Abstract
Environmentally transmitted opportunistic pathogens shuttle between two substantially different environments: outside-host and within-host habitats. These environments differ from each other especially with respect to nutrient availability. Consequently, the pathogens are required to regulate their behavior in response to environmental cues in order to survive, but how nutrients control the virulence in opportunistic pathogens is still poorly understood. In this study, we examined how nutrient level in the outside-host environment affects the gene expression of putative virulence factors of the opportunistic fish pathogen Flavobacterium columnare. The impact of environmental nutrient concentration on bacterial virulence was explored by cultivating the bacteria in various nutrient conditions, measuring the gene expression of putative virulence factors with RT-qPCR and, finally, experimentally challenging rainbow trout (Oncorhynchus mykiss) fry with these bacteria. Our results show that increased environmental nutrient concentration can increase the expression of putative virulence genes, chondroitinase (cslA) and collagenase, in the outside-host environment and may lead to more rapid fish mortality. These findings address that the environmental nutrients may act as significant triggers of virulence gene expression and therefore contribute to the interaction between an environmentally transmitted opportunistic pathogen and its host.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Environmentally transmitted opportunistic pathogens can often survive and replicate in the environment outside the host [5]. Thus, the nutrients of the surrounding environment may play a fundamental role in pathogen survival and significantly influence disease epidemics. High nutrient levels have been shown to be linked with increased virulence, possibly via an increased pathogen growth rate or decreased oxygen levels stressing the host [6, 33, 58]. Also, nutrient quality can have large-scale effects on host-pathogen dynamics in nature [17]. In environmentally transmitted pathogens, the transitions between the outside-host environment and the host expose the pathogens to various conditions, which may influence the emergence of disease outbreaks. Bacteria respond to environmental cues, such as elevated nutrient sources, and change their gene expression accordingly. The driving force behind bacterial pathogenicity is acquisition of host-derived nutrients for bacterial growth and survival. To achieve this, pathogens use virulence factors to colonize the host, avoid the host immune response, and finally, access the host tissues for nutrients [8]. Many bacterial pathogens secrete proteases that often have a role in bacterial pathogenesis [29]. These tissue-degrading enzymes provide nutrients for bacterial growth during infection and transmission ([30]; see also [43] for a review). So far, we have a limited understanding of how environmental nutrient levels affect the expression of bacterial virulence factors, for example, enzymes for host-tissue degradation, which may not be essential outside the host. Hence, understanding how the environment influences the production and regulation of virulence factors can provide tools for disease management and also deepen our knowledge of host-pathogen interactions.
Flavobacterium columnare (Bacteroidetes) is a member of the natural aquatic microbial community [22, 42], but it is also an important fish pathogen worldwide, causing significant economic losses for the freshwater aquaculture industry [11, 40, 56]. F. columnare has been shown to persist long periods in the environment outside the host [2, 49], and it has been suggested that environment can serve as a reservoir of the bacteria between the outbreak seasons [22, 36]. Despite its importance, the virulence mechanisms and their expression of F. columnare are still largely unknown. Especially in the case of columnaris disease, in which the symptoms can be seen as skin lesions, fin erosion, and gill necrosis [3, 54], enzymes targeting host tissue degradation may play a considerable role. Proteomics and suppression subtractive hybridization (SSH) [14, 32, 35] have revealed some potential factors involved with F. columnare virulence, such as the tissue-degrading enzymes chondroitinase (chondroitin AC lyase) and collagenase [35]. These enzymes degrade chondroitin sulfate and collagen, respectively, which are both widely found in animal connective tissues. Chondroitinase production has been connected to the virulence of F. columnare [23, 48, 51], and collagenolytic activity has been shown to be involved with bacterial virulence in many other pathogenic bacteria [18]. Furthermore, when grown under laboratory conditions, F. columnare can display three colony morphotypes: Rhizoid (Rz) and its derivatives Rough (R) and Soft (S). Of these colony types, only the ancestral Rz type is highly virulent [24, 27, 28]. Comparison of virulent and nonvirulent colony types in transcriptional level can help to identify genetic factors that are needed to produce bacterial infection.
In this study, we developed a reverse transcription quantitative PCR (RT-qPCR) protocol with two reference genes for studying the gene expression in F. columnare. RT-qPCR assays can be used to quantitatively measure the expression of specific genes of interest, such as those encoding proteases or other virulence factors. Primer design and optimization, high-quality template material, and normalization with more than one stably expressed reference gene are all crucial elements in RT-qPCR to generate accurate gene expression results [7]. The expression of the tissue-degrading enzymes chondroitinase and collagenase were determined in virulent (Rz) and nonvirulent (R and S) F. columnare B067 morphotypes [27] in liquid cultures and on agar plates, in which the nutrient concentration of the plates was manipulated. Furthermore, we analyzed whether the nutrient concentration of the culture medium has a direct effect on bacterial virulence using two F. columnare strains in an experimental infection of rainbow trout fry. The aim of this study was to identify the outside-host conditions favoring the expression of tissue-degrading enzymes in order to find factors that increase the virulence of this opportunistic pathogen.
