Abstract
The oral opportunistic pathogen Fusobacterium nucleatum is known to interact with a large number of different bacterial species residing in the oral cavity. It adheres to a variety of Gram-positive bacteria, including oral streptococci via the arginine-inhibitable adhesin RadD. In this study, we describe a novel protein encoded by the predicted open reading frame FN1253 that appears to play a role in interspecies interactions of F. nucleatum, particularly with oral streptococci and related Gram-positive species. We designated FN1253 as aid1 (Adherence Inducing Determinant 1). Expression analyses demonstrated that this gene was induced in F. nucleatum single species biofilms, while the presence of representative members of the oral microbiota known to adhere to F. nucleatum triggered its suppression. Inactivation as well as overexpression of aid1 affected the ability of F. nucleatum to coaggregate with oral streptococci and the closely related Enterococcus faecalis, but not other Gram-positive oral species tested. Furthermore, overexpression of aid1 led to a drastic change in the structure of dual species biofilms of F. nucleatum with oral streptococci. Aid1 function was abolished in the presence of arginine and found to be dependent on RadD. Interestingly, differential expression of aid1 did not affect messenger RNA and protein levels of RadD. These findings indicate that RadD-mediated adhesion to oral streptococci involves more complex cellular processes than the simple interaction of adhesins on the surface of partner strains. Aid1 could potentially play an important role in facilitating RadD-mediated interaction with oral streptococci by increasing binding specificity of F. nucleatum to other microbial species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The oral cavity is a great model system for studying polymicrobial interactions since it is home to over 600 different recognized species of bacteria [1] most of which are considered to be commensal [2]. The microorganisms in the oral biofilm have been categorized into early and late colonizers [3]. Early colonizing species are primarily Gram-positive, able to adhere directly to the tooth surface and form the basal layers of the oral biofilm. Late colonizers are comprised of mostly Gram-negative bacteria, including certain periodontal pathogens such as Treponema denticola, Tannerella forsythia, and Porphyromonas gingivalis as well as others. Bacteria within the oral biofilm, also known as the dental plaque, form a complex network of direct and indirect interactions. The spatial distribution of different bacterial species is important in oral biofilm formation and architecture. Many of the known oral bacterial species do not directly adhere to one another; instead they interact indirectly via their mutual attachment to Fusobacterium nucleatum [4]. F. nucleatum is a Gram-negative, anaerobic fusiform bacterium that has been associated with periodontal disease and a number of systemic diseases [5–11]. It is considered a “bridging organism” due to its ability to form a “colonization bridge” between species that do not directly interact, thus playing an integral role in the formation of a mature dental plaque. The physical attachment between interacting partner species is mediated by specific cellular adhesion proteins localized on their outer membranes. To characterize these important surface features in F. nucleatum on a molecular level, we employed a genetic system that was previously established in our laboratory and led to the discovery of the large outer membrane autotransporter protein RadD, which is required for effective binding to early colonizers [12]. In order to investigate the transcriptional responses of F. nucleatum upon interactions with other species, we conducted microarray analysis of F. nucleatum grown in the presence of representatives from both early and late colonizing species [13]. These microarray data revealed that a small hypothetical protein encoded by FN1253 according to annotation of F. nucleatum ATCC 25586 [14] is induced in F. nucleatum single species biofilms but ubiquitously repressed in the presence of both early and late colonizers. Downregulation of this gene in dual species biofilms was more pronounced upon interaction with early colonizing streptococci.
FN1253 homologues are highly conserved across all fusobacterial species sequenced to date, and with no homologues found in other species for which genome sequences are available, it appears to be unique to fusobacteria. In this study, we investigated the role of FN1253 in microbial interactions involving F. nucleatum. We demonstrated that FN1253, which we denoted as aid1 (Adherence Inducing Determinant gene 1), plays a role in interaction of F. nucleatum with oral streptococci. Aid1 function requires the presence of the previously identified adhesin RadD [12]. To the best of our knowledge, this is the first hypothetical protein in the F. nucleatum genome that has been characterized thus far with a role in interspecies interactions.
