Abstract
The community ecology, abundance, and diversity patterns of soil archaea are poorly understood—despite the fact that they are a major branch of life that is ubiquitous and important in nitrogen cycling in terrestrial ecosystems. We set out to investigate the elevational patterns of archaeal ecology, and how these compare with other groups of organisms. Many studies of different groups of organisms (plants, birds, etc.) have shown a series of distinct communities with elevation, and often a diversity maximum in mid-elevations. We investigated the soil archaeal communities on Mt. Norikura, Japan, using 454 pyrosequencing of the 16S ribosomal RNA (rRNA) gene. There was a strong mid-elevation maximum in diversity, and a mid-elevation maximum in abundance of soil archaea 16S rRNA and amoA genes. These diversity and abundance maximums could not be correlated with any identifiable soil parameter, nor plant diversity. Discrete, predictable communities of archaea occurred at each elevational level, also not explicable in terms of pH or major nutrients. When we compared the archaeal community and diversity patterns with those found in an earlier study of Mt Fuji, both mountains showed mid-elevation maximums in diversity and abundance of archaea, possibly a result of some common environmental factor such as soil disturbance frequency. However, they showed distinct sets of archaeal communities at similar elevational sampling points. Presumably, the difference reflects their distinct geology (Norikura being andesitic, while Fuji is basaltic) and the resulting combinations of soil chemistry and environmental conditions, although no explanatory variable was found. Clearly, many soil archaea have strongly defined niches and will only occur in a narrow subset of the range of possible climate and soil conditions. The findings of a mid-elevation diversity maximum on Norikura provides a further instance of how widespread this unexplained pattern is in nature, in a wide variety of groups of organisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Archaea [1] are now known to be ubiquitous in terrestrial environments [2–5]. It is remarkable that, as one of the three main branches of life on Earth, Archaea are so poorly understood in terms of their biology and ecology [6,7]. Partly, this is due to the difficulty of culturing them, as only a relative handful of archaeal species have been successfully grown in isolation [6,7]. Diversity and community composition of Archaea in nature can be studied using molecular methods, but there has been relatively little effort so far devoted to studying them. Hence, the nature of the niche, and the factors structuring communities, geographic distributions, and diversity of Archaea are still very poorly known. Despite being poorly understood in terms of their community ecology, there are indications that Archaea play a major role in terms of biogeochemical processes. In soil, Archaea are now thought to plant a major role in oxidizing ammonia to forms that make soil nitrogen more available to plants [8]. Hence, most work on soil Archaea to date has concentrated on their role as ammonia oxidizers [9–14]. There is strong evidence that most Archaea in mesic soils [15,16] are ammonia oxidizing archaea (AOA) belonging to the phylum Thaumarchaeota [17].
Here, we chose to study soil archaeal community variation for the following reasons:
-
Archaea are a major and ubiquitous branch of life whose community ecology, niche structuring, diversity, and biogeography is still poorly understood. Curiosity about the natural world leads us to explore these patterns.
-
It is interesting to understand whether soil Archaea follow the same general rules of community structure and diversity as other groups of organisms such as plants, animals, bacteria, and fungi. Essentially, this is a search for universality in patterns and general rules in ecology.
-
Understanding patterns in community structure and abundance of Archaea may give clues to patterns in their relative importance as a source of oxidized nitrogen in land ecosystems, and the extent to which this may be susceptible to environmental changes.
This particular study concentrated on a comparison of two mountain systems, one previously studied [24], the other newly sampled and studied, and offering a comparison and investigation of whether there are consistent general patterns in soil archaeal ecology. Mountain systems and their elevational gradients offer a promising study system for dissecting the components of community ecology and diversity [18–25]. Elevational gradients offer a more accessible substitute for geographical gradients, because their climate zones are so “compressed,” into a few kilometers vertically rather than thousands of kilometers horizontally; nevertheless, they may well give clues to the underlying factors which influence geographical-scale gradients.
Ecological studies on mountains around the world so far have mostly focused on eukaryotes, which have frequently been found to show mid-elevational diversity maxima [8]. The causes of such mid-elevational diversity maxima remain debatable but the most commonly invoked explanations are the mid-domain hypothesis [26,27], the intermediate disturbance hypothesis [28], and the intersecting effects of climate and ecosystem productivity [29–31]. It is also clear from many studies of how community composition varies with elevation [32,33], that niches are quite finely divided with respect to the climate or soil factors that vary with elevation—emphasizing the precision of environmental adaptation. Even if individual taxa occur across several elevational zones, their relative abundances clearly vary in predictable ways, constructing distinctive communities.
Most previous research on elevational diversity gradients in microbes has focused almost exclusively on bacterial taxa or particular functional groups of bacteria and archaea [14,34–40]. To our knowledge, there are no studies of archaeal elevational diversity that have thoroughly surveyed whole archaeal communities with replicated sets of samples across a wide elevational gradient, with the exception of our earlier study of Mt. Fuji [18]. Given the few existing studies of microbial communities in montane systems (and especially Archaea) it is important to add new work, to approach a better understanding of underlying mechanisms behind microbial patterns,. It is only when more observations of actual patterns of soil Archaea have been made (including extra mountain studies to test the generality of patterns), that a theoretical framework for archaeal richness/diversity on mountains can be formulated and discussed in broader ecological terms.
Earlier work by the present authors on Mt. Fuji, Japan, revealed strong elevational gradients in soil archaeal community composition, and a “maximum” in diversity in the mid-elevations [18]. This maximum was suggested as possibly being the result of intermediate levels of disturbance in this zone from frost heave and rain wash. The “intermediate disturbance principle” is already seen as being of great importance in the ecology of sedentary organisms such as trees, meadow plants, and corals [19]. Essentially, it suggests that a certain amount of disturbance (e.g., from soil or sediment movement, or fire) randomly knocks back populations of all organisms in the community, allowing a phase of recovery in which resources are abundant and competition is not intense. This allows relief from competitive exclusion by the strongest competitor species, allowing many species of weaker competitors to persist indefinitely in the community if the disturbance frequency is about optimum [28]. However, if disturbance occurs too frequently, or disturbance events are each too severe, very few species have the correct evolutionary pre-adaptions to maintain their populations in the face of frequent bottlenecks. Hence, there is lower diversity overall, despite the relative lack of competitive exclusion. Along a gradient of disturbance frequency or intensity, the result is a “humpback” diversity curve in the middle part of the gradient. This pattern has not yet been substantiated for microbes, but we suggested that it might be the cause of a mid-elevation diversity maximum on Fuji [18,40].
As an alternate hypothesis, we suggested the mid-elevation diversity maximum on Fuji could be the result of mixing of archaeal types from a sparsely vegetated upper zone with those from a densely vegetated lower zone.