Results
Validation of Reference Genes for qPCR Assay
The stability of five reference gene candidates (16S rRNA, 16S ribosomal RNA; gapdh, glyceraldehyde 3-phosphate dehydrogenase; glyA, serine hydroxymethyltransferase; rplQ, ribosomal protein; rpoD, RNA polymerase sigma factor) was evaluated in a sample set (N = 27) in GenEx v6.0 (MultiD Analyses) with the integrated geNorm and NormFinder methods [1, 55]. According to both methods, the best expression stability was obtained for the gapdh and glyA genes. M value of 0.52 for both genes was calculated with geNorm and standard deviations 0.19 and 0.26 for glyA and gapdh with NormFinder, respectively (Fig. S1). The stability was then calculated with geNorm method for the entire dataset, including the samples of Rz, R, and S types grown in 1× liquid Shieh broth and on 0.5×, 1×, and 2× Shieh agar, giving the M value of 0.69 for gapdh and glyA which were selected as reference genes and used for normalization of the gene expression data.
Expression of Chondroitinase and Collagenase in Liquid Culture
F. columnare morphotypes Rz, R, and S were grown in Shieh broth, and their gene expressions during the logarithmic growth phase were compared. The expression of chondroitinase (encoded by gene cslA) differed significantly between the F. columnare morphotypes (F = 21.189, df = 2, p < 0.001), being highest in the Rz morphotype as compared to the R and S types (LSD post hoc test, p = 0.002 and p < 0.001, respectively). Collagenase expression did not differ between the morphotypes (Fig. 1).
The DNA sequences of chondroitinase and collagenase were analyzed in all F. columnare B067 colony types (Rz, R, and S) to search for genetic differences. Chondroitinase and collagenase genes and their predicted promoter regions were 100 % identical in all three colony types. Sequences are submitted in GenBank under accession numbers KR014145 for chondroitinase and KR014146 for collagenase.
Expression of Chondroitinase and Collagenase on Agar Plates with Various Nutrient Levels
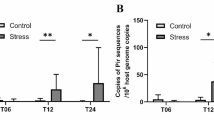
F. columnare morphotypes Rz, R, and S were grown on Shieh agar plates with various Shieh concentrations (0.5×, 1×, or 2×). After 2 days of incubation, the expressions of chondroitinase and collagenase in different colony types were compared. Colony type had a significant impact on the expression of chondroitinase (F 5.094, p = 0.010), the expression being highest in the virulent Rz type (Fig. 2; Table 1). In contrast to the liquid culture, on agar plates the collagenase expression differed between the colony types (F 7.283, p = 0.002), and the highest level of expression was observed in the nonvirulent R type. Interactions between the colony type and nutrients were not found (Table 1). Post hoc comparisons revealed that a high nutrient concentration significantly increased the expression of chondroitinase in all morphotypes (Table 2). Similarly, a significant increase in collagenase expression was observed in the virulent Rz morphotype as a response to increased nutrient level, but not in the nonvirulent R. Gene expression patterns of nonvirulent S type in various conditions were similar to that of Rz; however, nutrients did not have statistically significant impact on gene expression in S type (Fig. 2; Table 2).
Gene expression of chondroitinase and collagenase on agar plates in different colony types. Mean relative expressions (+/− S.E.) of a chondroitinase and b collagenase in Rz, R, and S colony morphotypes of Flavobacterium columnare B067 grown on diluted (0.5×), normal (1×), and concentrated (2×) Shieh agar. For statistics, see Tables 1 and 2
Environmental Nutrient Level Can Increase Bacterial Virulence in Experimental Infection of Rainbow Trout
The virulence of two F. columnare strains (B067 and B185), both Rz type, which were cultured in various nutrient conditions, 2 × N, 1×, or 0.5 × N Shieh (N referring to the modified concentration of peptone and yeast extract), was studied by infection of rainbow trout fry. The mortality of the infected fish in the 26-h experiment was 93.75–100 %. Bacteria that were cultured in high nutrient concentration (2 × N Shieh) caused significantly faster fish mortality and more rapid onset of disease in both bacterial strains (Table 3, Fig. 3). Fish mortality in the control group, which had been challenged with growth medium only, reached ∼45 %. However, the skin cultivations on Shieh agar plates from the infected fish were 98 % positive for F. columnare, while the bacterium was not detected in the cultivations from the control fish, indicating that the cause of death in the control group was most probably the stressful experimental conditions.