Materials and Methods
Bacterial Strains and Culture Conditions
F. nucleatum strains were grown on Columbia agar plates supplemented with 5 % sheep blood or in Columbia broth (Difco, Detroit, MI) under anaerobic conditions (5 % CO2, 5 % H2, 90 % N2). Thiamphenicol (MP Biomedicals, Irvine, CA) at 5 μg/ml was used for selection and maintenance of strains containing the catP determinant. Clindamycin (MP Biomedicals, Irvine, CA) at 0.4 μg/ml was used for selection and maintenance of strains possessing the ermB determinant. Streptococcus sanguinis ATCC 10556 and Streptococcus gordonii ATCC 10558 were grown anaerobically in Todd-Hewitt (TH) broth (BD Difco, Detroit, MI) at 37 °C. Enterococcus faecalis ATCC 19433 was grown aerobically at 37 °C with shaking in Brain Heart Infusion (BHI) (BD Difco, Sparks, MD) broth. Lactobacillus casei ATCC 393 was grown aerobically in the presence of 5 % CO2 in Luria-Bertani (LB) (BD Difco, Sparks, MD) broth supplemented with 1 % yeast extract. Staphylococcus epidermidis ATCC 12228 was grown aerobically at 37 °C with shaking at 250 rpm in Tryptic Soy Broth (TSB) (BD Difco, Sparks, MD). Veillonella atypica PK1910 was grown anaerobically in TH broth (BD Difco, Detroit, MI) supplemented with 0.06 % lactic acid at 37 °C. P. gingivalis W50 was grown in Columbia broth anaerobically at 37 °C. T. denticola ATCC 35405 was grown in TYGVS anaerobically at 37 °C as previously described [15]. T. forsythia ATCC 43037 was grown in NAM medium (ATCC) anaerobically at 37 °C.
Mutant Construction
In this study, we used F. nucleatum ATCC 23726, which in contrast to ATCC 25586, can be genetically modified [16]. FN1253 is annotated as HMPREF0397_0433 (GenBank ID: ADVK00000000.1, NCBI BioProject Accession: PRJNA31471 ID: 31471) in F. nucleatum ATCC 23726. The Δaid1 inactivation strain was constructed by double homologous recombination (Supplemental Fig. 1A). A sequence including aid1 and ~600–700 bp flanking regions (that contain only truncated portions of the respective upstream and downstream genes) was amplified from wild-type F. nucleatum ATCC 23726 strain with Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA) using forward (5′-TTTATTAAAACTTATGGGAGATAGATA-3′) and reverse (5′-TCCAGAAGGAAAACAACCATCA-3′) primers and subcloned into the pJET 2.1 cloning vector (Fermentas, Inc, Glen Burnie, MD, USA) to obtain construct pIP-aid1 (Supplemental Fig. 1A). The catP gene was amplified from plasmid pHS31 [16] using forward (5′-GTCACAGGATCCCAGTCGAAGTGGGCAAGT-3′) and reverse (5′-GTCACCGGATCCCCGTATTTCTACGATGTTTTTGC-3′) primers and subcloned into the pJET 2.1 vector. catP was excised with BamHI and ligated into a pIP-aid1 digested with BamHI resulting in an insertion at nucleotide 38 within the aid1 gene. The resulting plasmid pIP-aid1::catP was verified by restriction analysis and polymerase chain reaction (PCR). The plasmid was linearized with ScaI prior to transformation into F. nucleatum. For overexpression of aid1 in F. nucleatum, we constructed plasmid pEP-aid1 as follows: The fragment carrying aid1 including the upstream and downstream regions described above was excised from pIP-aid1 using XbaI and XhoI and ligated into linearized pHS58 shuttle plasmid (Haake, personal communication) that carries an ermB erythromycin/clindamycin resistance cassette to generate the pEP-aid1 expression vector. Plasmid pEP-aid1 was transformed into wild-type F. nucleatum strain ATCC 23726 as well as its derivative lacking radD according to previously described protocols [17] to yield Fn/pEP-aid1 and ∆radD/pEP-aid1, respectively.
Transcriptional Analysis
Genomic DNA was extracted from stationary-phase cells following standard protocols and used for generating reference standard curves. Total RNA was extracted using High-Pure RNA Isolation Kit (Roche, Palo Alto, CA, USA) according to the manufacturer’s instructions. Two micrograms of total RNA was used for cDNA synthesis using Transcriptor Universal cDNA Master (Roche, Palo Alto, CA, USA) following the manufacturer’s protocol. For quantitative real-time PCR (qRT-PCR), SYBR green (Bio-Rad, Hercules, CA, USA) was used for fluorescence detection with the iCycler real-time PCR system (Bio-Rad), according to the manufacturer’s instructions. aid1 cDNA was amplified using 5′-TACAGGAGGTGCCGTAGCAG-3′ forward and 5′-TTTTTGTTAATTCTCCAGCTCCA-3′ reverse primers. Expression levels of 16S rRNA were determined using 5′-TTGGACAATGGACCGAGAGT-3′ forward and 5′-GCCGTCACTTCTTCTGTTGG-3′ reverse primers for normalization of the qRT-PCR data.
