Abstract
In fungus-growing termites, fungi of the subgenus Pseudoxylaria threaten colony health through substrate competition with the termite fungus (Termitomyces). The potential mechanisms with which termites suppress Pseudoxylaria have remained unknown. Here we explore if Actinobacteria potentially play a role as defensive symbionts against Pseudoxylaria in fungus-growing termites. We sampled for Actinobacteria from 30 fungus-growing termite colonies, spanning the three main termite genera and two geographically distant sites. Our isolations yielded 360 Actinobacteria, from which we selected subsets for morphological (288 isolates, grouped in 44 morphotypes) and for 16S rRNA (35 isolates, spanning the majority of morphotypes) characterisation. Actinobacteria were found throughout all sampled nests and colony parts and, phylogenetically, they are interspersed with Actinobacteria from origins other than fungus-growing termites, indicating lack of specificity. Antibiotic-activity screening of 288 isolates against the fungal cultivar and competitor revealed that most of the Actinobacteria-produced molecules with antifungal activity. A more detailed bioassay on 53 isolates, to test the specificity of antibiotics, showed that many Actinobacteria inhibit both Pseudoxylaria and Termitomyces, and that the cultivar fungus generally is more susceptible to inhibition than the competitor. This suggests that either defensive symbionts are not present in the system or that they, if present, represent a subset of the community isolated. If so, the antibiotics must be used in a targeted fashion, being applied to specific areas by the termites. We describe the first discovery of an assembly of antibiotic-producing Actinobacteria occurring in fungus-growing termite nests. However, due to the diversity found, and the lack of both phylogenetic and bioactivity specificity, further work is necessary for a better understanding of the putative role of antibiotic-producing bacteria in the fungus-growing termite mutualistic system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In order to survive, organisms need to defend themselves against antagonism from other organisms, while antagonists try to overcome the defence. Thus, there is a continuous need for both organisms to evolve/adapt their strategy: formalised in the Red Queen hypothesis [46]. Defences can be behavioural, immunological or involve mutualisms with defensive symbionts. In the latter case, symbionts provide a benefit for their partner in the form of defence against parasites. Actinobacteria occur as defensive symbionts in several insect species: European beewolves (Philanthus species) harbour Actinobacteria in their antennae, where the bacteria produce antibiotics that help protect the wasp larvae from fungal infections [17, 20]; in Southern Pine Beetles (Dendroctonus frontalis) Streptomyces bacteria selectively inhibit a competitor fungus of the mutualistic fungus of the beetles [30, 40]. However, in other systems where Actinobacteria are known to be present, their ecological role has not yet been established, like in Ambrosia beetles [10] and bark beetles [14]. In fungus-growing ants, one of the two mutualistic symbioses between basidiomycete fungi and social insects (the other being fungus-growing termites), Actinobacteria are typically harboured in special structures on the ant cuticle, for defending their fungal cultivar against mycoparasitic fungi of the genus Escovopsis (Ascomycota: anamorphic Hypocreales) [4–6, 30]. In the case of the fungus-growing termites, the presence and potential role as defensive symbionts of Actinobacteria has never been investigated.
Fungus-growing termites (Blattodea—previously Isoptera: Termitidae: Macrotermitinae) live in mutualistic symbiosis with Termitomyces (Basidiomycota: Agaricales: Lyophyllaceae). This association is responsible for a large amount of plant material degradation in Sub-Saharan Africa and Southeast Asia [15, 19, 23, 25]. Enhanced by the warm, moist and stable climate of the termite mound, Termitomyces aids in degrading the plant material of faecal deposits, shaped into a comb by the termites, and produces nodules (primordial fruiting bodies). The termites eat the nodules and digested parts of this fungus comb—a nitrogen-rich food source compared to the original, often woody, plant material. Fungal cells from the nodules survive gut passage and act as the inocula for newly added comb substrate [24, 39, 53].
Individual nests harbour Termitomyces in monoculture [1, 18, 26, 42], but species of the Xylaria subgenus Pseudoxylaria (Ascomycota: Xylariales: Xylariaceae) are latently present in fungus-growing termite nests [11, 12, 50]. It is possible that other weed or competitor fungi, e.g. Fusarium or Trichoderma spp., may affect fungus-growing termite nests but, to our knowledge, no work has shown that those fungi play a role in the symbiosis (but see [45]). In contrast, fruiting bodies of Pseudoxylaria frequently occur in abandoned termite nests [35, 36], and fungus gardens without termites are rapidly overgrown by species of Pseudoxylaria [38, 45, 50]. Previous experiments have shown reduced growth of Termitomyces when interacting with Pseudoxylaria as well as overlap in carbon source use [49]. Thus, Pseudoxylaria may compete with Termitomyces for the substrate provided by the termites, thereby potentially having a negative impact on fungus garden productivity. Fungus-growing termites are thus predicted to have evolved strategies to suppress Pseudoxylaria within nests.