Effect of nutrient concentration of the bacterial culture on Flavobacterium columnare virulence in rainbow trout fry (Oncorhynchus mykiss). The fish longevity is measured after experimental infection of F. columnare strains a B067 Rz and b B185 Rz (final bacterial dose 5 × 103 CFU ml−1 in both experiments). The bacteria used for infections were cultured in 0.5 × N, 1×, or 2 × N Shieh medium (N refers to a modified nutrient concentration). Control fish received 1× Shieh only. Differences in fish mortality between the treatments were significant in all pairwise comparisons (p ≤ 0.006 for B185 and p ≤ 0.041 for B067); see full statistics in Table 3
Discussions
Opportunistic pathogens often have the ability to persist and transmit via the environment [5] which can substantially differ from the nutrient-rich within-host milieu. As the environment outside the host is unpredictable, the bacteria have to adapt rapidly to the surrounding conditions, e.g., temperature [16] or nutrients [21]. Furthermore, environmentally transmitted pathogens need to have capacity for the invasion when a potential host is encountered, but the production of virulence factors in the absence of a host is costly. Therefore, the expression of virulence factors has to be carefully regulated. Environmental signals can serve as triggers in switching the metabolic routes on and off, but the molecular-level knowledge of how nutrients in the outside-host environment affect the virulence mechanisms in environmentally transmitted pathogens is so far limited.
We developed an RT-qPCR method with two reference genes for studying the gene expression of the fish pathogen F. columnare. The expression of tissue-degrading enzymes chondroitinase and collagenase, which are likely involved in virulence [18, 23, 48, 51], was explored in the virulent Rz and nonvirulent R and S colony variants of F. columnare strain B067 (8–10). Furthermore, we examined how the nutrient conditions influence the expression of these enzymes in order to find associations with colony type and virulence. We found that the virulent Rz type bacteria expressed chondroitinase (cslA) significantly more as compared to the nonvirulent types, both in liquid and on agar plates. Surprisingly, the increased collagenase expression was not associated with the virulent Rz colony morphotype, but was observed in the nonvirulent R colony type. A significant increase in the expression of both tissue-degrading enzymes in response to increased nutrient concentration was detected, indicating that the gene expression may be regulated by the conditions in the outside-host environment. Finally, we found that the bacteria cultured in the high-nutrient medium were significantly more virulent in the natural fish host than those cultured in the low-nutrient medium.
Extracellularly secreted proteins and proteinases have a significant role in bacterial virulence because they act in the interface between the pathogen and the host organism [9, 30, 41]. Pathogenic species of phylum Bacteroidetes, such as Porphyromonas gingivalis, Bacteroides fragilis, and B. thetaiotaomicron, produce a variety of hydrolytic enzymes that degrade components of extracellular matrix of the host tissues [44, 53, 59]. Hence, in addition to acquisition of host-derived nutrients, these enzymes are important in host invasion and colonization [53]. The external symptoms caused by F. columnare infection, such as tissue lesions and erosion [40], reflect the importance of proteases for columnaris disease pathology. Indeed, proteolytic activities, including ability to degrade gelatin, casein, hemoglobin, fibrinogen, and elastin, have been reported in F. columnare [4]. However, thus far, only chondroitinase, an enzyme degrading chondroitin sulfates, the proteoglycan components of vertebrate connective tissues, has been connected to virulence of F. columnare [23, 48, 51]. Chondroitinase activity of the colony types has been studied previously, and it has been concluded that the Rz type exhibits the highest enzymatic activity of chondroitin AC lyase [23]. In accordance with these previous studies, our current study links the high chondroitinase (encoded by the gene cslA) expression with virulence. RT-qPCR revealed that the chondroitinase expression was significantly higher in the virulent colony type Rz as compared to the nonvirulent types. It was reported recently that F. columnare produces also a second chondroitin lyase gene, cslB [31]. However, a deletion of cslA resulted in significant reduction in the lytic activity of chondroitinase, whereas cslB possessed only a minor role in the total chondroitinase activity [31].
Many pathogenic bacteria produce proteases with collagenolytic activity [18]. These enzymes degrade collagens, constituents of extracellular matrix (ECM) found in vertebrate tissues, and contribute significantly to host tissue damage [18]. For instance, Arg-gingipains, which are virulence factors produced by a periodontal pathogen P. gingivalis, possess collagenolytic activity [19]. Collagenase has been suggested as a putative virulence factor in F. columnare based on its absence in F. johnsoniae, a nonvirulent member of Flavobacteria, as revealed by suppressive subtractive hybridization analysis [35]. However, the actual function and role of collagenase in F. columnare virulence have not been studied further. In the closely related fish pathogen, Flavobacterium psychrophilum, collagenase has been connected with virulence [34, 38] but opposite results have also been obtained [13, 50], and thus, its role in pathogenesis of F. psychrophilum has remained uncertain. Our results could not directly link collagenase with the virulent colony type of F. columnare, and it remains to be studied whether some other processes, such as temporal regulation of virulence factors (similar to, e.g., Staphylococcus aureus virulence factors [37]), are associated with collagenase expression. Collagenase is an important virulence factor also in Leptospira interrogans, and interestingly, its expression is induced by contact with cell cultures [20]. Therefore, our study cannot rule out the possible role of collagenase as a virulence factor in F. columnare, and its expression needs to be studied further in the presence of an appropriate substrate or host tissue. Furthermore, collagenolytic activity of F. columnare should be verified to find associations with virulence.