It would certainly be premature to draw general conclusions from our single previous study of Archaea on Fuji [24]. As with all aspects of ecology, there is a need for replicate studies on separate systems or different locations, which might either support or cast doubt upon the generality of the pattern observed on Fuji.
The present study adds new work on the soil archaeal communities of Mt. Norikura, whose climate is very similar to Mt. Fuji but which differs strongly in terms of its geology, offering detailed comparison with Mt. Fuji. We also add the perspective of quantitative PCR (qPCR) of archaeal 16S and amoA gene abundance on Fuji as well as Norikura, a factor which was not studied in the previous paper.
In this study, we set out to test following assumptions based upon what is already known of archaeal ecology and ecological theory in general:
-
(1)
That Mt. Norikura will show consistent differences from Mt. Fuji in its archaeal communities, due to its differing geology and resulting overall differences in soil chemistry. We anticipated that the effects of soil chemistry would also result in differences in archaeal community between the two mountains, even though archaea may be expected to differ in relation to climate/elevation, given different geological history on the two mountains.
-
(2)
That on each mountain, there will be characteristic communities that cluster according to elevational zone. In our earlier study [24], we found that on Fuji, there are relatively discrete, characteristic communities of archaea which occur repeatedly at each elevational zone. We hypothesized that other mountains, in this latter case, Norikura, sampled by elevation zone would also show strong evidence of differentiation of archaeal communities by elevational zone, emphasizing the generality of fine adaptation of archaeal niches to climate-related factors.
-
(3)
That despite differences in overall community composition, Norikura—like Fuji—will show a similar maximum in archaeal diversity in its mid-altitudes. We predicted that, despite differences in archaeal community composition between the two mountains because of the expected soil differences, that underlying selective processes in community structuring would produce parallel trends in community composition and diversity. We predicted that the mid-elevational diversity maximum would occur on Norikura as it does on Fuji, despite the differences in soils and history. We anticipate that this will be due to intermediate levels of disturbance or merging of upper and lower montane communities. These factors will result in a maximum in between the upper and lower regions of the mountain. If so, the common pattern would be a first step towards revealing general rules in archaeal ecology.
-
(4)
That archaeal abundance (measured as either 16S or amoA gene abundance) will also show a correlation with elevation on both Norikura and Fuji. We expected that there would be a decline in overall archaeal abundance with elevation [27], due to lower primary productivity on higher elevations, resulting in less litter production and decomposition which ultimately provide the nutrient and substrate for archaeal mineralization.
Methodology
Site Description
Mt. Norikura—the main site of new data presented in this paper—is a mountain of volcanic origin in central Japan, reaching 3026 meters above sea level (masl). Mt Fuji, against which the new data are contrasted, reaches 3776 masl. Both mountains have a cool temperate monsoon climate at their base at 1000 masl, with a mean annual temperature (MAT) of 9.9 °C for Fuji and 8.5 °C for Norikura, and mean annual precipitation (MAP) around 2100 mm (Fuji) and 2206 mm (Norikura), maximizing in summer (Supplementary Table S1: major climate parameters of Fuji and Norikura) [23,41]. Meteorological station data at higher elevations are lacking, but based on a mean lapse rate of 0.6 °C/100 masl [42], Fuji has a summit MAT of −5.3 °C, while Norikura’s somewhat lower summit will have a MAT around −3.5 °C.
Both Fuji and Norikura are of Quaternary age, with a late Quaternary volcanic cover [43]. Norikura’s volcanic cover is about 10,000 years old, but its lower parts, around 1000 masl, consist of Quaternary granite. Fuji’s volcanic cover is mostly around 15,000 years old, but the upper part, about 2500 masl, is younger, dating to around 6000 years ago. Fuji is a typical strato-volcano of entirely basaltic composition, while Norikura consists of more silica-rich andesitic material [44,45]. Neither volcano is currently active.
Vegetation
On their lowermost slopes, both Fuji and Norikura are covered by typical Japanese cool temperate forest, with a mix of mostly deciduous angiosperm trees and evergreen conifers [46–48]. This gives way to boreal-type conifer and birch forest in mid-elevations, with the forest cover thinning out above this level. The upper parts of Norikura are open pine scrub with a variety of small woody shrubs and herbaceous plants such as grasses occupying open ground. Fuji is similar to Norikura up to its mid-elevations, but its upper elevations are open lava fields with a sparse herbaceous vegetation cover, and no vegetation cover near the summit. Vegetation surveys have shown that both mountains have a steady decrease in tree and herbaceous plant species richness with elevation [46,48]. On both mountains, the vegetation cover above 1500 masl is little disturbed by humans, except in some areas right at the summit (we avoided sampling these areas). Vegetation below 1500 masl on both mountains is mostly secondary forests or plantations [46,48].
Sampling, Soil Analysis and DNA Extraction
Sampling on Norikura, as on Fuji in the earlier study, took place at the maximum of the summer warm season, during the last week in July 2010—a time when microbial activity at all elevations can be expected to be at around its maximum. Sampling on each mountain, respectively, was completed within a single day, along a broad transect. On Fuji, this was a north-eastern transect that paralleled the main Subaru line road and hiking trail system, whereas Norikura was sampled on its eastern slope along the Ecoh line and hiking trail. Sampling procedure and the soil analysis and DNA extraction procedure on Mt. Fuji was carried out as previously described by [24] and the same methodology was followed for the Mt. Norikura samples.
Quantitative PCR Analysis
The copy numbers of 16S ribosomal RNA (rRNA) and amoA gene of archaea were measured using real-time quantitative PCR (qPCR). Primer sets Arch-967F/Arch-1060R [49] and Arch-amoAF/Arch-amoAR [17] were used for the amplification of 16S rRNA gene and amoA gene fragments, respectively. Real-time qPCR was performed on an Eco Real-time PCR system (Illumina, San Diego, CA). The 10-μl-reaction volume contained 1 μl of DNA template (stock of 1 ng/μl), 1 μl of each primer (0.2 μM), and 5 μl of PerfeCta SYBR Green Fast Mix (Quanta BioSciences, Inc., Gaithersburg, MD). qPCR programs were as follows: 94 °C for 5 min, followed by 40 cycles of denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s (for 16S rRNA gene) and at 53 °C for 1 min (for amoA gene), and extension at 72 °C for 1 min. The amplification was followed by a melting curve analysis. Plasmids carrying the targeted gene (16S rRNA and amoA gene) were constructed by cloning the targeted gene fragments into plasmid pGEM-T Easy Vector (Promega, USA). Plasmid DNA was extracted using AxyPrep™ Plasmid Miniprep Kits (Axygen, USA) and the concentrations were determined on a NanoDrop ND-2000 spectrophotometer and the copy numbers of target genes were calculated directly from the concentration of the extracted plasmid DNA. Tenfold serial dilutions of the linearized plasmid DNA were applied to real-time PCR in triplicate to establish a standard curve for each gene. Amplification efficiencies were 90 and 93 % for 16S rRNA and amoA gene, respectively. Copy numbers per nanogram DNA were calculated based on the standard curves, and then transformed into gene copy numbers per gram of soil using the known DNA amount retrieved per gram of soil.