Coaggregation Assay
Coaggregation assays were performed in coaggregation buffer (CAB) 150 mM NaCl, 1 mM Tris, 0.1 mM CaCl2, and 0.1 mM MgCl2 [18]. Briefly, the cells were collected, washed, and resuspended in phosphate-buffered saline (PBS). Equal numbers of bacterial cells were diluted in CAB or 25 % pooled saliva in ddH2O to a final concentration of 2 × 109 cells ml−1 in a 200-μl clear PCR reaction tube. The tubes were vortexed for 5 s and graded on a 0–4 scale after 10 min based on the degree of coaggregation [19]. Scores were assigned as follows: 0—no visible coaggregation; 1—small aggregates that stay suspended; 2—larger aggregates that settle slowly and leave the supernatant turbid; 3—large aggregates that settle quickly but leave the supernatant turbid; and 4—complete sedimentation with a clear supernatant. No autoaggregation of individual strains was observed in our experimental controls. For coaggregation inhibition assays, 50 mM final concentration of either d-galactose, l-arginine, d-glucose, or N-acetylgalactosamine was added to the reaction tube containing the F. nucleatum strain and mixed by vortexing before the addition of the coaggregation partner strain.
Spectrophotometric Coaggregation Assay
Spectrophotometric coaggregation experiments were performed according to our published procedures [12]. Briefly, the cells were combined in CAB as described above, and after 10 min of incubation, the coaggregation reactions were centrifuged at low speed (100×g) for 1 min to pellet coaggregating cells while leaving the nonaggregated bacteria in suspension. The supernatant was then removed without disturbing the pellet, and the optical density of the recovered supernatant was measured at 600 nm. Relative coaggregation of species A and B was determined by dividing the difference between the total turbidity of each partner strain and the coaggregation supernatant turbidity by the total turbidity of each partner strain using the {[OD600(A) + OD600(B)] − OD600(A + B)} / [OD600(A) + OD600(B)] formula.
Biofilm Growth
Biofilms were grown in eight-well chambered coverglasses (Nunc, Rochester, NY) precoated with 100 μl of 50 % saliva diluted in ddH2O that was centrifuged for 5 min at 10,000×g to remove debris. The chambers were UV-sterilized for 1 h before inoculation. Overnight cultures of F. nucleatum (~107 cells), S. sanguinis, and S. gordonii (~105 cells) were inoculated into the growth chamber wells containing 400 μl of filter-sterilized BHI saliva broth (BHIS—25 % BHI and 25 % saliva). Samples were incubated overnight under anaerobic conditions (5 %H2, 5 % CO2, 90 %N2) at 37 °C. After 22–24 h, samples were fluorescently labeled with the nucleic acid staining dye SYTO9 (Invitrogen, Carlsbad, CA) and visualized using a PASCAL 5 confocal laser scanning microscope (CLSM) (Zeiss, Jena, Germany). The scanning module of the system was mounted onto an inverted microscope (Axiovert 200M), and the samples were viewed through a ×40 oil immersion objective (Achroplan/N.A. 1.3). Excitation of 488 nm with an argon laser in combination with a 505–530-nm bandpass emission filter was used for SYTO9 fluorescence imaging.
Statistical Analysis
Student’s t test was performed using Excel 2010 (Microsoft, Seattle, WA).
Results
Confirmation of the aid1 Gene Expression Profile
Previous microarray analysis in F. nucleatum strain ATCC 23726 revealed a unique expression pattern for an ORF that corresponds to FN1253 in the published genome of F. nucleatum strain ATCC 25586 [13]. F. nucleatum ATCC 23726 was used for further characterization since it can be genetically manipulated [16]. Homology searches with the recently published draft genome of F. nucleatum ATCC 23726 confirmed the presence of the corresponding ORF (HMPREF0397_0433) in this strain, albeit the original annotation indicated a truncated version of the protein. Analysis of flanking sequences revealed that HMPREF0397_0433 exhibits 100 % homology with FN1253 indicating a mis-annotation in the ATCC 25586 genome, where the protein is missing the N-terminal portion.
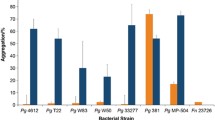
Expression of FN1253 was induced in single species biofilms and repressed in the presence of representative species of both early and late colonizers [13]. Conditions tested included dual species biofilms of F. nucleatum ATCC 23726 with S. sanguinis ATCC 10556 and S. gordonii ATCC 10558 representing early colonizing streptococci, as well as T. denticola ATCC 35405 and T. forsythia ATCC 43037 as representatives of Gram-negative late colonizing species. The microarray data were validated via qRT-PCR with aid1-specific primers, which confirmed the differential regulation of aid1 in single and dual species biofilms (Fig. 1). Repression of aid1 was most pronounced upon interaction with the streptococci.