Bacteria sense properties of their physical and biological environment to take the best advantage of the available resources. Hence, local environmental cues have wide influences on bacterial physiology [46]. Environmental signals are recognized by systems that function in global coordination of gene regulation, such as carbon catabolite repression [15], nitrogen phosphotransferase system [39], and ppGpp-mediated regulation [10], all of which are found in several bacterial species. With these multi-functional systems, it has been exemplified that virulence pathways are often integrated in the general metabolism of bacteria and exploit the signaling pathways crucial for metabolism of carbon and nitrogen, for example [10, 15, 39]. The presence of the host can be a signal for a pathogen to turn on the virulence factor expression. In B. thetaiotaomicron, the chondroitin lyase activity is induced by the presence of its substrate, chondroitin sulfate A [45], similarly as the collagenase expression is in L. interrogans mentioned above [20]. Given that the outside- and within-host habitats are different especially with respect to nutrient concentration, it is possible that nutrients contribute to the regulation of virulence gene expression. In general, the increase of nutrients is expected to influence disease dynamics via accelerating the pathogen growth rate and infective dose [26, 27]. However, our study indicates a possibility that high nutrient availability during outside-host growth could increase the virulence factor (cslA) expression and directly prime bacterial virulence even in the absence of host. Changes in virulence were observed with bacteria grown under altered peptone and yeast extract concentrations, both of which are complex nutrient resources. Therefore, a more detailed exploration is needed to determine the specific components that contribute to the regulation of chondroitinase expression. Nevertheless, it has been shown that F. columnare can maintain high virulence also during starvation [49], but how a long-scale exposure to nutrient-limited conditions affect the virulence factor expression in F. columnare is not known.
The expression of chondroitinase and collagenase was measured using RT-qPCR, which can be used for messenger RNA (mRNA) quantification. However, the final enzymatic activity is dependent on the following steps, including the post-transcriptional and post-translational regulation and the accurate folding and modifications of the protein. Furthermore, the proteolytic enzymes that are needed for the host tissue destruction need to be successfully secreted to their final subcellular location, for which a functional secretion system is required. Therefore, the future studies may include biochemical quantification of the enzyme activity in different conditions to explore whether the high mRNA level reflects the true enzyme activity of the extracellular environment. In addition, a broad-scale approach, such as transcriptome sequencing, could help to understand the wider role of environmental nutrients in the gene expression and regulation of F. columnare.
The pathogen-environment interaction is complex, and both the quantity and quality of the available nutrients can influence bacterial virulence [21]. In the current study, the increase in the general nutrient concentration significantly increased not only the expression of tissue-degrading enzymes but also bacterial virulence. These findings may have significant impacts at the applied level, as the uneaten fish food, feces, and carcasses offer nutrient resources for outside-host growth of F. columnare in the aquaculture rearing units [25, 57]. Furthermore, these conditions may preload the virulence machineries and thereby directly alter the disease dynamics.
Materials and Methods
qPCR Primer Design and Validation
Primers for amplifying and sequencing the genes of interest were designed using the whole genome sequence of F. columnare ATCC 49512 [52], as well as our own shotgun sequencing results. The primers were designed with Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/). The sequences of the primers used in this study are listed in Table S1. The specificities of the primer pairs were verified by inspecting the length of the PCR product in agarose gel electrophoresis and by melting curve analysis in CFX Manager™ Software v3.0 (for melt curves, see Fig. S2). Specific primer binding was also confirmed by sequencing the qPCR amplicon with an appropriate primer using Sanger method (see below). The resulting DNA sequences were imported into BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi), and correct binding was ensured if sequence originated from the relevant gene. The efficiency of each primer pair was determined by making a standard curve from a 1:5 diluted dilution series as a template and analyzed with CFX Manager™ Software v3.0 (Bio-Rad; Table S1).
The preliminary expression stability testing was first performed with five reference gene candidates (16S rRNA; gapdh; glyA; rplQ; rpoD) using a test sample set (N = 27) that included samples from Rz, R, and S colony types grown on 1× Shieh agar plates and in 1× Shieh liquid medium. The expression stabilities were measured via the geNorm [55] and NormFinder [1] methods, which are integrated in GenEx v6.0 (MultiD Analyses) (Fig. S1). According to both methods, the gapdh and glyA genes possessed the best stabilities and were chosen to be used as reference genes in the expression analysis of the entire dataset. Finally, the stability of the chosen reference genes was measured again for the whole dataset with geNorm method to cover all the nutrient conditions that were used in this study.
Flavobacterium columnare Growth Conditions
F. columnare strains B067 and B185 were originally isolated from an infected brown trout (Salmo trutta) and from rearing tank water during columnaris disease outbreak, respectively [26], on Shieh medium supplemented with tobramycin [12]. The ancestral Rz bacteria were exposed to lytic phage as described in Laanto et al. to obtain the R colony morphotype [28]. The S colony morphotype appeared spontaneously among the original Rz colonies in a plate culture. The virulence of these different morphotypes of the strain B067 was reported in an earlier study [27]. Throughout this study, Shieh medium with small modifications [47] was used for bacterial cultivations and as a basis of the nutrient-modified media. The bacteria of different colony morphotypes were stored frozen at −80°C with 10 % glycerol and 10 % fetal calf serum. For the analyses, the bacteria were revived from the freezer in Shieh medium at +26 °C under constant shaking (150 rpm) for 48 h to obtain turbid cultures, after which they were used for the experiments. The revived bacterial cultures were enriched in fresh Shieh medium or spread on Shieh agar plates for the studies described below.