The presence of termite workers affects the incidence of Pseudoxylaria on the fungus comb, with Pseudoxylaria only appearing when workers are absent, suggesting active suppression of Pseudoxylaria by the termites [42, 48]. Chemical secretions from the termites (e.g. antimicrobial peptides) may be used for this purpose [8, 21], but their effects have not yet been tested on Pseudoxylaria. Consequently, although termite workers suppress Pseudoxylaria, the underlying mechanism by which this is achieved, i.e. weeding/grazing, secretions like antimicrobial peptides, compounds produced by additional symbionts or a combination of several or all of these, has remained unresolved.
Because fungus gardens likely attract exploiters, fungus-growing insects are expected to employ symbionts as defence against parasites [16] and so are fungus-growing termites. Actinobacteria are good candidate defensive symbionts in fungus-growing termites as they are well-known antibiotic producers and occur as defensive symbionts in other insect–fungus symbioses [6, 40]. We address this hypothesis by exploring the presence of Actinobacteria in the three main genera of fungus-growing termites in South Africa. The majority (288 isolates) of the isolated Actinobacteria (360) was screened for their selective antibiotic effect against Pseudoxylaria using a single Pseudoxylaria and Termitomyces strain. In order to explore the specificity of antibiotic effect in more detail, we subsequently tested a subset (53) of the Actinobacteria against four Pseudoxylaria and six Termitomyces strains. We discuss the presence, distribution, specificity and potential of Actinobacteria isolated from fungus-growing termite nests as defensive symbionts in this symbiosis.
Materials and Methods
Colonies Sampled
Termite colonies of Macrotermes natalensis (9), Microtermes sp. (16) and Odontotermes sp. (5) were sampled in January 2010 from two locations in South Africa: Pretoria (S 25°43′47.1″ E 28°14′07.2″, elevation 1,345 m) and Mookgophong (previously Naboomspruit, S 24°40′30.5″ E 28°47′50.4″, elevation 1,045 m). Microtermes colonies were all collected from the walls of Macrotermes mounds. Fungus comb and termites were collected in clean plastic bags, stored at 5°C, and processed within 1 day after collecting. See Supplementary Table S1 for an overview of the sampled colonies and isolated fungal strains.
Microbial Isolations
Isolations for Actinobacteria were made both from termite workers and from fungus comb material. The termites were individually washed in demineralised water (DEMI), and subsequently separated into abdomen and head (including pronotum). Each termite sample was processed separately and thoroughly fragmented and mixed in 700 μl of DEMI. The same procedure was used for fungus comb samples (using about 0.1 cm3 per sample). Bacteria were isolated by plating 350 μl of the mixtures described above on two different selective low-nutrient media: chitin (per litre: 4 g chitin, 0.7 g K2HPO4, 0.3 g KH2PO4, 0.5 g MgSO4·5H2O, 0.01 g FeSO4·7H20, 0.001 g ZnSO4, 0.001 g MnCl2 and 20 g of agar [13]) and microcrystalline (per litre: 5 g microcrystalline and 20 g of agar) medium. Suspensions resulting from the initial wash, one per worker, were plated in the same way, representing bacteria present on the exoskeleton.
Isolates with Actinobacteria-like morphology on these low-nutrient media were transferred to a richer malt yeast extract agar medium (MYA, see [50]), and sub-cultured until pure. This resulted in a total of 360 Actinobacteria isolates, which were morphologically divided into 44 morphotypes (Supplementary Table S2). To assess if morphotype was a good proxy for classifying strains, we amplified a region of the 16S rDNA gene for 35 strains using general primers [8F and 1540R or 27F and 1492R [7, 22]] and previously published DNA extraction and PCR protocols [4, 33]. The obtained PCR products were subsequently direct-sequenced at the University of Wisconsin Biotechnology Center (http://www.biotech.wisc.edu/).
Pseudoxylaria was isolated from hyphal cords or stroma appearing on fragments of ∼15 g of fungus comb that had been incubated for 7–14 days, in the absence of termites, in sealed styrofoam cups with paper tissue soaked in DEMI to preserve humidity. Termitomyces strains were obtained by placing nodules from fresh fungus comb directly onto MYA. In some cases, one or more transfers to new plates were needed to obtain a pure culture.
All incubations of bacteria and fungi were kept in the dark at 25°C.
Screening Bioassay
To explore the antifungal effects of the Actinobacteria isolates, we screened 288 Actinobacteria cultures (selected based on capability to grow on MYA) for their effect against one Pseudoxylaria (P2) and one Termitomyces strain (T1), both isolated from a Macrotermes natalensis nest. The fungal strains belong to the largest clades in their respective phylogenetic trees [2, 28, 50].