PCR Amplification and Pyrosequencing
PCR amplification used bar-coded primers targeting the V1 to V3 region of the 16S rRNA gene, with PCR conditions and primers as previously described by Hur et al. [50]. Triplicate PCR reactions were performed in 50-μl reactions, pooled, purified, and quantified before sending 50 ng for each sample combined in a single tube and sent to ChunLab Inc. (Seoul, Korea) for pyrosequencing using Roche 454 GS FLX Titanium platform.
Processing and Pyrosequencing Data and Taxonomic Analysis
The sequence data obtained after pyrosequencing were processed following Mothur platform’s 454 SOP analysis example [51]. To begin with, sff files obtained after pyrosequencing were used to extract the fasta and quality files. Sequences shorter than 150 nt with homo-polymers longer than 8 nt and all reads containing ambiguous base calls were also removed. Next, the sequences were aligned against the EzTaxon archaeal database [52] and then screened, so that subsequent analyses were constrained to the same portion of the 16S rRNA gene (V1–V3 region). The remaining reads were pre-clustered (http://www.mothur.org/wiki/Pre.cluster) to remove erroneous sequences derived from sequencing errors and then chimeric sequences were detected and removed using UCHIME [53]. Next, we generated a distance matrix (cutoff of 0.25), before clustering these sequences into operational taxonomic units (OTUs) using average algorithm. Taxonomic classification of each OTU (clustered at ≥97 % sequence similarity) was obtained by classifying alignments against EzTaxon reference archaeal taxonomy and non-redundant nucleotide archaeal databases files [52] at 80 % Bayesian bootstrap cutoff with 1000 iterations. DNA pyrosequences are available under the following GenBank SRA Accession No. SRA050374.1 (Mt. Fuji) and SUB452555 (Mt. Norikura) respectively.
Statistical Processing and Analysis of Results
To assess the relationship between soil archaeal species richness/diversity and elevation, as well as with edaphic factors such as pH, and other soil properties, the number of OTUs and other diversity indices were calculated for samples standardized to 529 reads (size decided by default, set to the smallest sample size) as diversity is directly correlated with the number of sequences collected (Supplementary Table S2). Two samples from Mt. Norikura (1000 and 2469 masl) and one from Mt. Fuji (1000 masl) were removed due to low numbers of reads. Soil archaeal diversity was estimated using non-parametric Shannon index and Faith’s phylogenetic diversity (PD) [54,55]. To assess the relationship of elevation with OTU richness, non-parametric Shannon and Faith’s PD indices of the total archaeal community, we fitted a linear, cubic, or quadratic regression (Sigma plot v10). Relative abundances of the four most abundant sub-phyla [i.e., groups I.1a, I.1b, I.1c (Thaumarchaea) and Thermoplasmata (Euryarchaea)] were also fitted against elevation, extractable ammonium (NH4–N), and pH. Similarly, archaeal 16S rRNA gene and amoA gene abundance (qPCR results) were fitted against elevation, NH4–H, and pH. Goodness of fit was evaluated by means of adjusted R 2 and root mean square error (RMSE).
Community composition (Wisconsin double standardized; Bray-Curtis distance) was analyzed by non-metric multidimensional scaling (NMDS) using the standard metaMDS function in the vegan package in R [56]. The envfit function in the vegan package was then used to fit the environmental parameters on the NMDS ordination, which produces vectors that point in the direction a variable is most rapidly changing and that have lengths proportional to the strength of the correlation between the ordination and a given variable; significance was calculated using 999 permutations. Before applying envfit to the dataset, we looked for redundant edaphic factors using the Varclus procedure in the Hmisc package [57] in the R platform. Since MAT was calculated by using a mean lapse rate of 0.6 °C/100 masl, it showed a complete correlation with elevation and thus was removed. Similarly, total carbon (TC) and total nitrogen (TN): (Spearman’s ρ 2 ≥ 0.86; on both the mts) were highly correlated with elevation. NH4–N was the next most correlated (Spearman’s ρ 2 = 0.78) on Fuji and extractable potassium (K) was the next most correlated (Spearman’s ρ 2 = 0.79) on Norikura and therefore, we removed MAT, TC, TN, and NH4–N (Fuji only) and K (Norikura only) from the analysis, and used the remaining environmental variables for further analysis (elevation, pH, K, P205, and NO3–N: Fuji; elevation, pH, K, P205, and NH4–N). Elevation was also plotted as smoothed surfaces across the ordination using the thin-plate splines in generalized additive models (gam) implemented by ordisurf (Vegan) [56].
Finally, to check how much the different environmental variables are delimiting the archaeal community, we used a multiple regression on matrices (MRM) approach [58]. Here, Bray-Curtis and UniFrac dissimilarity matrices (obtained using Mothur) were used as a response matrix along with environmental distance matrices as an explanatory matrix. Euclidean distance matrix of each environmental variable were calculated using Primer-6 [59]. Non-significant factors were removed sequentially and the MRM analysis was repeated until only significant factors were left in the model. Significance was tested by permutations (999) and P values of the two-tailed tests are reported for this analysis.
We performed a non-metric multi dimensional scaling (NMDS) using Primer v6 [59] with the community composition data for both the mountains incorporated into a single file (square root transformed and standardized by total) to explore patterns in species composition using a Bray-Curtis similarity matrix.
To assess if the relative abundance of the four most abundant phyla differed significantly between the two mountains, we performed linear models for normally distributed data (normal), or generalized linear models for non-normally distributed data (non-normal), with site, elevation, and their interaction as factors.
To investigate differences in diversity of archaeal community in relation to vegetation, we again used linear model (normal) and generalized linear model (non-normal), respectively; vegetation was entered as a factor in each model. Subalpine coniferous forest was the only type of vegetation which was common in both the forests. Post hoc Tukey’s HSD test was used for pairwise comparisons when the effect of vegetation was significant.
Results
Broad Taxonomic Features of Archaeal Community on Norikura
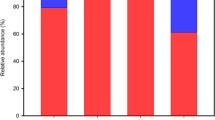
A total of 118,345 quality sequences were classified into 850 OTUs at ≥97 % similarity level, distributed across all 23 samples. On average, 39 OTUs (±2.09 SE) were found in each biological replicate standardized at 529 reads. As expected, Thaumarchaeota was the most abundant phylum, accounting for approximately 97.57 % (115,470 seq) of the total sequences obtained, followed by Euryarchaeota with around 1.95 % (2302 seq) of the total sequences (Supplementary Fig. S1 (B), Supplementary Table S3). Crenarchaeota constituted approximately 0.48 % or 565 sequences (Supplementary Table S3).
