Abstract
In this study, hydrocarbon (HC) degradation activity of a HC-rich marine sediment was assessed in anaerobic microcosms during a 224 days incubation period. Natural TOC/N/P ratio of the sediment porewater (1,000/5/1) was gradually decreased to 1,000/40/6 which resulted in approximately ninefold increase in gas production (CH4+CO2) and HC removal. Addition of external HCs to the microcosms was also resulted in approximately twofold higher gas production and HC removal. A high proportion (92%) of aromatic HCs and all n-alkanes were removed from the microcosms under unlimited nutrient supply conditions without external HC addition. The microorganisms of the sediment degraded a wide range of aliphatic (n-C9–31 alkanes and acyclic isoprenoids) and aromatic (18 different one- to five-ring aromatics) HCs. Monitoring functional gene and transcript abundances revealed that methanogenesis and dissimilatory sulfate reduction took place simultaneously during the first 126 days, afterwards, only the syntrophic methanogenic consortium was active. Genes and transcripts related to initial activation of HCs were highly abundant throughout the incubation period showing that fumarate addition was the main pathway of anaerobic HC degradation. In conclusion, biostimulation of highly polluted anoxic marine sediments via nutrient amendment is effective and may constitute a suitable and cost-effective field-scale bioremediation strategy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Petroleum hydrocarbons (HCs) are one of the most important organic pollutants in marine environments [19, 21]. Although anaerobic HC degradation is widespread and has been reported under nitrate-, iron-, manganese- and sulfate-reducing and methanogenic conditions [14, 18, 62], natural attenuation of oil in anoxic marine sediments is slow and oil deposits persist for many years [14, 19, 26, 35]. Biological HC removal from the sediments can be accelerated through addition of limiting nutrients (biostimulation) or HC-degrading microbes (bioaugmentation) [38].
Bioaugmentation with bacteria capable of anaerobic degradation is untested. This is because added microbes are at a disadvantage in competition with the indigenous microbiota and, even under aerobic conditions, successful bioaugmentation trials are sparse [14].
Since the type and concentration of terminal electron acceptors (TEAs) have a major effect on the outcome of bioremediation, TEA addition is the most studied way of accelerating HC biodegradation in anoxic environments. Addition of sulfate [2] and nitrate [49] was shown to enhance in situ degradation of mono aromatic HCs in petroleum-contaminated aquifers. Three independent studies reported that amending with both nitrate and sulfate rather than the just one was advantageous due to existence of a heterogeneous microbial population: (1) Cunningham et al. [11] accelerated the natural attenuation of petroleum-contaminated groundwater; (2) in situ phenanthrene biodegradation was enhanced threefold in a marine sediment [55]; (3) the rate of diesel fuel degradation was the highest under mixed electron acceptor conditions in marine sediment microcosms [5]. Overall outcomes of these studies pointed out that a response to TAE amendment depends on indigenous microbiota and redox conditions, and the contaminated environment must be well characterized to apply a site-specific biostimulation strategy.
HC-polluted environments usually experience complex mixtures of contaminants that can influence the success of TAE amendment. In a survey of various aquatic sediments incubated under four different redox conditions, Phelps and Young [41] found that degradation of a mixture of benzene, toluene, ethylbenzene, and m + p + o-xylene (BTEX) compounds in cultures amended with aliphatic HCs was slower and incomplete. This negative effect contrasts with the stimulatory effect of aliphatic HCs on aromatic HC degradation in microcosms under methanogenic and sulfate-reducing conditions [44]. These conflicting observations emphasize the unpredictability of site-specific responses to mixed substrates.
The stimulatory effect of providing nutrients, such as fixed nitrogen and/or phosphate, has not been as thoroughly studied under anaerobic conditions as under aerobic conditions [14, 38]. Two cases show the benefit of fertilizing nutrient-poor anaerobic environments contaminated with diesel fuel: Cross et al. [10] observed enhanced anaerobic degradation when contaminated groundwater microcosms were amended with ammonium, nitrate, and phosphate, and Powell et al. [43] noted the stimulatory effect of nitrate, ammonium, phosphate and calcium on denitrifying HC degraders in nutrient-poor Antarctic soils. The fertilization to increase anaerobic HC degradation activity of marine microbenthos has not been tested yet.