Termitomyces mycelium and nodules were placed in an Eppendorf tube with 0.5 ml saline solution (0.8% NaCl w/v), after which the material was fragmented and suspended by mashing and twisting with a pestle. Pseudoxylaria inoculum was grown in Erlenmeyer flasks with ±125 ml of liquid broth (malt 2% and yeast 0.2% w/v). The broth was inoculated with a piece of MYA with Pseudoxylaria mycelium and macerated with a blender to fragment and mix the inoculum. Macerating was repeated 3 and 4 days after inoculation. Fifty microlitres of the mycelium suspensions of either Termitomyces or Pseudoxylaria were used to inoculate bioassay plates (with MYA, diameter 85 mm), and the inoculated suspensions were spread on plates by shaking with 5–15 sterile glass beads (diameter 3 mm). The glass beads were removed and the plates were incubated overnight before Actinobacteria were added; this allowed for plates to dry and prevented Actinobacteria from floating across the plate.
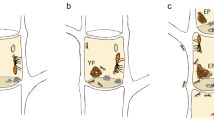
Actinobacteria were inoculated by placing a 3 × 3 ×3-mm cube of 2–3-week old MYA cultures upside-down on the Pseudoxylaria and Termitomyces plates. Groups of five Actinobacteria were tested on each plate, at 10 mm from the edge of the plate and at equal distance from each other (Fig. 3). The effects of Actinobacteria secretions on fungal growth were evaluated 8 days after inoculation of the bacteria. Measurements were done using the edge of the bacterial colony as the point of reference (Fig. 3a). The zone of effect (ZOE) was equal to the distance between the bacteria and the point where the fungus grew normally, and often included a zone where the fungus was completely inhibited (only shown in supplementary tables).
Detailed Bioassay
To further explore the specificity of antibiotic effect, we tested a subset of the Actinobacteria against four Pseudoxylaria and six Termitomyces strains. Actinobacteria strains (a total of 53) were selected based on their effects in the screening bioassay (Supplementary Table S3): a group of 19 bacterial strains with a large effect on Pseudoxylaria but no (or little) effect on Termitomyces (selection P), 21 with a large effect on both Pseudoxylaria and Termitomyces (selection P and T) and 13 that had an effect on Termitomyces but no effect on Pseudoxylaria (selection T).
For both fungi, representative strains from three different termite genera were used: Macrotermes, Microtermes and Odontotermes (Supplementary Table S1). The choice of Pseudoxylaria and Termitomyces strains was based on their respective phylogenetic placement [28, 50]. The methodology for inoculations and for activity assessment was as described above for the screening bioassay.
The bioassays described above are different from those published for other fungus-growing insects [4, 30, 32, 40] in that the target of the candidate defensive symbiont was inoculated on the whole surface of the test plates.
Primary Antibiotic Production Assay
To explore antibiotic effects caused by metabolites produced by the Actinobacteria in the absence of another organism (primary antibiotics), we tested agar plugs obtained in close proximity to Actinobacteria colonies growing in pure culture on one Pseudoxylaria and one Termitomyces strain. This was done simultaneously with the screening bioassay. We randomly chose nine Actinobacteria strains, although only strains with colonies far enough apart to allow plugs being taken without including bacteria could be used. The plugs were placed in the same positions on the fungal plates, and measurements were done in the same way as described in the screening bioassay.
Data Analysis
Isolates were analysed for specificity to their origin (location, host, colony and colony part). A phylogenetic analysis was conducted with the 16S sequences obtained from termite-associated strains (GenBank accession numbers JN409351-385), as well as the top hit for each of the termite strains from a BLASTn search in GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi), and the most closely related type strain for each termite-strain obtained from a type-strain search in the Ribosomal Database Project (RDP; https://rdp.cme.msu.edu/). Actinobacteria strains from Ambrosia beetles ([10]; GenBank accession number GL877172) and Southern Pine beetles ([40]; GenBank accession numbers EU798707-8) were also included. A neighbour-joining (NJ) distance tree [37] was estimated using the software MEGA5.02 [44], after automatic and manual alignment of the sequences, and bootstrap support under maximum likelihood conditions were obtained after 100 pseudo-replications, also using MEGA5.02.
Statistical tests were done in SPSS Inc PASW Statistics version 17. A paired t test with H1: ZOE Pseudoxylaria > ZOE Termitomyces was done to test the hypothesis that Actinobacteria selectively suppress Pseudoxylaria. To test for differences between Actinobacteria with respect to their origin, ANOVA was done for differences in ZOE between termite genera, between fungus comb and different termite body parts, and between isolation media. Student’s t tests were done to further explore the difference in ZOE between Actinobacteria from Microtermes and those isolated from the other two termite genera.
Results
Occurrence and Distribution of Actinobacteria with Fungus-Growing Termites
Actinobacteria were isolated from both geographic locations, all termite genera and colonies, and all types of colony parts that were sampled (Supplementary Table S1 and S2). An overview of the 360 Actinobacteria isolated is given in Table 1. Isolates showed no apparent specificity for origins—Actinobacteria were frequently isolated from each geographical location, each termite genus, each type of colony part sampled—and the number of Actinobacteria isolated showed no bias towards one of the isolation media.