The most abundant single phylotype belonged to the group I.1c (Thaumarchaeota) with a total of 24,442 sequences (20.7 %). The most abundant phylotype which could be classified up to species level was AOA Nitrososphaera gargensis, represented by a total of 25 sequences.
Comparison of two Mountains
Pyrosequencing Results
Although the two mountains were quite similar in terms of community composition of the dominant phyla, their elevational distribution of these was considerably different when broken down to sub-phylum level (Fig. 1). On Mt. Fuji, the thaumarchaeal group I.1b was most abundant at higher elevations (2500 masl and above, 99.16 % of reads, Fig. 1) whereas on Mt. Norikura, its abundance was greatest at the lowest elevation (1000 masl, 90.73 %, Fig. 1). Especially noteworthy was that more than half of the archaeal community on Mt. Fuji was composed of group I.1b (56.6 %, 49,126 seq), whereas it occupied a much smaller portion of the community on Mt. Norikura (30.03 %, 35,535 seq). Regarding other thaumarchaeal sub-taxa, group I.1a and I.1c were more abundant on Mt. Norikura (group I.1a ~ 17.9 %, 21,179 seq; group I.1c ~ 49.7 %, 58,755 seq) as compared to Mt. Fuji (group I.1a ∼7.0 %, 6075 seq; group I.1c ∼32.6 %, 28,289 seq).
The most abundant single phylotype on Mt. Fuji was from group I.1b (Thaumarchaeota) with a total of 33,763 sequences (around 38.9 %) whereas on Mt. Norikura, the most abundant phylotype was from group I.1c (Thaumarchaeota). Most abundant phylotypes which could be classified up to species level, on both mountains, were AOA from same genus Nitrososphaera but belonging to different species on the two different mountains, being Nitrososphaera. viennensis represented by only 55 sequences in total on Mt. Fuji.
Comparison of the relative abundance for the four most abundant classes between the two mountains revealed certain striking and consistent differences (Fig. 1, Supplementary Table S3). On Mt. Fuji, thaumarchaeal group I.1b was more abundant (pyrosequencing results) than on the Mt. Norikura but was explained by elevation as a factor (F 4, 46 = 5.14, P < 0.01) rather than site. The interaction between site and elevation was also highly significant (F 4, 46 = 22.78, P < 0.0001). Similarly, euryarchaeal class Thermoplasmata was also more abundant on Mt. Fuji which was significantly explained by an interaction between site and elevation (F 4, 46 = 7.55, P < 0.001). High abundance of thaumarchaeal group I.1c on Mt. Norikura was significantly explained by elevation (F 4, 46 = 5.61, P < 0.01). The interaction between site and elevation was also significant for group I.1c (F 4, 46 = 33.72, P < 0.0001). Finally, for thaumarchaeal group I.1a the interaction between site and elevation was significant (F 4, 46 = 8.93, P < 0.0001), (See Fig. 1).
The results also show a significant maximum of diversity/richness with elevation along the elevational gradient on the two mountains (Fig. 2). On Mt. Fuji, there was a maximum in richness in mid-elevations at around 1500 masl (27.7 % of total OTUs, 140/505 OTUs) whereas for Mt. Norikura, this maximum was observed at 2000 masl (49.1 % of total OTUs, 136/277 OTUs) (Fig. 2).
Relationship between elevation and phylotype richness (a), phylogenetic diversity (b), and phylotype diversity (c) for the whole archaeal community on Mt. Fuji (top) and Mt. Norikura (bottom). We tested three models (linear, quadratic, and cubic) to describe the relationships and model selection was carried out based on adjusted R 2 and RMSE (root mean square error; value not shown). Significance level is shown at ***P < 0.001; **P < 0.01; and *P < 0.05
The results show that the archaeal communities were strikingly different between different elevational zones on each of the mountains. When we further examined the percent relative abundance of the four most abundant archaeal phyla individually, most of them were significantly correlated with elevation on both mountains. But unlike the consistently similar trends found earlier for the whole community on both mountains, with prominent maximums at mid-elevations (Fig. 3), sub-phyla behaved differently along transect. Soil pH was also significantly correlated with the relative abundance of three sub-phyla (thaumarchaeal groups I.1b and I.1c and euryarchaeal Thermoplasmata) but only on Mt. Fuji except for group I.1b, which correlated with pH on both mountains (Supplementary Fig. S2).
An NMDS performed on the Bray-Curtis similarity matrix, calculated from the total community, assesses changes in archaeal community composition, incorporating both taxon abundance and identity. The NMDS showed that composition of the archaeal communities was highly variable between different elevations (Fig. 4, ANOSIM results ∼ Fuji r 2 = 0.85, P = 0.001; Norikura r 2 = 0.82, P = 0.001). Both mountains showed significant variability in community composition along the elevational gradient; however, the clustering pattern observed along the two transects was somewhat different. Norikura’s lower elevational samples were somewhat more clustered into a single group whereas Fuji’s samples were spread into small clusters (each representing a single elevational level) that were spaced out from one another more evenly throughout the gradient.
MRM was able to explain a significant portion of the variability in archaeal community composition on both mountains (Fuji, Bray-Curtis: R 2 = 70 %, P = 0.001, UniFrac: R 2 = 47 %, P = 0.001; Norikura, Bray-Curtis: R 2 = 58 %, P = 0.001, UniFrac: R 2 = 59 %, P = 0.001) (Fig. 5). Elevation along with pH explained a significant amount of the variation on both mountains (Table 1, Fig. 5) whereas K concentration had significant effect on the soil archaeal community separately on Fuji and NO3–N concentration on Norikura, respectively (Table 1).
NMDS ordination illustrating dissimilarity of community composition between samples of different elevational gradients overlaid with a smooth fitted vectors of significant environmental variables and contours showing the elevational gradient fitted using a generalized additive model to the ordination plot using function ordisurf in R. Fuji (a); Norikura (b)
qPCR Results
Quantification of Archaeal 16S rRNA and amoA Genes
Quantification of 16S rRNA and amoA genes revealed high copy numbers; higher 16S rRNA gene copies were found at mid-elevations for both mountains (Fuji 1.02E + 04 to 1.05E + 08 copies g−1 soil; Norikura 8.86E + 05 to 2.19E + 08 copies g−1 soil). A statistically significant humpbacked trend in abundance was seen when archaeal abundance was plotted against the elevation on both Fuji (r 2 = 0.42, P < 0.001) and Norikura (r 2 = 0.70, P < 0.001; Fig. 6, top). For archaeal amoA gene, higher abundance was found at mid-elevations for Mt. Fuji only (0 to 9.39E + 05 copies g−1 soil) with three out of five biological replicates at 1000 masl giving negative amplification (Fig. 6, bottom). On Mt. Norikura for archaeal amoA gene, higher abundances were instead found at lowest and higher elevation samples (3.90E + 04 to 3.06E + 06 copies g−1 soil). It appeared that archaeal amoA gene abundance was loosely following the abundance of thaumarchaeal group I.1b (Supplementary Fig. S3).