Differential expression of aid1 in F. nucleatum single and dual species biofilms. F. nucleatum ATCC23726 cells were grown under biofilm conditions for 24 h either alone or in the presence of known interacting partners. aid1 gene expression was analyzed by quantitative real-time PCR (qRT-PCR). Three independent experiments were performed
Repression of aid1 is Time and Contact Dependent
Next, to determine whether physical interaction of F. nucleatum with streptococci plays a role in the regulation of aid1, we analyzed the aid1 messenger RNA (mRNA) levels in mixed culture. Cells were mixed together with and without arginine, a known inhibitor of interactions of F. nucleatum with oral streptococci [12], and spun down into a pellet, since only few F. nucleatum cells start forming biofilms at early time points. qRT-PCR revealed a small decrease in aid1 expression after 1 h of incubation (Fig. 2). At the 20-h time point, the expression of aid1 was significantly decreased. When 50 mM arginine was added to the cell mixture, there was no significant gene regulation present at both time points. Neither co-culture with S. sanguinis nor the addition of arginine affected the growth rate of F. nucleatum. Since fusobacterial interactions with streptococci are predominantly mediated by RadD, we performed similar analysis with a F. nucleatum mutant strain lacking this major adhesin. However, inactivation of radD did not have an effect on aid1 levels, while the addition of 50 mM of arginine to the mixture blocked repression of aid1 in a manner comparable to the wild-type parent.
aid1 gene expression is time and touch dependent. aid1 gene expression was monitored by qRT-PCR at early (1 h) and late (20 h) time points when F. nucleatum was coincubated with S. sanguinis after being pelleted by centrifugation. Arginine was added as a negative control to block physical interactions between the cells. The data represent the average of three independent experiments
Construction and Basic Characterization of aid1 Gene Inactivation and Overexpression Derivatives
In order to characterize aid1 on a molecular level, we constructed a gene inactivation mutant as well as a strain overexpressing the gene in wild-type F. nucleatum ATCC 23726. Multiple attempts to obtain an inactivation mutant in which the gene was fully deleted were unsuccessful. As an alternative, we disrupted aid1 by inserting a chloramphenicol/thiamphenicol resistance cassette (catP) 38 nucleotides downstream of the gene start site. The mutation was introduced into the F. nucleatum wild-type ATCC 23726 strain via double homologous recombination of a construct carrying the catP gene and the corresponding chromosomal regions (aid1::catP) (Supplemental Fig. 1A). This results in a nonfunctional truncated and out-of-frame final gene product. The overexpression mutant was constructed by introducing an expression vector carrying aid1 under its own endogenous promoter (pEP-aid1) into F. nucleatum ATCC 23726 (Supplemental Fig. 1B). The respective mutant derivatives were selected with appropriate antibiotics, thiamphenicol for the inactivation mutant and clindamycin for the overexpression strain. Inactivation of aid1 was confirmed by PCR (Fig. 3a). Both mutants had growth rates comparable to the wild-type strain (data not shown). Expression levels of aid1 were examined by qRT-PCR and the overexpression strain was found to produce approximately three times more aid1 mRNA than the wild-type parent strain (Fig. 3b).
Analysis and verification of aid1 mutants. a Analysis of Δaid1 mutant strain. Diagram of gene inactivation linear vector introduced into WT Fn ATCC 23726 to obtain the Δaid1 inactivation mutant. Confirmation of insertion into aid1 gene by PCR analysis. Arrows indicate the location of the primers used for PCR amplification. Fragments were amplified from the mutant strain but not from the wild-type control. b Gene expression of aid1 in mutant strains and verification of aid1 overexpression strain. aid1 gene expression was verified using quantitative real-time PCR (qRT-PCR) using aid1-specific primers
aid1 Modulates the Coaggregation Ability of F. nucleatum with Oral Streptococci and Related Species in a RadD-Dependent Manner
The aid1 encoding gene is noticeably repressed in the presence of oral streptococci and Gram-negative late colonizers. We therefore investigated the involvement of aid1 in the ability of F. nucleatum to coaggregate with a wide array of oral bacterial species. Since F. nucleatum does not autoaggregate using standard protocols, we first tested if the lack or overexpression of aid1 would alter this behavior and found that this is not the case (Table 1). We then investigated the ability of the aid1 mutants to interact with other species using a standard coaggregation assay [18]. Equal numbers of cells were added into a tube with coaggregation buffer (CAB) and coaggregation was scored on a 0–4 scale after 10 min as described in the “Materials and Methods” section. The aid1 inactivation strain displayed a reduced ability to aggregate with oral streptococci as well as closely related E. faecalis, while its ability to coaggregate with other Gram-positive and Gram-negative species was not affected. The F. nucleatum strain overexpressing aid1 exhibited an increased aggregation ability with oral streptococci, a feature that was not apparent during interaction with other Gram-positive and Gram-negative oral species (Table 1). Based on this data, FN1253 was designated as aid1 (Adherence Inducing Determinant gene 1). To further characterize the nature of this enhanced interaction with oral streptococci, we performed coaggregation assays with S. sanguinis as representative species in the presence of known inhibitors of fusobacterial interactions with other species as well as carbohydrates that generally do not interfere with coaggregation [20]. Enhanced coaggregation of the aid1-overexpressing mutant derivative with streptococci was only blocked by arginine (Table 2).