In the phylogenetic analysis, Actinobacteria from fungus-growing termites did not form a monophyletic group, but were interspersed with Actinobacteria from other origins (frequently from soil), but also with clades containing Actinobacteria from fungus-growing Ambrosia and Southern Pine Beetles (Supplementary Table S4, Fig. 1). The assigned morphotypes were not supported by the sequencing data: strains with the same morphotype occurred in different clades of the phylogenetic tree, while sequences of strains assigned to different morphotypes occurred in the same terminal branches (Fig. 1, Supplementary Table S4). Thus, morphotypes were not used in further analyses.
An unrooted neighbour-joining distance tree showing the phylogenetic placement of a subset of the Actinobacteria obtained from fungus-growing termites interspersed with strains from other origins (for origin details, see Supplementary Table S4). Termite strains are highlighted in bold, and host names (M. natalensis, Microtermes sp. and Odontotermes sp.) are given as identifiers. Colony number, worker number and isolation origins are also given. In addition to termite Actinobacteria, we include the top hit for each of the termite strains from a BLASTn search in GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi; see Supplementary Table S4), the most closely related type strain for each termite-strain from the Ribosomal Database Project (https://rdp.cme.msu.edu/; see Supplementary Table S4), and Streptomyces Actinobacteria associated with Ambrosia and Southern Pine Beetles (see Supplementary Table S4). Bootstrap support values above 50 after 100 pseudo-replications under ML conditions are given
Antibiotic Effect of Actinobacteria on Pseudoxylaria and Termitomyces
In the screening bioassay with 288 Actinobacteria, Pseudoxylaria was overall significantly less affected than Termitomyces (Fig. 2, Supplementary Table S3, p = 0.0001). In the detailed bioassay with 53 Actinobacteria, average ZOE on Pseudoxylaria strains was again less than the ZOE on Termitomyces strains (t = −4.795, df = 52, p < 0.0001; Supplementary Table S5), and this difference remained apparent even at a detailed level when Actinobacteria were grouped according to isolation origin (Fig. 3).
Results of the detailed bioassay. Examples of effect of Actinobacteria on Pseudoxylaria (A) and Termitomyces (B), and bar graphs showing the zone of effect (ZOE) caused by Actinobacteria averaged for each fungal strain per termite host genus (C, D) and per origin material (E, F) from which the bacteria were isolated. Whiskers on bars show the standard error of the mean. Ma M. natalensis, Mi Microtermes, Od Odontotermes sp
Table 2 summarises the effects of Actinobacteria by showing only ZOE values that exceeded 2% of the total ZOE values observed within each of the fungal strains concerned. Twelve Actinobacteria that did not have ZOE values exceeding this 2% threshold for any of the ten fungal strains tested are thus not shown (see Supplementary Table S5 for the complete dataset). Only two Actinobacteria had a pronounced and consistent antibiotic effect exclusively on Pseudoxylaria strains, and three had a strong effect exclusively on Termitomyces (top and bottom rows of Table 2). Single Actinobacteria strains varied considerably in their effect on Pseudoxylaria and Termitomyces; the categories by which Actinobacteria were selected in the screening bioassay (with one strain for each fungus), did not match the results of the detailed bioassay in half of the cases (Table 2). Certain Actinobacteria caused a large ZOE for only a part of the Pseudoxylaria strains, not affecting other Pseudoxylaria strains, and the same was observed for Termitomyces strains. Placement of Actinobacteria in the NJ tree was not correlated with the effect on Pseudoxylaria or Termitomyces in the screening bioassay (Fig. 1).
Actinobacteria did not show specific activity against fungi isolated from the same host (Fig. 3c–f). The only trend observed was that Actinobacteria from Microtermes colonies seemed to have a stronger effect on average on all fungal strains (Fig. 3c, d). Consequently, the effects of Actinobacteria differed significantly between termite genera in the detailed bioassay (F = 3.338, df = 50, p = 0.044). ZOE of Actinobacteria isolated from Microtermes was significantly higher with both Pseudoxylaria and Termitomyces (t = 2.355, df = 51, p = 0.022 and t = 2.602, df = 51, p = 0.012), but no significant effects were found for Microtermes Actinobacteria in the screening assay. There were no significant differences in the average antibiotic effect between Actinobacteria strains isolated from comb, head (including pronotum) or abdomen; and neither was there a difference in effect concerning the medium on which Actinobacteria were isolated.
In the primary antibiotic production assay, agar blocks cut from positions adjacent to pure Actinobacteria colonies had effects on Pseudoxylaria and Termitomyces that were similar to the effects of the Actinobacteria themselves (Table 3).
Discussion
Actinobacteria in Fungus-Growing Insect Nests
Comparisons of the fungus-growing termite symbiosis with other fungus-growing insects are frequently done, in particular with the New World fungus-growing ants (e.g. [27, 29]). The independent origins of fungus-growing termites and other fungus-farming mutualisms make comparisons particularly valuable, because it is possible to test if the same “solutions” to evolutionary problems have independently arisen multiple times, or if different solutions arose in different associations.