Effect of Vegetation
Our results (Fig. 7) showed that irrespective of elevation, vegetation type had a significant effect on phylogenetic diversity (F 7, 52 = 4.39, P < 0.001), Shannon index (F 7, 52 = 10.58, P < 0.0001), and number of OTUs (F 7, 52 = 3.08, P < 0.05).
a Archaeal Richness (OTUs). b Diversity (Shannon Index). c Phylogenetic diversity (Faith’s PD) for the main vegetation types sampled at both mountains. Mean ± SD. A temperate mixed deciduous, B Montane forest, C subalpine coniferous forest*, D alpine zone, E volcanic desert, F montane deciduous broadleaved forest, G subalpine coniferous forest with dwarf bamboo, H scrub-type forest with alpine dwarf pine. *It was the only vegetation type which was common between both the mountains
Discussion
Differences Between the two Mountains, Norikura and Fuji
The two mountains showed distinct sets of archaeal communities (Figs. 1 and 4), even at similar elevations. This is despite the fact that these two mountains have very similar climates [46,47]. Such between-mountain differences in archaeal communities thus likely reflect the different geology and the resulting distinct combinations of soil conditions (Supplementary Fig. S4, Ordination of soil variables). Various soil factors differ between the two mountains, along with elevation (which was highly correlated to TC and TN on both mountains, see VARCLUS results; Materials and Methods section) were able to explain a significant proportion of the variation (Table 1). Soil pH, which is apparently the major factor controlling community composition of soil bacteria and fungi on a broader geographical scale [60,61], also explained a portion of variation on these two mountains, especially on Mt. Norikura (Table 1). We discuss the correlation between soil archaeal abundance patterns and pH in more details in the next section.
What is clear from these between-mountain differences is that soil archaeal niches are sensitive to the overall soil environment beyond pH, such that different geological substrate types will produce different communities under similar climate and pH levels.
Distinct Communities at Each Elevational Level
Both Norikura and Fuji conform to a pattern of distinctive clustered communities of soil archaea being present at each elevational level, even though those communities are different between similar elevations on the two mountains (Fig. 1). Phylum Crenarchaea was found only on Mt. Norikura, and was mostly composed of unclassified and distinctive sequences from the miscellaneous crenarchaeal group (MCG), which are normally abundant in marine environments rather than terrestrial environments [62].
A notable difference between the two mountains could be seen in the distribution of group I.1c (Fig. 1). Thaumarchaeal group I.1c generally has an overwhelming presence in acidic forest soils [4,63] and a previous study [64] observed a massive decline of the abundance of group I.1c with increased pH (between pH 4.5 and 6.0). On Mt. Fuji, this group became less abundant with increasing pH, but on Mt. Norikura, it was more abundant at mid and higher elevations despite the increase in pH (see Fig. 1; on both the mountains, pH and vegetative richness linearly increase and decrease with elevation, respectively; see Supplementary Table S1). This inconsistent response to pH along the elevational gradient could be due to the fact that group I.1c appear to be associated with mycorrhizal hyphae and roots [65], so their distribution might actually be controlled by biotic vegetation factors rather than just soil pH. Also, a recent study by Weber, Lehtovirta, Prosser and Gubry-Rangin [66] reported that there is no evidence of ammonia oxidation by group I.1c while suggesting a heterotrophic lifestyle for the group. Group I.1c could possibly be playing different ecosystem functions unlike other thaumarchaeal groups and their distribution along the elevational transects here is not being simply dictated by a couple of factors like pH and ammonia.
Vegetation cover on the two mountains has a different distribution; on upper elevations of Fuji, there is open lava and ash fields with a sparse herbaceous vegetation cover and no vegetation cover at all near the summit. By contrast, the upper parts of Norikura are open pine scrub with a variety of small woody shrubs and herbaceous plants such as grasses. Our ANOVA results showed that the archaeal OTU richness and diversity differed substantially and consistently among vegetation types on both the mountains irrespective of elevation (Fig. 7).
Another notable difference between the two mountains was the group I.1b composition at different elevations (Fig. 1). Thaumarchaeal groups I.1a and I.1b are usually rare in acidic soils rich in organic matter [4,64,67]. Groups I.1a and I.1b followed this expected pattern along the elevational gradient on both mountains except for group I.1b on Mt. Norikura (Fig. 1; on both mountains, pH and vegetative richness linearly increase and decrease with elevation simultaneously, see Supplementary Table S1). Group I.1b was unusually abundant (>90 % of total archaeal abundance) at the 1000 masl level on Mt. Norikura, which is quite the opposite of what would be expected on an acidic soil rich in organic matter. Due to this inconsistency, we hypothesized that the OTUs shared between this elevational sampling area (N10) and other elevations on both the mountains might be quite different. Indeed, out of the total 68 OTUs at N10, only 25 were shared (2.82 %) with the remaining 843 OTUs from all other samples taken together. Although, these shared OTUs made up for around 86.92 % of total sequences belonging to group I.1b (see supplementary Fig. S5).
The finding of such varied and distinctive soil communities, which nevertheless cluster consistently strongly by elevation on the same mountain, suggests that the niches of many soil archaea may be quite specialized relative to the overall range of soil environments. In the same set of conditions (same elevation, on the same geological substrate), the community is strongly predictable and distinct from communities found elsewhere. Exactly what it is that determines the limits of these niches, whether temperature, moisture content, or some aspect of soil chemistry, is unknown. Even the fundamental mode of living of most soil Thaumarchaea (whether autotrophic, heterotrophic, or a mixed) is little understood, although those successfully cultured so far have all proven to be autotrophic ammonia oxidizers [28,29]. Despite the huge unknowns, such investigation of the ecology of this unexplored group is a necessary step towards understanding them.
Similar Patterns in Overall Diversity Between the two Mountains
Despite the strong differences in community composition, it is intriguing that overall richness at the OTU level, and the diversity (both Shannon and Faith’s PD), is so similar between Norikura and Fuji (Fig. 2).
The strong elevational trend in diversity on each mountain, with a mid-elevational diversity maximum, did not relate to any single identifiable soil parameter but instead to a set of different soil and climatic parameters (Table 1), as well as a proportion that was explained by the plant diversity (see “Effect of Vegetation” section, Fig. 7). Most important, as a predictor of diversity along with pH was temperature, which is highly correlated with the elevation on both mountains. The importance of this factor is not particularly surprising since declining temperatures with increasing elevation can directly influence community properties through limiting metabolism and process rates for soil microbes [68]. Elevational gradients represent natural experiments, which provide information on community responses to variation in temperature at the landscape-scale when most other abiotic factors remain relatively constant [69,70].