Culture Conditions of qPCR Samples
-
1.
Chondroitinase and Collagenase Expression in Liquid Culture
B067 Rz, R, and S were cultured in 15–20 ml of liquid Shieh medium. The growth was monitored by measuring the optical density at 570 nm every 0.5–4 h. After reaching the logarithmic growth phase (5–8.5 h post-inoculation), duplicate samples of 0.5 ml were collected, and the RNA of the samples was stabilized with RNA Protect™ Bacteria Reagent (Qiagen). The samples were forwarded to the RNA isolation step during the same day. Alternatively, 1–1.5 ml of bacterial culture was centrifuged (3 min, 10,000g), and the pellet was stored in RNAlater RNA Stabilization Reagent (Qiagen) at −20 °C to be processed later. A minimum of three independent liquid cultures were grown per colony type (see Table S2).
-
2.
Chondroitinase and Collagenase Expression on Agar Plate Culture
To prepare samples of bacteria grown on a solid surface, diluted overnight culture of B067 Rz, R, or S grown in 1× liquid Shieh medium was spread on Shieh agar plates where all the components of the medium were either diluted (with deionized and sterilized water) to 50 % (0.5×), normal 100 % (1×), or concentrated 200 % (2×). After incubating for 48 h at room temperature (+24 °C), bacterial lawns from plates with separate colonies were chosen for RNA isolation. RNA was stabilized and stored for later processing with RNA Protect™ Bacteria Reagent (Qiagen) according to the manufacturer’s instructions. From each sample type (colony type-nutrient concentration combination), a minimum of four plates were analyzed (see Table S2).
Sample Preparation and RT-qPCR
The total RNA from the liquid and plate culture samples was extracted using an RNeasy Mini Kit (Qiagen), and residual genomic DNA was removed with DNA-free™ (Ambion by Life Technologies) according to the manufacturers’ instructions. RNA concentration was measured with a NanoDrop® ND-1000 Spectrophotometer. To ensure that the isolated RNA was intact, the RNA samples were run on an Agilent RNA 6000 Nano Chip in an Agilent 2100 Bioanalyzer platform (Agilent Technologies). RNA integrity numbers (RINs) were analyzed with 2100 Expert Software (Agilent Technologies). Only high-quality RNA was accepted into the following step, resulting in RINs within a scale of 8.3 to 10.0, with a mean of 9.73 (N = 75). Qualified samples proceeded to complementary DNA (cDNA) synthesis immediately after RNA quality validation. Forty nanograms of DNAse-treated RNA was used as a template in triplicate 20-μl cDNA synthesis reactions, including iScript™ reverse transcriptase in a 1× iScript™ Reaction Mix from an iScript™ cDNA Synthesis Kit (Bio-Rad). cDNA synthesis reactions were incubated under the following conditions: 5 min at 25 °C, 30 min at 42 °C, and 5 min at 85 °C. Three replicate cDNA reactions were pooled. Each sample was run with each primer pair in triplicate. Twenty-microliter qPCR reactions contained 40 ng of cDNA as a template, 0.5 μM of both forward and reverse primers, and 1× iQ™ SYBR Green Supermix (Bio-Rad) as a reaction mix, including iTaq DNA polymerase (25 U/ml). The thermocycling conditions for the qPCR reactions were as follows: 95 °C for 3 min, followed by 40 cycles of 95 °C for 10 s, Tm °C for 20 s, and 72 °C for 20 s, where Tm (melting temperature) was dependent on the primer pair in question (see Table S1). The qPCR runs were performed in a CFX96™ Real-Time System in C1000™ and C1000™ Touch Thermal Cyclers (Bio-Rad) on 96-well Hard-Shell® PCR plates (Bio-Rad). To reduce the variation between plates, two interplate calibrator (IPC) samples were run in triplicates on each plate.
Relative Expression Analysis
Missing Cq (quantification cycle) values were replaced with the average Cq of a sample’s qPCR replicates. Cq values were calibrated with IPC samples within each gene and corrected with the efficiency determined specifically for each primer pair. The average Cq of qPCR repeats was calculated, after which the Cq values of the target genes, chondroitinase (cslA) and collagenase, were normalized against those of two reference genes, gapdh and glyA. Finally, the Cq values were converted into relative gene expression. All qPCR data analyses described above were performed with GenEx v5.3.4 (MultiD Analyses). To maintain a normal distribution, the relative quantities were log-transformed for the statistical analyses.