We reported previously that an arginine-inhibitable adhesion RadD plays an important role in mediating the coaggregation between F. nucleatum and a variety of Gram-positive oral bacteria, including oral streptococci [12]. To investigate whether Aid1 requires RadD for its function, we introduced the pEP-aid1 plasmid into a radD mutant derivative of ATCC 23726, which is unable to coaggregate and form biofilms with oral streptococci [12]. Despite overexpression of aid1, the lack of radD still abolished coaggregation of F. nucleatum with oral streptococci (Table 1). All coaggregation data were quantified by performing a spectrophotometric coaggregation assay, which verified the differences in coaggregation under the various conditions tested in the visual coaggregation assay (Fig. 4).
Quantitative coaggregation assay between F. nucleatum strains and S. sanguinis. Wild-type F. nucleatum and aid1 mutant derivatives were mixed with equal number of S. sanguinis cells in coaggregation buffer (CAB) and allowed to aggregate for 10 min. OD600 absorption was measured before and after cells were allowed to aggregate. The data represent the average of three independent experiments
aid1 Overexpression Enhances the Ability of F. nucleatum to Form Biofilms with Oral Streptococci and Alters Biofilm Morphology
Since aid1 levels are significantly decreased after 20 h of coincubation with S. sanguinis, we investigated the ability of aid1 mutant derivatives to form biofilms with S. sanguinis. Lack of aid1 did not affect the ability of F. nucleatum to form dual species biofilms with S. sanguinis. The biofilms formed with the aid1 inactivation mutant were indistinguishable from the ones formed by S. sanguinis and wild-type F. nucleatum. In contrast, biofilms produced by the aid1 overexpression mutant in the presence of S. sanguinis were consistently taller and morphologically different. Streptococcal cells were more evenly distributed throughout the height of the dual species biofilm, as opposed to localizing closer to the bottom of the biofilm when grown with wild-type F. nucleatum (Fig. 5).
Biofilm growth and morphology of aid1 mutants with oral streptococci. Dual species biofilms were grown with wild-type F. nucleatum, mutants lacking and overexpressing aid1 together with S. sangunis. Biofilms were grown for 24 h under anaerobic conditions and stained with SYTO9. Sections from 20 μm above the growth surface demonstrate the variation in biofilm morphology as well as the differences in the height of the biofilm. Representative images chosen from three independent experiments are shown
Discussion
In this study, we discovered that a small conserved hypothetical protein in F. nucleatum ATCC 23726, which we designated as Aid1 (Adhesion Inducing Determinant 1) plays a role in fusobacterial interspecies interactions and biofilm formation. According to the corresponding F. nucleatum ATCC 25586 annotation [14], Aid1 is encoded by a homologue of ORF FN1253. This ORF first caught our attention during microarray analysis, since it was significantly induced in F. nucleatum monospecies biofilms and repressed in the presence of other oral bacterial species [13]. We confirmed the expression pattern of aid1 by qRT-PCR and demonstrated a much greater reduction of gene expression in the presence of the Gram-positive oral streptococci compared to Gram-negative species (Fig. 1). Neither inactivation nor overexpression of aid1 had any discernable effect on the general cell physiology, appearance, overall membrane composition or single species biofilm growth (data not shown), and interaction with Gram-negative species (Table 1). Aid1-dependent differences in coaggregation appear to be limited to oral streptococci and closely related species, since coaggregation of the F. nucleatum aid1 mutant derivatives with more distantly related Gram-positive species such as L. casei and S. epidermidis was indistinguishable from the wild-type parent behavior. This apparent differential effect of Aid1 on binding to partner species was especially intriguing considering that F. nucleatum interacts with numerous oral bacterial species and serves as a colonization bridge between species that cannot attach to each other [3, 21]. This unique characteristic of fusobacteria plays a major role in the formation and architecture of oral biofilms, and fusobacterial interactions with other microorganisms have previously been characterized by specific inhibition with a variety of amino acids and carbohydrates [20, 19]. Furthermore, we recently identified the large outer membrane protein RadD as the major adhesin for interactions with a number of Gram-positive oral bacterial species including oral streptococci and demonstrated that the lack of this large outer membrane protein disrupts dual species biofilm architecture [12]. In this study, we found that Aid1-mediated effects are dependent on the presence of RadD (Table 1).