Over the course of evolution, the ubiquitous Actinobacteria have become integrated in microbial defence in several symbiotic associations [6, 16, 17, 40]. Here, we investigated whether the fungus-growing termite symbiosis involves Actinobacteria with the potential to defend colonies against Pseudoxylaria, a competitor of the termite fungus. Although we take a culture-based approach, and consequently miss unculturable microbes (cf. [34, 47]), our results show that Actinobacteria with antifungal properties are abundant in fungus-growing termite nests: isolates were obtained from (1) colonies from two geographically distant locations, (2) three termite genera, (3) all colonies examined and (4) all colony parts examined. This indicates that Actinobacteria are present, and thus have the potential to play a role in the fungus-growing termite mutualism.
Specificity of Actinobacteria for Fungus-Growing Termite Nests
We found no indication of specificity of Actinobacteria to fungus-growing termites, either at the broad level (i.e. a specific group of bacteria associating across different termite genera) or at the species or genus levels (i.e. specific groups of bacteria associating with specific fungus-growing termites). Instead, our phylogenetic analysis showed that Actinobacteria isolated from fungus-growing termites are genetically intermingled with Actinobacteria occurring outside termite nests (e.g. isolates from soil), and that grouping isolates based on morphotype is insufficient for distinguishing isolates to being the same strain/species.
Actinobacteria are ubiquitous in soil and related substrates, suggesting that their presence in the system might represent environmental strains entering fungus-growing termite nests via workers performing nest-building and foraging activities. However, given that 16S was sequenced for only 35 of the 360 strains of Actinobacteria obtained, and because 16S rRNA sequencing provides limited phylogenetic resolution [43], we cannot reject the hypothesis that additional sequencing would reveal specific phylogenetic clades of Actinobacteria associated with the termites. A comparison of the Actinobacteria communities between the termites and the soil environment could be an important next step towards understanding the level of specificity with the termites.
The Role of Actinobacteria in Fungus-Growing Termite Nests
Our bioassays, exploring whether fungus-growing termite-associated Actinobacteria inhibit the invasive fungus Pseudoxylaria, and whether they affect the cultivar fungus Termitomyces, revealed a high degree of bioactivity. Both bioassays showed that most of the isolated Actinobacteria secrete compounds with antibiotic properties, some of which inhibit the invasive fungus Pseudoxylaria. Agar plugs taken adjacent to pure Actinobacteria cultures caused similar, but not always identical, inhibition of both fungi, suggesting constitutive production of antibiotics, irrespective of the presence of another microorganism. Generally, Actinobacteria inhibited the termite cultivar fungus Termitomyces more often and more severely than Pseudoxylaria. A possible explanation for Pseudoxylaria being less susceptible than Termitomyces is the origin of the fungi in the association with the termites: if Pseudoxylaria occurs metabolically active in niches (i.e. plant-biomass-degrading systems) that involve competition with Actinobacteria [9], and only relatively recently—compared to Termitomyces—engaged in the association with fungus-growing termites, it is conceivable that Pseudoxylaria has been under stronger selection to evolve resistance to Actinobacteria-produced compounds than Termitomyces.
The bioassays did not establish Actinobacteria as specific defensive symbionts targeting Pseudoxylaria, as the Actinobacteria caused stronger inhibition of Termitomyces. However, we need to acknowledge the possibility that they may still play a specific defensive role under natural conditions, including against other potential fungal weeds or competitors that enter termite nests. Actinobacteria typically only produce a small fraction of the small molecules encoded in their genomes under artificial conditions on an artificial medium (e.g. [51, 52]), so we may miss molecules mediating these interactions in the in vitro assays. Another reason why in vitro antagonism observed in Petri plate assays may not fully reflect natural interactions is the expectation that antibiotic dose in pure cultures of bacteria is much higher than in bacterial populations in the environment (cf. [32]). Nonetheless, previous work has shown that observations in Petri plates (in vitro) can match what happens in miniature colonies (in vivo) and thus can mimic some of the dynamics within nests [31].
Even if the in vitro effects observed are stronger on Termitomyces than Pseudoxylaria, the responsible compounds may play a role in the suppression of Pseudoxylaria if they are applied in a directed way, i.e. in a way that allows for the suppression of Pseudoxylaria without affecting Termitomyces. Active directed application has been suggested for Actinobacteria-derived antibiotics in fungus-growing ants [3, 32], where the bacterial secretions also have inhibitory properties against the ants’ cultivar fungus in vitro [31, 41], but apparently not in vivo [32]. Whether the secretions with in vitro inhibitory properties against Termitomyces also affect the mutualistic fungus in vivo remains to be tested.
There are some notable major differences in the biology of the two convergent cases of fungus-growing social insects, which lead to the prediction that fewer additional symbionts are present in fungus-growing termites (as noted in [29]). First, in fungus-growing termites the substrate passes through the termite gut before it is being deposited on the fungus garden. Potentially, this gut passage facilitates a higher control over the fungus garden (either by the termites themselves or by gut symbionts). Second, most fungus-growing termites have horizontal symbiont transmission, associated with sexual reproduction, contrasting with fungus-growing ants, which by default transmit the fungus clonally and vertically. Theory predicts that the consequently higher genetic variability of the termite fungi provides a benefit in the arms races with parasites, compared to clonal vertically transmitted fungi.