It is intriguing that many mountain systems around the world, for many different taxa (plants, birds, etc.), have been found to show a similar mid-elevational diversity maximum [35]. The mid-elevational diversity and abundance maximum for Archaea might reflect intermediate disturbance levels between the less vegetated upper slopes more subject to frost heave and rain wash, and the more stable and densely vegetated lower slopes. “Humpbacked” curves have been observed along disturbance intensity gradients [25]; however, no ready measure of disturbance frequency on these mountains is available, so the role of disturbance as a cause of this trend is conjectural.
Alternatively, the humpbacked trend might relate to elevational trends in ammonia supply in soil. There is evidence that AOA are less abundant in conditions of abundant NH4 supply, due to out-competition by ammonia oxidizing bacteria (AOB) [26]. The low diversity of Archaea at lower elevations on both mountains could relate to a more active ecosystem (higher primary productivity supplying more material for decomposition) which supplies more NH4 to soil, resulting in fewer niches open to AOA. The effect could be similar to the humpback diversity observed within many plant communities along resource gradients (Huston 1994): at the highest levels of nutrients or of growth rate, competition intensifies and fewer species can coexist. In this case, the competition may come from outside the group (AOB).
On both Norikura and Fuji, there was also a mid-elevation maximum in abundance of soil Archaea, as determined by qPCR for the archaeal 16S rRNA. Archaeal 16S abundance was highly predictable by elevational zone, and the maximum of archaeal abundance, as assessed by this criterion, occurred at approximately the same elevation on both mountains. This was despite the quite different communities of Archaea that occur at similar elevations on the two mountains. There was a consistently higher abundance of archaeal 16S rRNA copies compared to amoA gene copy numbers across the elevational gradient.
Although similar to the 16S result, the amoA gene abundance did not follow exactly the same pattern on both the mountains (Fig. 6). Also, unlike 16S, amoA gene abundance did not correlate with ammonium and pH values on both mountains (Fig. 6), except for Fuji, where there was a significant pH relationship. This was contradictory to previous findings where AOA have been proposed to make up the majority of soil archaea [11] and seem relatively more abundant at low pH [15,16] and ammonium concentrations. Although AOA seem to make majority of the soil archaea, another noticeable pattern in our results is that amoA gene abundance with elevation most closely follows the abundance of group I.1b (Supplementary Fig S3) on both the mountains—suggesting that this particular subgroup made up most of the AOA present on these mountains. However, it is necessary to bear in mind that the high abundance of amoA genes at specific elevations does not conclusively prove that there is high AOA activity [8].
Essentially, then, the exact cause of the mid-elevation archaeal abundance maximum on these two mountains is unknown. It is necessary to emphasize again the possibility that the archaeal abundance trend with elevation is produced by a complex combination of environmental gradients working simultaneously on both mountains, including pH, ammonia/ammonium ion concentrations, and other soil nutrients and climate parameters, and also biotic factors such as vegetation. A decrease in soil pH is generally correlated with exponential reduction in ammonia availability, through ionization to ammonium ion [71], which in effect, reduces the growth and activity of ammonia oxidizers at low pH as it decreases NH3 diffusion and increases the requirement for energy-dependent transport of ammonium ion. Acidophilic and near neutrophilic archaeal ammonia oxidizers have been reported as more abundant than AOB under acidic conditions which have a much higher affinity for ammonia as compared to AOB [28,72,73]. This indicates that AOA may be physiologically adapted to ammonia oxidation in environments with low concentrations of ammonia [26,74]. This phenomenon might be at work here as the pH linearly increases on the both the mountains with elevation while making optimal ammonia/ammonium ion concentration at near mid-elevations for the ammonia oxidizing archaeal sub populations, suggesting that the archaeal ammonia oxidizer subgroups have distinct physiological characteristics and ecological niches, with consequences for nitrification in acidic soils.
There also needs to be further work to assess how archaeal abundance, diversity and community composition varies with elevation on different mountains located in a range of geographical regions and climates. As “natural laboratories” of archaeal ecology in relation to environment, understanding mountain systems will offer broader clues to the nature of the archaeal niche and community structure in land systems in general.
General Conclusions
Both the parallels and differences between the archaeal communities of Norikura and Fuji are intriguing. There are many unknowns in soil archaeal ecology, and this study represents an initial exploratory phase. It is however clear that: (1) soil archaeal communities differ between substrate types, in ways that relate to underlying geology. Although pH (which is usually seen as dominating soil archaeal community composition) is a contributing factor, it cannot wholly explain the differences between these two mountains. (2) In each broad set of substrate conditions (i.e., within each mountain), archaeal communities differ between elevational zones, indicating an additional dimension to soil archaeal community ecology. It appears that the niches of many soil Archaea must be finely adjusted to the prevailing conditions, although we cannot at present know what underlying environmental parameters are important.
Equally intriguing is the mid-elevational diversity and abundance maximum, almost identical on both mountains. This suggests some common cause, perhaps intermediate disturbance rate. Finding this same diversity pattern on two different mountains, even when their archaeal communities are quite distinct, implies that this might be widespread among archaeal communities on mountains. It adds to the large number of observations of mid-elevational diversity maxima in different groups of organisms around the world.