Investigation of Aid1, the novel protein that appears to modulate in particular fusobacterial interactions with oral streptococci and the closely related E. faecalis, led to the hypothesis that interspecies interactions of F. nucleatum are more complex than the simple binding of a fusobacterial surface adhesin with the corresponding adhesin on its partner species. Specifically, we propose that this important bridging organism distinguishes its binding partners by employing large outer membrane proteins for general attachment to larger groups of bacteria and fine-tuning these interactions by providing binding specificity via differential expression of small membrane-associated proteins such as Aid1. The idea that F. nucleatum can distinguish its binding partners is also supported by a previous work on the inhibition of coaggregation, which demonstrated that F. nucleatum interacts with oral streptococci in a multimodal manner since different combinations of inhibitors were necessary to block these interactions [22].
Even though the presence of RadD is required for Aid1 function, both proteins overlap in their involvement in the same process on intercellular interactions, and their gene expression is independent of each other. Increased abundance of aid1 appears to induce the ability of F. nucleatum to bind to oral streptococci without significantly increasing radD mRNA or protein levels (data not shown). Furthermore, expression of aid1 is regulated in a contact- and time-dependent manner that is RadD independent (Fig. 2). Addition of arginine completely blocks all cellular interactions between F. nucleatum and S. sanguinis (Table 2, Fig. 4) even when cells are coincubated in pellet form and abolishes the typical dramatic decrease of aid1 mRNA in the presence of this partner species (Fig. 2), which suggests that cellular contact is required for activation of the signaling pathway resulting in the regulation of the aid1 gene. At the same time, inactivation of RadD, while still dramatic, does not completely eliminate coaggregation between F. nucleatum and S. sanguinis. This observation is more obvious in the more sensitive spectrophotometric coaggregation assay (Fig. 4). Our data suggest that the reduced cell-cell contact in the absence of RadD is still sufficient to trigger the signaling cascade leading to repression of aid1, while the addition of arginine completely blocks all interactions and thus the downstream transcriptional effects. These findings further support that RadD and Aid1 act independently of each other but are involved in the same intracellular interaction process. The aid1 gene seems to be required for initial interaction with oral streptococci, since the Δaid1 mutant displays decreased coaggregation with S. sanguinis ability (Fig. 4, Table 1). Downregulation of aid1 occurs over time, and at the time of initial contact with S. sanguinis, the protein is still present in its unchanged amounts in the cell; therefore, in the coaggregation assay, the lack of Aid1 leads to reduction in binding ability. Based on our data, Aid1 appears to allow F. nucleatum cells to distinguish between different Gram-positive species. RadD, while being the primary adhesin, does not possess the necessary specificity to distinguish between different Gram-positive species including different streptococci, and the interactions rely on additional proteins that facilitate cell-cell recognition and further attachment.
The interactions between different bacterial species are important in the formation and maintenance of oral biofilms. The oral biofilm is a structured bacterial community of cells growing attached directly to tooth and tissue surfaces [23]. Within the oral biofilm architecture, each species occupies a certain niche, which is crucial in the formation and maintenance of the mature plaque. The importance of the spatial distribution of the different species has been described both in vitro [24] and more recently in vivo [25]. Gram-positive species, primarily streptococci, usually occupy the basal layers of the biofilm and are able to directly attach to the tooth surface, while F. nucleatum is distributed throughout the biofilm, providing a scaffold by binding different species. To date, only the fusobacterial adhesin RadD has been shown to play a role in the formation of a dual species biofilm between F. nucleatum and S. sanguinis [12]. Our data indicate that aid1 also appears to play a role in dual species biofilm architecture of F. nucleatum and S. sanguinis. The observed spatial distribution of S. sangunis in a dual species biofilm with F. nucleatum strain overexpressing aid1 (Fig. 5) suggests that the downregulation of aid1 is necessary for the normal formation of the biofilm involving F. nucleatum and oral streptococci and overexpressing the gene that disrupts this natural architecture of the oral biofilm and alters the proper distribution of the streptococcal species. This can have potential detrimental effects on the downstream maturation of the biofilm by not allowing other species, primarily late colonizers, to adhere and integrate into the growing plaque. Inactivation of aid1 had no significant effect on biofilm formation and structure, which is consistent with its role in interactions with oral streptococci and the repression of the gene in the presence of species belonging to this genus.
The aid1 gene is predicted to encode a small hypothetical protein comprised of 67 amino acids. BLAST searches against available databases revealed that it is highly conserved across oral fusobacterial species, while no significant homology to any gene within other species sequenced to date was apparent. Protein analysis tools such as InterProScan (version 4.8), PredictProtein, and SABLE protein structure prediction server predict that Aid1 is membrane-associated based on the presence of a predicted short N-terminal signal sequence, short helices that are indicative of interaction with the cellular membrane as well as a lipoprotein motif at the N-terminal end of the protein followed by a glycine zipper motif. Glycine zipper sequences are common motifs in membrane proteins and are usually found in transmembrane domains [26]. These domains are also thought to be involved in protein oligomerization [27, 28] that may allow Aid1 to form a larger protein complex on the bacterial membrane. While Aid1 is the first small hypothetical protein being characterized in F. nucleatum interspecies interactions, another small fusobacterial membrane protein FadA has been previously identified and characterized for its ability to attach to eukaryotic cells [29]. FadA has been shown to oligomerize and thus form a large protein complex via leucine zipper motifs that allows it to act as an adhesin required in the attachment and invasion of epithelial cells [30].