Concluding Remarks
Our work describes the first discovery of a large assembly of Actinobacteria occurring in fungus-growing termite nests. Actinobacteria were found throughout all sampled nests and materials, and the bioassays showed that many strains inhibit both the substrate competitor Pseudoxylaria and the termite cultivar Termitomyces. This, in combination with the high Actinobacteria diversity and lack of phylogenetic specificity, means that the role of Actinobacteria as defensive symbionts with fungus-growing termites remains unproven. Nevertheless, documenting the presence of antibiotic-producing Actinobacteria in the termite environment implies the potential for an influence of bacteria on the fungus-growing termites mutualistic system. It is therefore a first step towards gaining a better understanding of additional associates potentially playing a role in the symbiosis. Future work is needed for a better understanding of the antibiotic-producing bacteria in this system. Finally, even if none of the Actinobacteria found are specialised defensive symbionts within the fungus-growing termites system, it is conceivable that Actinobacteria—by being present in the mounds, surrounding soil and forage material—can be beneficial to the mutualistic system if useful antibiotics are produced and can be obtained by the termites.
References
Aanen DK, de Fine Licht HH, Debets AJM, Kerstes NAG, Hoekstra RF, Boomsma JJ (2009) High symbiont relatedness stabilizes mutualistic cooperation in fungus-growing termites. Science 326:1103–1106
Aanen DK, Ros VID, de Fine Licht HH, Mitchell J, de Beer ZW, Slippers B, Rouland-Lefèvre C, Boomsma JJ (2007) Patterns of interaction specificity of fungus-growing termites and Termitomyces symbionts in South Africa. BMC Evol Biol 7:115. doi:10.1186/1471-2148-7-115
Boomsma JJ, Aanen DK (2009) Rethinking crop-disease management in fungus-growing ants. Proc Natl Acad Sci U S A 106:17611–17612
Cafaro MJ, Poulsen M, Little AE, Price SL, Gerardo NM, Wong B, Stuart AE, Larget B, Abbot P, Currie CR (2011) Specificity in the symbiotic association between fungus-growing ants and protective Pseudonocardia bacteria. Proc R Soc B 278(1713):1814–1822
Currie CR, Poulsen M, Mendenhall J, Boomsma JJ, Billen J (2006) Coevolved crypts and exocrine glands support mutualistic bacteria in fungus-growing ants. Science 311:81–83
Currie CR, Scott JA, Summerbell RC, Malloch D (1999) Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 398:701–704
Fields MW, Yan TF, Rhee SK, Carroll SL, Jardine PM, Watson DB, Criddle CS, Zhou JZ (2005) Impacts on microbial communities and cultivable isolates from groundwater contaminated with high levels of nitric acid–uranium waste. FEMS Microbiol Ecol 53:417–428
Fuller CA (2007) Fungistatic activity of freshly killed termite, Nasutitermes acajutlae, soldiers in the Caribbean. J Insect Sci 7:1–8
Goodfellow M, Williams ST (1983) Ecology of Actinomycetes. Annu Rev Microbiol 37:189–216
Grubbs KJ, Biedermann PH, Suen G, Adams SM, Moeller JA, Klassen JL, Goodwin LA, Woyke T, Munk AC, Bruce D, Detter C, Tapia R, Han CS, Currie CR (2011) Genome sequence of Streptomyces griseus strain XylebKG-1, an Ambrosia beetle-associated actinomycete. J Bacteriol 193:2890–2891
Guedegbe HJ, Miambi E, Pando A, Houngnandan P, Rouland-Lefèvre C (2009) Molecular diversity and host specificity of termite-associated Xylaria. Mycologia 101:686–691
Hsieh HM, Lin CR, Fang MJ, Rogers JD, Fournier J, Lechat C, Ju YM (2010) Phylogenetic status of Xylaria subgenus Pseudoxylaria among taxa of the subfamily Xylarioideae (Xylariaceae) and phylogeny of the taxa involved in the subfamily. Mol Phylogenet Evol 54:957–969
Hsu SC, Lockwood JL (1975) Powdered chitin as a selective medium for enumeration of actinomycetes in water and soil. Appl Microbiol 29:422–426
Hulcr J, Adams AS, Raffa K, Hofstetter RW, Klepzig KD, Currie CR (2011) Presence and diversity of Streptomyces in Dendroctonus and sympatric bark beetle galleries across North America. Microb Ecol 61:759–768
Jones JA (1990) Termites, soil fertility and carbon cycling in dry tropical Africa: a hypothesis. J Trop Ecol 6:291–305
Kaltenpoth M (2009) Actinobacteria as mutualists: general healthcare for insects? Trends Microbiol 17:529–535
Kaltenpoth M, Göttler W, Herzner G, Strohm E (2005) Symbiotic bacteria protect wasp larvae from fungal infestation. Curr Biol 15:475–479