References
Woese CR, Kandler O, Wheelis ML (1990) Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A 87:4576–4579
Bintrim SB, Donohue TJ, Handelsman J, Roberts GP, Goodman RM (1997) Molecular phylogeny of archaea from soil. Proc Natl Acad Sci U S A 94:277–282
Buckley DH, Graber JR, Schmidt TM (1998) Phylogenetic analysis of nonthermophilic members of the kingdom Crenarchaeota and their diversity and abundance in soils. Appl Environ Microbiol 64:4333–4339
Oline DK, Schmidt SK, Grant MC (2006) Biogeography and landscape-scale diversity of the dominant crenarchaeota of soil. Microb Ecol 52:480–490. doi:10.1007/s00248-006-9101-5
Ochsenreiter T, Selezi D, Quaiser A, Bonch-Osmolovskaya L, Schleper C (2003) Diversity and abundance of Crenarchaeota in terrestrial habitats studied by 16S RNA surveys and real time PCR. Environ Microbiol 5:787–797. doi:10.1046/j.1462-2920.2003.00476.x
Khelaifia S, Raoult D, Drancourt M (2013) A Versatile Medium for Cultivating Methanogenic Archaea. Plos One 8. doi: ARTN e61563 DOI 10.1371/journal.pone.0061563
Wolfe RS, Metcalf WW (2010) A vacuum-vortex technique for preparation of anoxic solutions or liquid culture media in small volumes for cultivating methanogens or other strict anaerobes. Anaerobe 16:216–219. doi:10.1016/j.anaerobe.2009.11.005
Prosser JI, Nicol GW (2008) Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ Microbiol 10:2931–2941
Offre P, Spang A, Schleper C (2013) Archaea in biogeochemical cycles. Annu Rev Microbiol 67:437–457. doi:10.1146/annurev-micro-092412-155614
Karner MB, DeLong EF, Karl DM (2001) Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409:507–510. doi:10.1038/35054051
Bates ST, Berg-Lyons D, Caporaso JG, Walters WA, Knight R, Fierer N (2011) Examining the global distribution of dominant archaeal populations in soil. ISME J 5:908–917. doi:10.1038/ismej.2010.171
Lipp JS, Morono Y, Inagaki F, Hinrichs KU (2008) Significant contribution of Archaea to extant biomass in marine subsurface sediments. Nature 454:991–994. doi:10.1038/nature07174
Thauer RK (2011) Anaerobic oxidation of methane with sulfate: on the reversibility of the reactions that are catalyzed by enzymes also involved in methanogenesis from CO2. Curr Opin Microbiol 14:292–299. doi:10.1016/j.mib.2011.03.003
Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R (2008) Methanogenic archaea: ecologically relevant differences in energy conservation. Nat Rev Microbiol 6:579–591. doi:10.1038/nrmicro1931
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809. doi:10.1038/Nature04983
Erguder TH, Boon N, Wittebolle L, Marzorati M, Verstraete W (2009) Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiol Rev 33:855–869. doi:10.1111/j.1574-6976.2009.00179.x
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci U S A 102:14683–14688
Bryant JA, Lamanna C, Morlon H, Kerkhoff AJ, Enquist BJ, Green JL (2008) Microbes on mountainsides: contrasting elevational patterns of bacterial and plant diversity. Proc Natl Acad Sci U S A 105:11505–11511. doi:10.1073/pnas.0801920105
Wang JJ, Soininen J, He JZ, Shen J (2012) Phylogenetic clustering increases with elevation for microbes. Environ Microbiol Rep 4:217–226. doi:10.1111/j.1758-2229.2011.00324.x
Shen C, Xiong J, Zhang H, Feng Y, Lin X, Li X, Linag W, Chu H (2012) Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol Biochem. doi:10.1016/j.soilbio.2012.07.013
Singh D, Lee-Cruz L, Kim WS, Kerfahi D, Chun JH, Adams JM (2014) Strong elevational trends in soil bacterial community composition on Mt. Ha lla, South Korea. Soil Biol Biochem 68:140–149
Yang YF, Gao Y, Wang SP, Xu DP, Yu H, Wu LW, Lin QY, Hu YG, Li XZ, He ZL, Deng Y, Zhou JZ (2014) The microbial gene diversity along an elevation gradient of the Tibetan grassland. ISME J 8:430–440. doi:10.1038/ismej.2013.146
Singh D, Takahashi K, Kim M, Chun J, Adams JM (2012) A hump-backed trend in bacterial diversity with elevation on Mount Fuji, Japan. Microb Ecol 63:429–437. doi:10.1007/s00248-011-9900-1
Singh D, Takahashi K, Adams JM (2012a) Elevational patterns in archaeal diversity on Mt. Fuji. Plos One 7. doi: ARTN e44494 DOI 10.1371/journal.pone.0044494
Huston MA (1994) Biological diversity: the coexistence of species on changing landscapes. Cambridge University Press, Cambridge
Verhamme DT, Prosser JI, Nicol GW (2011) Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. ISME J 5:1067–1071
Zhang LM, Wang M, Prosser JI, Zheng YM, He JZ (2009) Altitude ammonia-oxidizing bacteria and archaea in soils of Mount Everest. FEMS Microbiol Ecol 70:208–217. doi:10.1111/j.1574-6941.2009.00775.x
Tourna M, Stieglmeier M, Spang A, Konneke M, Schintlmeister A, Urich T, Engel M, Schloter M, Wagner M, Richter A, Schleper C (2011) Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proc Natl Acad Sci U S A 108:8420–8425. doi:10.1073/pnas.1013488108
Kim JG, Jung MY, Park SJ, Rijpstra WIC, Sinninghe Damste JS, Madsen EL, Min D, Kim JS, Kim GJ, Rhee SK (2012) Cultivation of a highly enriched ammonia-oxidizing archaeon of thaumarchaeotal group I.1b from an agricultural soil. Environ Microbiol 14:1528–1543
Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P (2008) Mesophilic crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol 6:245–252. doi:10.1038/Nrmicro1852
Sanders NJ, Rahbek C (2012) The patterns and causes of elevational diversity gradients. Ecography 35:1–3. doi:10.1111/j.1600-0587.2011.07338.x
Colwell RK, Hurtt GC (1994) Nonbiological gradients in species richness and a spurious rapoport effect. Am Nat 144:570–595. doi:10.1086/285695
Colwell RK, Lees DC (2000) The mid-domain effect: geometric constraints on the geography of species richness. Trends Ecol Evol 15:70–76. doi:10.1016/S0169-5347(99)01767-X
Wilson JB (1990) Mechanisms of species coexistence—12 explanations for Hutchinson paradox of the plankton - evidence from New Zealand plant-communities. N Z J Ecol 13:17–42
Rahbek C (1995) The elevational gradient of species richness: a uniform pattern? Ecography 18:200–205
Grytnes JA (2003) Species-richness patterns of vascular plants along seven altitudinal transects in Norway. Ecography 26:291–300. doi:10.1034/j.1600-0587.2003.03358.x
Wang XP, Fang JY, Sanders NJ, White PS, Tang ZY (2009) Relative importance of climate vs local factors in shaping the regional patterns of forest plant richness across northeast China. Ecography 32:133–142. doi:10.1111/j.1600-0587.2008.05507.x
Rahbek C (2005) The role of spatial scale and the perception of large-scale species-richness patterns. Ecol Lett 8:224–239. doi:10.1111/j.1461-0248.2004.00701.x
McCain CM (2009) Global analysis of bird elevational diversity. Glob Ecol Biogeogr 18:346–360. doi:10.1111/j.1466-8238.2008.00443.x