Sequencing of Chondroitinase and Collagenase Genes
Chondroitinase and collagenase genes, including the upstream regions (approximately 100 bp) assumably covering the promoter regions, were sequenced from F. columnare B067 colony types Rz, R, and S. The DNA sequences were determined in an automatic sequencer (Applied Biosystems 3130xl Genetic Analyzer) with the Sanger sequencing method using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems). Sequencing Analysis Software v6.0 (Applied Biosystems) was used to analyze the raw sequencing data, and the gene sequences were aligned to find differences between the colony types using Vector NTI Advance® 11.5.0 (Invitrogen).
Virulence Experiments with Rainbow Trout
For the experimental infections, Rz colony types of F. columnare strains B067 and B185 were grown in the conditions described above. The compositions of the growth media were manipulated by altering only the concentration of yeast extract and peptone. The bacteria were cultivated in three different nutrient concentrations, 2 × N, 1×, or 0.5 × N Shieh. N refers to the concentrations of peptone and yeast extract that were doubled, the same or halved (when compared to 1× Shieh), respectively. Detailed compositions of the growth media used in this study are presented in Table S3.
Apparently healthy rainbow trout (Oncorhynchus mykiss) fry with no previous contact with F. columnare were obtained from a fish farm in Central Finland in spring, before the warm water season. Prior to the experiments, the fish were maintained in aerated groundwater at 17 °C in 250-l flow-through aquaria. For the infection experiments, the water temperature was gradually elevated to 24 °C during 7 days. Rainbow trout fry (n = 16 per each treatment, mean weight 0.195 g (n = 111)) were placed individually in 0.75-l aquaria containing 500 ml of groundwater (T = 24.1 °C). The fish were challenged by adding bacterial culture (7.5–12 μl of original culture diluted in total of 400 μl of Shieh medium) directly into aquaria to reach an infective dose of 5 × 103 CFU ml−1 of F. columnare. In this continuous infection, bacteria are present in the aquaria throughout the experiment. Control fish were exposed to sterile 1× Shieh medium (n = 15). The fish longevity was monitored in intervals of 30–60 min, starting from the addition of bacterial culture. To meet the requirements of ethical endpoint of the experimental animals, morbid fish that did not respond to external stimuli were considered dead, removed from the experiment and euthanized. To determine the presence or absence of F. columnare on the fish, skin cultivations on Shieh agar supplemented with tobramycin [12] were taken from the moribund fish and from the fish that were alive in the end of the experiment.
Statistics
The effects of colony type and nutrient concentration on chondroitinase and collagenase of log-transformed gene expression were analyzed via ANOVA, and post hoc analyses within each colony type were Bonferroni-corrected. Bacterial virulence after culturing in various nutrient conditions was analyzed from rainbow trout longevity data using Kaplan-Meier survival analysis. All statistical analyses were performed with IBM SPSS Statistics 22.
References
Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245
Arias CR, Lafrentz S, Cai W, Olivares-Fuster O (2012) Adaptive response to starvation in the fish pathogen Flavobacterium columnare: cell viability and ultrastructural changes. BMC Microbiol 12:266
Bernardet JF (1997) Immunization with bacterial antigens: Flavobacterium and Flexibacter infections. Dev Biol Stand 90:179
Bertolini JM, Rohovec JS (1992) Electrophoretic detection of proteases from different Flexibacter-Columnaris strains and assessment of their variability. Dis Aquat Org 12:121
Brown SP, Cornforth DM, Mideo N (2012) Evolution of virulence in opportunistic pathogens: generalism, plasticity, and control. Trends Microbiol 20:336
Bruno J, Petes L, Harvell C, Hettinger A (2003) Nutrient enrichment can increase the severity of coral diseases. Ecol Lett 6:1056
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611
Casadevall A, Pirofski L (2001) Host-pathogen interactions: the attributes of virulence. J Infect Dis 184:337
Costa TR, Felisberto-Rodrigues C, Meir A, Prevost MS, Redzej A, Trokter M, Waksman G (2015) Secretion systems in gram-negative bacteria: structural and mechanistic insights. Nat Rev Microbiol 13:343
Dalebroux ZD, Svensson SL, Gaynor EC, Swanson MS (2010) ppGpp conjures bacterial virulence. Microbiol Mol Biol Rev 74:171
Declercq AM, Haesebrouck F, Van den Broeck W, Bossier P, Decostere A (2013) Columnaris disease in fish: a review with emphasis on bacterium-host interactions. Vet Res 44:27