In summary, the nature of bacterial interactions involving F. nucleatum appears to be more complex than the contact between individual adhesins on the cell surfaces of the partner species. It appears that small proteins like Aid1 are able to define and fine-tune the specificity of large adhesins such as RadD. To the best of our knowledge, aid1 is currently the first hypothetical fusobacterial protein identified to have a specific role in interspecies interactions of this important bridging organism with other species in the oral cavity. It appears to be required by F. nucleatum for recognition and discrimination for Gram-positive oral bacterial species. This study sheds light on the complexity of bacterial interactions in the oral cavity, suggesting that most of these interactions involve more than one adhesin. Bacteria in the oral cavity need mechanisms to identify and select their binding partners to create the large network of interactions that is present in dental plaque. Other proteins may be involved in recognition of different streptococcal groups as well as specific species. Studies are currently underway to understand the deeper implications of aid1 in the formation and maintenance of the oral biofilm as well as identification of other proteins involved in specific interactions of F. nucleatum with different oral bacterial species.
References
Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG (2010) The human oral microbiome. J Bacteriol 192(19):5002–5017. doi:10.1128/JB.00542-10
Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE (2005) Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43(11):5721–5732. doi:10.1128/JCM.43.11.5721-5732.2005
Kolenbrander PE, London J (1993) Adhere today, here tomorrow: oral bacterial adherence. J Bacteriol 175(11):3247–3252
Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ (2002) Communication among oral bacteria. Microbiol Mol Biol Rev 66(3):486–505. doi:10.1128/mmbr.66.3.486-505.2002
Bastos JA, Diniz CG, Bastos MG, Vilela EM, Silva VL, Chaoubah A, Souza-Costa DC, Andrade LC (2011) Identification of periodontal pathogens and severity of periodontitis in patients with and without chronic kidney disease. Arch Oral Biol 56(8):804–811. doi:10.1016/j.archoralbio.2010.12.006
Gonzalez OA, Li M, Ebersole JL, Huang CB (2010) HIV-1 reactivation induced by the periodontal pathogens Fusobacterium nucleatum and Porphyromonas gingivalis involves toll-like receptor 2 [corrected] and 9 activation in monocytes/macrophages. CVI 17(9):1417–1427. doi:10.1128/CVI.00009-10
Hsiao WW, Li KL, Liu Z, Jones C, Fraser-Liggett CM, Fouad AF (2012) Microbial transformation from normal oral microbiota to acute endodontic infections. BMC Genomics 13:345. doi:10.1186/1471-2164-13-345
Lee HR, Jun HK, Kim HD, Lee SH, Choi BK (2012) Fusobacterium nucleatum GroEL induces risk factors of atherosclerosis in human microvascular endothelial cells and ApoE(−/−) mice. Mol Oral Microbiol 27(2):109–123. doi:10.1111/j.2041-1014.2011.00636.x
Okada M, Awane S, Suzuki J, Hino T, Takemoto T, Kurihara H, Miura K (2002) Microbiological, immunological and genetic factors in family members with periodontitis as a manifestation of systemic disease, associated with hematological disorders. J Periodontal Res 37(4):307–315
Sparks Stein P, Steffen MJ, Smith C, Jicha G, Ebersole JL, Abner E, Dawson D 3rd (2012) Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement JAlzheimer Assoc 8(3):196–203. doi:10.1016/j.jalz.2011.04.006
Han YW, Wang X (2013) Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res 92(6):485–491
Kaplan CW, Lux R, Haake SK, Shi W (2009) The Fusobacterium nucleatum outer membrane protein RadD is an arginine-inhibitable adhesin required for inter-species adherence and the structured architecture of multispecies biofilm. Mol Microbiol 71(1):35–47. doi:10.1111/j.1365-2958.2008.06503.x
McHardy IH (2011) Analysis of interspecies interactions of periodontal bacteria using microarrays. Ph D, UCLA
Kapatral V, Anderson I, Ivanova N, Reznik G, Los T, Lykidis A, Bhattacharyya A, Bartman A, Gardner W, Grechkin G, Zhu L, Vasieva O, Chu L, Kogan Y, Chaga O, Goltsman E, Bernal A, Larsen N, D'Souza M, Walunas T, Pusch G, Haselkorn R, Fonstein M, Kyrpides N, Overbeek R (2002) Genome sequence and analysis of the oral bacterium Fusobacterium nucleatum strain ATCC 25586. J Bacteriol 184(7):2005–2018