Katoh H, Miura T, Maekawa K, Shinzato N, Matsumoto T (2002) Genetic variation of symbiotic fungi cultivated by the macrotermitine termite Odontotermes formosanus (Isoptera: Termitidae) in the Ryukyu Archipelago. Mol Ecol 11:1565–1572
Konaté S, Le Roux X, Verdier B, Lepage M (2003) Effect of underground fungus-growing termites on carbon dioxide emission at the point- and landscape-scales in an African savanna. Funct Ecol 17:305–314
Kroiss J, Kaltenpoth M, Schneider B, Schwinger MG, Hertweck C, Maddula RK, Strohm E, Svatos A (2010) Symbiotic streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat Chem Biol 6:261–263
Lamberty M, Zachary D, Lanot R, Bordereau C, Robert A, Hoffmann JA, Bulet P (2001) Insect Immunity. Constitutive expression of a cysteine-rich antifungal and a linear antibacterial peptide in a termite insect. J Biol Chem 276:4085–4092
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–175
Lepage M (1984) Distribution, density and evolution of Macrotermes bellicosus nests (Isoptera, Macrotermitinae) in the northeast of Ivory-Coast. J Anim Ecol 53:107–117
Leuthold RH, Badertscher S, Imboden H (1989) The inoculation of newly formed fungus comb with Termitomyces in Macrotermes colonies (Isoptera, Macrotermitinae). Insectes Sociaux 36:328–338
Mando A, Brussaard L (1999) Contribution of termites to the breakdown of straw under Sahelian conditions. Biol Fertil Soils 29:332–334
Moriya S, Inoue T, Ohkuma M, Yaovapa T, Johjima T, Suwanarit P, Sangwanit U, Vongkaluang C, Noparatnaraporn N, Kudo T (2005) Fungal community analysis of fungus gardens in termite nests. Microb Environ 20:243–252
Mueller UG, Gerardo NM, Aanen DK, Six DL, Schultz TR (2005) The evolution of agriculture in insects. Annu Rev Ecol Evol Syst 36:563–595
Nobre T, Kone NA, Konate S, Linsenmair KE, Aanen DK (2011) Dating the fungus-growing termites’ mutualism shows a mixture between ancient codiversification and recent symbiont dispersal across divergent hosts. Mol Ecol 20:2619–2627
Nobre T, Rouland-Lefevre C, Aanen D (2011) Comparative biology of fungus cultivation in termites and ants. In: Bignell DE, Roisin Y, Lo N (eds) Biology of termites: a modern synthesis. Springer, New York, p 576
Oh DC, Poulsen M, Currie CR, Clardy J (2009) Dentigerumycin: a bacterial mediator of an ant–fungus symbiosis. Nat Chem Biol 5:391–393
Poulsen M, Cafaro MJ, Erhardt DP, Little AEF, Gerardo NM, Tebbets B, Klein BS, Currie CR (2010) Variation in Pseudonocardia antibiotic defence helps govern parasite-induced morbidity in Acromyrmex leaf-cutting ants. Environ Microbiol Rep 2:534–540
Poulsen M, Currie CR (2010) Symbiont interactions in a tripartite mutualism: exploring the presence and impact of antagonism between two fungus-growing ant mutualists. PLoS One 5:e8748
Poulsen M, Erhardt DP, Molinaro DJ, Lin TL, Currie CR (2007) Antagonistic bacterial interactions help shape host-symbiont dynamics within the fungus-growing ant-microbe mutualism. PLoS One 2:e960
Riesenfeld CS, Schloss PD, Handelsman J (2004) Metagenomics: genomic analysis of microbial communities. Annu Rev Genet 38:525–552
Rogers JD (2000) Thoughts and musings on tropical Xylariaceae. Mycol Res 104:1412–1420
Rogers JD, Ju YM, Lehmann J (2005) Some Xylaria species on termite nests. Mycologia 97:914–923
Saitou N, Nei M (1987) The neighbor-joining method—a new method for reconstructing phylogenetic tress. Mol Biol Evol 4:406–425
Sands WA (1960) The initiation of fungus comb construction in laboratory colonies of Ancistrotermes guineensis (Silvestri). Insectes Sociaux 7:251–259
Sands WA (1969) The association of termites and fungi. In: Krishna K, Weesner FM (eds) Biology of termites. Vol. 1. Academic, New York, pp 495–524
Scott JJ, Oh DC, Yuceer MC, Klepzig KD, Clardy J, Currie CR (2008) Bacterial protection of beetle–fungus mutualism. Science 322:63
Sen R, Ishak HD, Estrada D, Dowd SE, Hong EK, Mueller UG (2009) Generalized antifungal activity and 454-screening of Pseudonocardia and Amycolatopsis bacteria in nests of fungus-growing ants. Proc Natl Acad Sci U S A 106:17805–17810
Shinzato N, Muramatsu M, Watanabe Y, Matsui T (2005) Termite-regulated fungal monoculture in fungus combs of a macrotermitine termite Odontotermes formosanus. Zool Sci 22:917–922
Staley JT (2006) The bacterial species dilemma and the genomic-phylogenetic species concept. Phil Trans R Soc B 361:1899–1909