Decostere A, Haesebrouck F, Devriese LA (1997) Shieh medium supplemented with tobramycin for selective isolation of Flavobacterium columnare (Flexibacter columnaris) from diseased fish. J Clin Microbiol 35:322
Duchaud E, Boussaha M, Loux V, Bernardet JF, Michel C, Kerouault B, Mondot S, Nicolas P, Bossy R, Caron C, Bessieres P, Gibrat JF, Claverol S, Dumetz F, Le Henaff M, Benmansour A (2007) Complete genome sequence of the fish pathogen Flavobacterium psychrophilum. Nat Biotechnol 25:763
Dumpala PR, Gulsoy N, Lawrence ML, Karsi A (2010) Proteomic analysis of the fish pathogen Flavobacterium columnare. Proteome Sci 8:26
Gorke B, Stulke J (2008) Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6:613
Guijarro JA, Cascales D, Garcia-Torrico AI, Garcia-Dominguez M, Mendez J (2015) Temperature-dependent expression of virulence genes in fish-pathogenic bacteria. Front Microbiol 6:700
Hall SR, Knight CJ, Becker CR, Duffy MA, Tessier AJ, Caceres CE (2009) Quality matters: resource quality for hosts and the timing of epidemics. Ecol Lett 12:118
Harrington DJ (1996) Bacterial collagenases and collagen-degrading enzymes and their potential role in human disease. Infect Immun 64:1885
Houle MA, Grenier D, Plamondon P, Nakayama K (2003) The collagenase activity of Porphyromonas gingivalis is due to Arg-gingipain. FEMS Microbiol Lett 221:181
Kassegne K, Hu W, Ojcius DM, Sun D, Ge Y, Zhao J, Yang XF, Li L, Yan J (2014) Identification of collagenase as a critical virulence factor for invasiveness and transmission of pathogenic Leptospira species. J Infect Dis 209:1105
Ketola T, Mikonranta L, Laakso J, Mappes J (2016) Different food sources elicit fast changes to bacterial virulence. Biol Lett 12:0150660
Kunttu HM, Sundberg L-R, Pulkkinen K, Valtonen ET (2012) Environment may be the source of Flavobacterium columnare outbreaks at fish farms. Environ Microbiol Rep 4:398
Kunttu HM, Jokinen EI, Valtonen ET, Sundberg L-R (2011) Virulent and nonvirulent Flavobacterium columnare colony morphologies: characterization of chondroitin AC lyase activity and adhesion to polystyrene. J Appl Microbiol 111:1319
Kunttu HM, Suomalainen L-R, Jokinen EI, Valtonen ET (2009) Flavobacterium columnare colony types: connection to adhesion and virulence? Microb Pathog 46:21
Kunttu HM, Valtonen ET, Jokinen EI, Suomalainen L-R (2009) Saprophytism of a fish pathogen as a transmission strategy. Epidemics 1:96
Laanto E, Sundberg L-R, Bamford JKH (2011) Phage specificity of the freshwater fish pathogen Flavobacterium columnare. Appl Environ Microbiol 77:7868
Laanto E, Penttinen RK, Bamford JKH, Sundberg L-R (2014) Comparing the different morphotypes of a fish pathogen—implications for key virulence factors in Flavobacterium columnare. BMC Microbiol 14:170
Laanto E, Bamford JKH, Laakso J, Sundberg L-R (2012) Phage-driven loss of virulence in a fish pathogenic bacterium. Plos One 7:e53157
Lantz M (1997) Are bacterial proteases important virulence factors? J Periodont Res 32:126
Lebrun I, Marques-Porto R, Pereira AS, Pereira A, Perpetuo EA (2009) Bacterial toxins: an overview on bacterial proteases and their action as virulence factors. Mini-Rev Med Chem 9:820
Li N, Qin T, Zhang XL, Huang B, Liu ZX, Xie HX, Zhang J, McBride MJ, Nie P (2015) Gene deletion strategy to examine the involvement of the two chondroitin lyases in Flavobacterium columnare virulence. Appl Environ Microbiol 81:7394
Li N, Zhang J, Zhang LQ, Nie P (2010) Difference in genes between a high virulence strain G(4) and a low virulence strain G(18) of Flavobacterium columnare by using suppression subtractive hybridization. J Fish Dis 33:403
McKenzie VJ, Townsend AR (2007) Parasitic and infectious disease responses to changing global nutrient cycles. Ecohealth 4:384
Nakayama H, Tanaka K, Teramura N, Hattori S (2015) Expression of collagenase in Flavobacterium psychrophilum isolated from cold-water disease-affected ayu (Plecoglossus altivelis). Biosci Biotechnol Biochem 80:135
Olivares-Fuster O, Arias CR (2008) Use of suppressive subtractive hybridization to identify Flavobacterium columnare DNA sequences not shared with Flavobacterium johnsoniae. Lett Appl Microbiol 46:605
Olivares-Fuster O, Baker JL, Terhune JS, Shoemaker CA, Klesius PH, Arias CR (2007) Host-specific association between Flavobacterium columnare genomovars and fish species. Syst Appl Microbiol 30:624
Oogai Y, Matsuo M, Hashimoto M, Kato F, Sugai M, Komatsuzawa H (2011) Expression of virulence factors by Staphylococcus aureus grown in serum. Appl Environ Microbiol 77:8097
Ostland VE, Byrne PJ, Hoover G, Ferguson HW (2000) Necrotic myositis of rainbow trout, Oncorhynchus mykiss (Walbaum): proteolytic characteristics of a crude extracellular preparation from Flavobacterium psychrophilum. J Fish Dis 23:329