Ohta K, Makinen KK, Loesche WJ (1986) Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun 53(1):213–220
Kaplan CW, Lux R, Huynh T, Jewett A, Shi W, Haake SK (2005) Fusobacterium nucleatum apoptosis-inducing outer membrane protein. J Dent Res 84(8):700–704
Haake SK, Yoder SC, Attarian G, Podkaminer K (2000) Native plasmids of Fusobacterium nucleatum: characterization and use in development of genetic systems. J Bacteriol 182(4):1176–1180
Cisar JO, Kolenbrander PE, McIntire FC (1979) Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun 24(3):742–752
Kolenbrander PE, Andersen RN, Moore LV (1990) Intrageneric coaggregation among strains of human oral bacteria: potential role in primary colonization of the tooth surface. Appl Environ Microbiol 56(12):3890–3894
Kolenbrander PE, Andersen RN (1989) Inhibition of coaggregation between Fusobacterium nucleatum and Porphyromonas (Bacteroides) gingivalis by lactose and related sugars. Infect Immun 57(10):3204–3209
Whittaker CJ, Klier CM, Kolenbrander PE (1996) Mechanisms of adhesion by oral bacteria. Annu Rev Microbiol 50:513–552. doi:10.1146/annurev.micro.50.1.513
Takemoto T, Hino T, Yoshida M, Nakanishi K, Shirakawa M, Okamoto H (1995) Characteristics of multimodal co-aggregation between Fusobacterium nucleatum and streptococci. J Periodontal Res 30(4):252–257
ten Cate JM (2006) Biofilms, a new approach to the microbiology of dental plaque. Odontology 94(1):1–9. doi:10.1007/s10266-006-0063-3
Kolenbrander PE (1993) Coaggregation of human oral bacteria: potential role in the accretion of dental plaque. J Appl Bacteriol 74(Suppl):79S–86S
Zijnge V, van Leeuwen MB, Degener JE, Abbas F, Thurnheer T, Gmur R, Harmsen HJ (2010) Oral biofilm architecture on natural teeth. PLoS ONE 5(2):e9321. doi:10.1371/journal.pone.0009321
Kim S, Jeon TJ, Oberai A, Yang D, Schmidt JJ, Bowie JU (2005) Transmembrane glycine zippers: physiological and pathological roles in membrane proteins. Proc Natl Acad Sci U S A 102(40):14278–14283. doi:10.1073/pnas.0501234102
Barwe SP, Kim S, Rajasekaran SA, Bowie JU, Rajasekaran AK (2007) Janus model of the Na, K-ATPase beta-subunit transmembrane domain: distinct faces mediate alpha/beta assembly and beta-beta homo-oligomerization. J Mol Biol 365(3):706–714. doi:10.1016/j.jmb.2006.10.029
Plotkowski ML, Kim S, Phillips ML, Partridge AW, Deber CM, Bowie JU (2007) Transmembrane domain of myelin protein zero can form dimers: possible implications for myelin construction. Biochemistry 46(43):12164–12173. doi:10.1021/bi701066h
Han YW, Ikegami A, Rajanna C, Kawsar HI, Zhou Y, Li M, Sojar HT, Genco RJ, Kuramitsu HK, Deng CX (2005) Identification and characterization of a novel adhesin unique to oral fusobacteria. J Bacteriol 187(15):5330–5340
Xu M, Yamada M, Li M, Liu H, Chen SG, Han YW (2007) FadA from Fusobacterium nucleatum utilizes both secreted and nonsecreted forms for functional oligomerization for attachment and invasion of host cells. J Biol Chem 282(34):25000–25009
Acknowledgments
The pHS58 shuttle plasmid was kindly provided by Dr. Susan Kinder Haake. This work is supported by the Whitcome Pre-doctoral Training Grant to AK and NIH/NIDCR grant DE021108 to RL.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Fig. 1
Construction of aid1 mutant strains. A. Construction of Δaid1 inactivation strain. aid1 was inactivated via double homologous recombination by inserting a catP thiamphenicol/chloramphenicol resistance cassette into the aid1 gene. B. Construction of Fn/pEP-aid1 and radD/pEP-aid1 overexpression strains. aid1 was overexpressed in wild type F. nucleatum ATCC 23726 and in radD strains by introducing a shuttle plasmid carrying aid1 gene with its own endogenous promoter into wild type F. nucleatum ATCC 23726 and ΔradD strains respectively. (PPTX 52 kb)
Rights and permissions
About this article
Cite this article
Kaplan, A., Kaplan, C.W., He, X. et al. Characterization of aid1, a Novel Gene Involved in Fusobacterium nucleatum Interspecies Interactions. Microb Ecol 68, 379–387 (2014). https://doi.org/10.1007/s00248-014-0400-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-014-0400-y