Massive C loads from oil spills or chronic HC pollutions can be removed from marine sediments by microorganisms as long as (1) dissolution and hydrolysis of particulate nutrient forms are not rate limiting for microbial growth and (2) dissolved C/N/P ratio of the porewater is close to that of marine bacterioplankton (100/20/3) [60]. Although excessive N–P inputs from anthropogenic pollution sources change the nutrient balance toward low C ratios in the sediments, C/N/P ratio of the porewaters might still be much higher than 100/20/3 due to very slow dissolution/hydrolysis of particulate N–P forms. This has been the case for Marmara Sea, which has very high C/N/P ratios in the porewaters (∼100/0.5/0.1) despite the low C ratios in the sediments (∼100/50/10) [24]. N and P amendment is an option to increase microbial HC removal in this ecosystem.
Marmara Sea connects Black Sea to Aegean Sea via Bosphorus Strait, covering an area of 214,000 km2. It has been over polluted with petroleum HCs [29]. The pollution has been originated from: highly polluted Black Sea, spills from oil tankers (∼35 accidents/year), discharges during marine transportation (∼50,000 tankers/year), municipal wastes (∼20 million people), industrial wastes (40% of the Turkish Industry), atmospheric deposition, and urban surface and river runoff [40, 59]. The extreme pollution has led to approximately fivefold increase in anoxic areas during the last 30 years (Turkish Water Agency, personal communication).
Halic Bay is located in Istanbul, at a junction between Bospshorus Strait and Marmara Sea. The 8-km long bay has a water surface area of 2.6 million m2. Its depth changes between 2 and 60 m depending on the regional waste input level and the deep water flow rate. Aromatic and aliphatic hydrocarbon levels in the sediments were in ranges of 4,000–6,000 and 1,500–3,000 ppm, respectively, which were similar to that of the extremely hydrocarbon-contaminated marine environments [1, 57]. The high and chronic pollution has resulted in formation of a highly anaerobic deep sludge over the years [23].
Water and Sewerage Administration of Istanbul (ISKI) and Istanbul Metropolitan Municipality (IBB) started “Halic Cleaning Project” in the late 1980s (http://www.iski.gov.tr/Web/statik.aspx?KID=1000470); ∼5-million-m3 sludge was collected by physical screening, and then the water body was aerated. The water quality was improved significantly in a very short time period (15 months); on the other hand, the remediation cost was remarkably high. Halic Bay ecosystem slowly returned to its over polluted status after the completion of the project (during the last 20 years).
A cost-effective remediation strategy that can be sustained for a long time with a minimal human intervention is needed to overcome the chronic HC pollution in Halic Bay. The best candidate for this purpose is bioremediation under anaerobic/anoxic conditions as long as oil-degrading anaerobes are abundant and active in the sediments, and there is a way to increase activity of this population [21]. In order to assess the anaerobic bioremediation feasibility of Halic Bay, a Turkish Academy of Sciences (TUBITAK) project was carried out [24]. Monitoring the physicochemical and microbiological sediment characteristics revealed that (1) the total petroleum HC levels (the sum of aliphatics, aromatics, asphaltenes, and resenes; 11,000–18,000 ppm) were similar to those from extremely polluted marine environments [30]; (2) the microbial cell contents were very high (9–12 × 1010 cells/ml) compared with the other marine environments [29]; (3) the sediments were dominated by methanogens and anaerobic HC degraders, and these microbes were active [30]; and (4) N and P were limited in the porewaters for biological activity [30]. These indications raised the question that anaerobic HC degradation activity of Halic Bay sediments can be increased by N–P amendment under methanogenic conditions. This paper describes assessment of this hypothesis through anaerobic HC degradation microcosms.