Pfluger-Grau K, Gorke B (2010) Regulatory roles of the bacterial nitrogen-related phosphotransferase system. Trends Microbiol 18:205
Pulkkinen K, Suomalainen L-R, Read AF, Ebert D, Rintamaki P, Valtonen ET (2010) Intensive fish farming and the evolution of pathogen virulence: the case of columnaris disease in Finland. Proc Biol Sci 277:593
Rao M, Tanksale A, Ghatge M, Deshpande V (1998) Molecular and biotechnological aspects of microbial proteases. Microbiol Mol Biol Rev 62:597
Revetta RP, Rodgers MR, Kinkle BK (2005) Isolation and identification of freshwater bacteria antagonistic to Giardia intestinalis cysts. J Water Health 3:83
Rohmer L, Hocquet D, Miller SI (2011) Are pathogenic bacteria just looking for food? Metabolism and microbial pathogenesis. Trends Microbiol 19:341
Rudek W, Haque R (1976) Extracellular enzymes of genus Bacteroides. J Clin Microbiol 4:458
Salyers AA, Kotarski SF (1980) Induction of chondroitin sulfate lyase activity in Bacteroides thetaiotaomicron. J Bacteriol 143:781
Somerville GA, Proctor RA (2009) At the crossroads of bacterial metabolism and virulence factor synthesis in Staphylococci. Microbiol Mol Biol Rev 73:233
Song Y, Fryer J, Rohover J (1988) Comparison of six media for the cultivation of Flexibacter-Columnaris. Fish Pathol 23:91
Stringer-Roth KM, Yunghans W, Caslake LF (2002) Differences in chondroitin AC lyase activity of Flavobacterium columnare isolates. J Fish Dis 25:687
Sundberg L-R, Kunttu HM, Valtonen ET (2014) Starvation can diversify the population structure and virulence strategies of an environmentally transmitting fish pathogen. BMC Microbiol 14:67
Sundell K, Wiklund T (2015) Characteristics of epidemic and sporadic Flavobacterium psychrophilum sequence types. Aquaculture 441:51
Suomalainen L-R, Tiirola M, Valtonen ET (2006) Chondroitin AC lyase activity is related to virulence of fish pathogenic Flavobacterium columnare. J Fish Dis 29:757
Tekedar HC, Karsi A, Gillaspy AF, Dyer DW, Benton NR, Zaitshik J, Vamenta S, Banes MM, Gulsoy N, Aboko-Cole M, Waldbieser GC, Lawrence ML (2012) Genome sequence of the fish pathogen Flavobacterium columnare ATCC 49512. J Bacteriol 194:2763
Thomas F, Hehemann J, Rebuffet E, Czjzek M, Michel G (2011) Environmental and gut Bacteroidetes: the food connection. Front Microbiol 2:93
Tripathi NK, Latimer KS, Gregory CR, Ritchie BW, Wooley RE, Walker RL (2005) Development and evaluation of an experimental model of cutaneous columnaris disease in koi Cyprinus carpio. J Vet Diagn Invest 17:45
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:RESEARCH0034
Wagner B, Wise D, Khoo L, Terhune J (2002) The epidemiology of bacterial diseases in food-size channel catfish. J Aquat Anim Health 14:263
Wakabayashi H (1991) Effect of environmental conditions on the infectivity of Flexibacter-Columnaris to fish. J Fish Dis 14:279
Wedekind C, Gessner MO, Vazquez F, Maerki M, Steiner D (2010) Elevated resource availability sufficient to turn opportunistic into virulent fish pathogens. Ecology 91:1251
Zenobia C, Hajishengallis G (2015) Porphyromonas gingivalis virulence factors involved in subversion of leukocytes and microbial dysbiosis. Virulence 6:236
Acknowledgments
The authors would like to thank Prof. Annemie Decostere for commenting on the manuscript and Ms Irene Helkala, Ville Hoikkala, MSc, Dr. Elina Laanto, Katja Neuvonen, MSc, Marjut Paljakka, MSc, Mr Petri Papponen, Dr. Katja Pulkkinen, and Dr. Ilona Rissanen, for assistance in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The fish experiments were conducted according to the Finnish Act on Use of Animals for Experimental Purposes, under permission ESAVI-3940/04.10.07/2015 granted for L-RS by the National Animal Experiment Board at the Regional State Administrative Agency for Southern Finland.
Funding Information
This work was funded by Academy of Finland grant nos. 252411 (the Centre of Excellence in Biological Interactions 2012–2017) and 272995 (for L-RS), Maj and Tor Nessling Foundation, and the Doctoral Programme in Biological and Environmental Science (University of Jyväskylä). The funders had no role in study design, data collection, and interpretation, or the decision to submit the work for publication in Microbial Ecology.
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 306 kb)
Rights and permissions
About this article
Cite this article
Penttinen, R., Kinnula, H., Lipponen, A. et al. High Nutrient Concentration Can Induce Virulence Factor Expression and Cause Higher Virulence in an Environmentally Transmitted Pathogen. Microb Ecol 72, 955–964 (2016). https://doi.org/10.1007/s00248-016-0781-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-016-0781-1