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) Mega5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Thomas RJ (1987) Distribution of Termitomyces Heim and other fungi in the nests and major workers of Macrotermes bellicosus (Smeathman) in Nigeria. Soil Biol Biochem 19:329–333
van Valen L (1973) A new evolutionary law. Evol Theory 1:1–30
Vartoukian SR, Palmer RM, Wade WG (2010) Strategies for culture of ‘unculturable’ bacteria. FEMS Microbiol Lett 309:1574–6968
Visser AA (2011) On the ecology and evolution of microorganisms associated with fungus-growing termites. PhD-thesis. university of Wageningen, 176 pp
Visser AA, Kooij P, Debets AJM, Kuyper TW, Aanen DK (2011) Pseudoxylaria as stowaway of the fungus-growing termite nest: interaction asymmetry between Pseudoxylaria, Termitomyces and free-living relatives. Fungal Ecol 4:322–332
Visser AA, Ros VID, de Beer ZW, Debets AJM, Hartog E, Kuyper TW, Læssøe T, Slippers B, Aanen DK (2009) Levels of specificity of Xylaria species associated with fungus-growing termites: a phylogenetic approach. Mol Ecol 18:553–567
Waksman SA, Schatz A (1945) Strain specificity and production of antibiotic substances. VI. Strain variation and production of streptothricin by Actinomyces lavendulae. Proc Natl Acad Sci U S A 31:208–214
Waksman SA, Schatz AR, Reynolds DM (2010) Production of antibiotic substances by Actinomycetes. Ann N Y Acad Sci 1213:112–124
Wood TG, Thomas RJ (1989) The mutualistic association between Macrotermitinae and Termitomyces. In: Wilding N, Collins NM, Hammond PM, Webber JF (eds) Insect–fungus interactions. Academic, London, pp 69–92
Acknowledgements
We thank Thomas W. Kuyper for valuable comments on the manuscript. We are grateful to Michael J. Wingfield, Z. Wilhelm de Beer and colleagues at the Forestry and Agricultural Biotechnology Institute (FABI), South Africa, for hosting and welcoming us to use the laboratory facilities at FABI. Thanks go to Jannette D. Mitchell for showing us sampling sites and to the Oerlemans family for allowing us to sample termite mounds on their property. A.A.V. was supported by a fellowship from the C.T. de Wit Graduate School of Production Ecology & Resource Conservation (PE&RC), Wageningen University, the Netherlands; D.K.A. was funded by a Vidi grant by the Dutch Science Foundation (NWO-ALW) and a grant of the C.T. de Wit Graduate School PE&RC; T.N. was funded by a Marie Curie Intra-European Fellowship within the 7th European Community Framework Programme (IEF Project No. 220077), C.R.C. and M.P. were supported by the US DOE Great Lakes Bioenergy Research Center (DOE BER Office of Science DE-FC02-07ER64494) and by a National Science Foundation grant DEB-0747002 awarded to C.R.C, and M.P. was supported by the Carlsberg Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary TableS1
Overview of sampled termite colonies and isolated strains. *Not sampled for Actinobacteria. (DOC 76 kb)
Supplementary Table S2
Overview of Actinobacteria showing isolation details, assigned morphotype (see main text for details), and strain codes used in the bioassays. (DOC 740 kb)
Supplementary Table S3
Complete data of screening bioassay. Effects (in millimetres) of Actinobacteria AV151-AV288 on Pseudoxylaria (P2) and Termitomyces (T1). Strains that were also tested in the detailed bioassay, selected because of their effect in this screening assay on either Pseudoxylaria (P), Termitomyces (T) or both (P and T), are shown in bold. (DOC 355 kb)
Supplementary Table S4
Sequenced Actinobacteria strains from fungus-growing termite included in the estimation of the Neighbour Joining tree: First BLAST-hit of sequenced strains with strain name, ecological and geographical origins (if known). The table also shows the closest match to type strains from an RDP Type strain search. (DOC 128 kb)
Supplementary Table S5
Complete data for the detailed bioassay. Average effects of Actinobacteria on Pseudoxylaria and Termitomyces. Page 1 shows the average effects of Actinobacteria on Pseudoxylaria and Termitomyces in millimetres zone of inhibition and zone of effect. Page 2 gives the effects per individual fungal strain, in addition to the total zone of effect per strain, all in millimetres. (DOC 251 kb)
Rights and permissions
About this article
Cite this article
Visser, A.A., Nobre, T., Currie, C.R. et al. Exploring the Potential for Actinobacteria as Defensive Symbionts in Fungus-Growing Termites. Microb Ecol 63, 975–985 (2012). https://doi.org/10.1007/s00248-011-9987-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-011-9987-4