kruskal–Wallis test was applied to show the effect of plant identity (PI) and fertilization (F) on the abundance of OTU10 (a), OTU12 (b), OTU46 (e), and OTU50 (f), which did not satisfy the homogeneity of variance, followed by pairwise comparisons at P < 0.05. Two-way ANOVA revealed the effect of PI, F, and their interaction (PI × F) on the abundance of OTU25 (c) and OTU39 (d), which satisfied the homogeneity of variance, followed by Tukey’s HSD test at P < 0.05. Data are means ± SE. Shared letters above bars (lowercase) and lines (uppercase) denote no significant difference among nine treatments and three plant species, respectively. ns, P ≥ 0.05; *P < 0.05; **P < 0.01; ***P < 0.001. (DOC 115 kb)
Figure S2
Abundance (a–d) and OTU richness (e–g) of arbuscular mycorrhizal fungal families in different treatments. Non-parametric Kruskal–Wallis test was applied to show the effect of plant identity (PI) and fertilization (F) on the abundance of Archaeosporaceae (a) and Gigasporaceae (d) and the richness of Archaeosporaceae (e), which did not satisfy the homogeneity of variance, followed by pairwise comparisons at P < 0.05. Two-way ANOVA revealed the effect of PI, F, and their interaction (PI × F) on the abundance of Claroideoglomeraceae (b) and Diversisporaceae (c) and the richness of Diversisporaceae (f) and Glomeraceae (g), which satisfied the homogeneity of variance, followed by Tukey’s HSD test at P < 0.05. Data are means ± SE. Shared letters above bars (lowercase) and lines (uppercase) denote no significant differences among nine treatments and three plant species, respectively. ns, P ≥ 0.05; *P < 0.05; **P < 0.01; ***P < 0.001. (DOC 140 kb)
ESM 3
(DOC 49 kb)
ESM 4
(DOC 24 kb)
Rights and permissions
About this article
Cite this article
Zheng, Y., Chen, L., Luo, CY. et al. Plant Identity Exerts Stronger Effect than Fertilization on Soil Arbuscular Mycorrhizal Fungi in a Sown Pasture. Microb Ecol 72, 647–658 (2016). https://doi.org/10.1007/s00248-016-0817-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-016-0817-6