Fierer N, McCain CM, Meir P, Zimmermann M, Rapp JM, Silaman MR, Knight R (2011) Microbes do not follow the elevational diversity patterns of plants and animals. Ecology 92:797–804. doi:10.1890/10-1170.1
Takahashi K, Azuma H, Yasue K (2003) Effects of climate on the radial growth of tree species in the upper and lower distribution limits of an altitudinal ecotone on Mount Norikura, central Japan. Ecol Res 18:549–558
Huzimura I (1971) The climate and weather of Mt. Fuji. In: Tsuya, H (ed.) Rep of the Scientific Survey of Mt Fuji, Tokyo, pp. 211–345
Nakano S, Fukuoka T, Aramaki S (1987) Trace-element abundances in the quaternary volcanic-rocks of the Norikura Volcanic Chain, Central Honshu, Japan. Geochem J 21:159–172
Tsuya H (1968) Geology of volcano Mt. Fuji. Explanatory text of geological map 1:50,000 scale, Geological Survey of Japan
Nakano S, Otsuka T, Adachi M, Harayama S, Yoshioka T (1995) Geology of the Norikuradake district, quadrangle series. Kanazawa 10, Geol. Surv. Japan (in Japanese with English abstract)
Ohsawa M (1984) Differentiation of vegetation zones and species strategies in the subalpine region of Mt Fuji. Vegetation 57:15–52
Miyajima Y, Takahashi K (2007) Changes with altitude of the stand structure of temperate forests on Mount Norikura, central Japan. J For Res-Jpn 12:187–192
Miyajima Y, Sato T, Takahashi K (2007) Altitudinal changes in vegetation of tree, herb and fern species on Mount Norikura, central Japan. J Veg Sci 24:29–40
Cadillo-Quiroz H, Brauer S, Yashiro E, Sun C, Yavitt J, Zinder S (2006) Vertical profiles of methanogenesis and methanogens in two contrasting acidic peatlands in central New York State, USA. Environ Microbiol 8:1428–1440
Hur M, Kim Y, Song HR, Kim JM, Choi YI, Yi H (2011) Effect of genetically modified poplars on soil microbial communities during the phytoremediation of waste mine tailings. Appl Environ Microbiol 77:7611–7619. doi:10.1128/AEM.06102-11
Schloss PD, Gevers D, Westcott SL (2011) Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS ONE 6
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721. doi:10.1099/ijs.0.038075-0
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. doi:10.1093/bioinformatics/btr381
Hill TCJ, Walsh KA, Harris JA, Moffett BF (2003) Using ecological diversity measures with bacterial communities. FEMS Microbiol Ecol 43:1–11. doi:10.1016/S0168-6496(02)00449-X
Faith DP (1992) Conservation evaluation and phylogenetic diversity. Biol Conserv 61:1–10
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2013) Vegan: community ecology package. R package version 2.0-8
Sarle WS (1990) The VARCLUS procedure. SAS/STAT User’s Guide. SAS Institute, Inc., Cary NC
Legendre P, Lapointe FJ, Casgrain P (1994) Modeling brain evolution from behavior: a permutational regression approach. Evolution 48:1487–1499
Clarke KR, Gorley RN (2006) Primer v6: user manual/tutorials. Primer-E Ltd, Plymouth, UK
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. Proc Natl Acad Sci U S A 103:626–631. doi:10.1073/pnas.0507535103
Rousk J, Baath E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351. doi:10.1038/ismej.2010.58
Kubo K, Lloyd KG, Biddle JF, Amann R, Teske A, Knittel K (2012) Archaea of the miscellaneous crenarchaeotal group are abundant, diverse and widespread in marine sediments. ISME J 6:1949–1965
Timonen S, Bomberg M (2009) Archaea in dry soil environments. Phytochem Rev 8:505–518
Lehtovirta LE, Prosser JI, Nicol GW (2009) Soil pH regulates the abundance and diversity of Group 1.1c Crenarchaeota. FEMS Microbiol Ecol 70:367–376. doi:10.1111/j.1574-6941.2009.00748.x
Bomberg M, Timonen S (2009) Effect of tree species and mycorrhizal colonization on the archaeal population of Boreal Forest Rhizospheres. Appl Environ Microbiol 75:308–315
Weber EB, Lehtovirta LE, Prosser JI, Gubry-Rangin C (2015) Ammonia oxidation is not required for growth of Group 1.1c soil Thaumarchaeota. FEMS Microbiol Ecol 91. doi: http://dx.doi.org/10.1093/femsec/fiv001
Kemnitz D, Kolb S, Conrad R (2007) High abundance of Crenarchaeota in a temperate acidic forest soil. FEMS Microbiol Ecol 60:442–448
Margesin R, Jud M, Tscherko D, Schinner F (2009) Microbial communities and activities in alpine and subalpine soils. FEMS Microbiol Ecol 67:208–218. doi:10.1111/j.1574-6941.2008.00620.x
Sundqvist MK, Liu Z, Giesler R, Wardle DA (2013) Plant and microbial responses to nitrogen and phosphorus addition across an elevational gradient in subarctic tundra. Ecology. doi:10.1890/13-0869.1
Korner C (2007) The use of ‘altitude’ in ecological research. Trends Ecol Evol 22:569–574. doi:10.1016/j.tree.2007.09.006
Frijlink MJ, Abee T, Laanbroek HJ, Deboer W, Konings WN (1992) The bioenergetics of ammonia and hydroxylamine oxidation in nitrosomonas-europaea at acid and alkaline Ph. Arch Microbiol 157:194–199
Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA (2009) Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461:976–U234
Park HD, Noguera DR (2007) Characterization of two ammonia-oxidizing bacteria isolated from reactors operated with low dissolved oxygen concentrations. J Appl Microbiol 102:1401–1417
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2010) Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiol Ecol 72:386–394. doi:10.1111/j.1574-6941.2010.00861.x
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
Archaeal phyla breakdown for Mt. Fuji (a) and Mt. Norikura (b) (GIF 35 kb)
Supplementary Fig. S2
Relative abundance of the four most abundant archaeal sub-phyla on Mt. Fuji (a) and Mt. Norikura (b) (Mean ± SE) in relation to pH. Significance level is shown at ***P < 0.001, **P < 0.01, and *P < 0.05. (GIF 38 kb)
Supplementary Fig. S3
Distribution of Thaumarchaeal group I.1b percent relative abundance (solid line; pyrosequencing results) vs. amoA gene copies per gram of soil (dashed line; qPCR results) in relation to elevation on Mt. Fuji (a) and Mt. Norikura (b). Data from biological replicates at each elevational zones were averaged prior to statistical analysis. (GIF 17 kb)
Supplementary Fig. S4
NMDS plot on the Euclidean distances from normalized environmental variables. (GIF 14 kb)
Supplementary Fig. S5
Venn diagram representing overall overlap of OTUs for Thaumarchaeal Group I.1b (GIF 44 kb)
ESM 1
(XLSX 20 kb)
ESM 2
(XLSX 17 kb)
ESM 3
(XLSX 13 kb)
Rights and permissions
About this article
Cite this article
Singh, D., Takahashi, K., Park, J. et al. Similarities and Contrasts in the Archaeal Community of Two Japanese Mountains: Mt. Norikura Compared to Mt. Fuji. Microb Ecol 71, 428–441 (2016). https://doi.org/10.1007/s00248-015-0681-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-015-0681-9