Methods
Sampling and Characterization of the Sediment
Sediment samples were taken from Halic Bay (41°33.66′ N and 28°56.64′ E), in the northwest of Marmara Sea, at a water depth of 2 m. The samples were taken using a Van Veen grab with a volume of 3.5 L and a penetration depth of 15 cm on the day of microcosm set up (December 2008). The samples were taken in three replicates and then subdivided for molecular and chemical analyses and stored at −20°C.
The samples had a grayish-black color and a fine-grained nature being rich in mud (>90%) and poor in sand. The porewater was brackish (12 ± 0.8 psu). The bottom water temperature, pH, and redox potential were 19°C, 7.8 ± 0.3, and −230 ± 18 mV, respectively. The porewater NO −3 concentration (300 ± 21 μM) was considerably higher than that of typical seawater (10–50 μM) while the SO 2−4 level (13 ± 1.2 mM) was lower than the typical levels (26–32 mM) [34]. No oxygen was detected in the porewater.
TOC/N/P ratio of the sediment (100/35/8) was very low compared with Redfield ratio (106/16/1) indicating pollution from anthropogenic sources. N and P were limited in the porewaters for biological activity which was evident from much lower TOC/N/P ratio of the porewater (862/5/1) compared with that of the exponentially growing marine bacterioplankton (100/20/3) [60]. Total aromatic and aliphatic HC level in the sediment (8,120 ± 450 ppm) was similar to those from highly polluted marine environments [1, 57].
Microcosm Setup
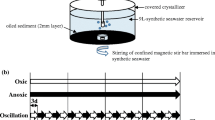
An anaerobic cabinet (Coy Laboratory Products) fitted with an oxygen sensor and with a regulated atmosphere of nitrogen (100%) was used in the preparation and incubation of the anaerobic microcosms. The microcosms were set up in glass 120-ml serum bottles sealed with butyl rubber stoppers and aluminum crimps (Aldrich). The total volume of liquid was 100 ml with 20 ml of headspace volume. Each microcosm comprised a carbonate-buffered nutrient medium containing sources of nitrogen and phosphorus, vitamins and trace minerals, made up in deionized water, according to the brackish medium of Widdel and Bak [61]. Methanogenic conditions were established by the exclusion of the exogenous electron acceptors.
Microcosms were seeded with 10 g of the Halic Bay sediment and amended with 200 mg of the HC mix (HC composition on day 0 in Figs. 6 and 7) except the controls, which were used to determine the extent of methanogenesis on natural HC content of the sediments. Composition of the HC mix was defined based on the detected HC types in the sediments during the 2-year monitoring study [30].
The overall TOC/N/P ratio of Halic Bay sediments (∼1,000/5/1) was chosen as a nutrient limited condition. The unlimited nutrient condition was calculated as 1,000/40/6 (C/N/P) based on the following assumptions: (1) molecular formula of the HC mix was C5nH8n (derived from the HC composition); (2) the maximum biomass yield was as high as 0.2 g cell/g HC mix [16]; and (3) C/N/P ratio of the marine microbes was 100/20/3 [60]. Hence, the nutrient amendment was done by gradually decreasing TOC/N/P ratio from 1,000/5/1 to 1,000/40/6.
Microcosms were prepared in triplicate. In addition to the control microcosms without external HC addition, two control microcosms were included: (1) Na2MoO4 was added to inhibit dissimilatory sulfate reduction; (2) NaN 3 (1 g/L) treatment was applied to suppress microbial activity.
The experimental conditions and controls, and abbreviations of the sample names were summarized in Table 1. Five sets of each condition were prepared for destructive sampling. Total of 360 microcosms were set up.
Gas productions in the microcosms were monitored periodically (2 weeks) and the incubations were done till the all gas productions stopped (224 days). The destructive samplings were carried out on days 0, 84, 126, 168, and 224. The samples on days 84, 126, and 168 were taken because of the discernable increases in gas production (Figs. 1 and 2).
Genomic DNA and Total RNA Extraction, and cDNA Synthesis
Genomic DNA was extracted using the FastDNA Spin Kit for Soil (Qbiogene, UK), and total RNA was extracted using the ChargeSwitch® Total RNA Cell Kit (Invitrogen, Germany) by following the manufacturer’s instructions. To test for a DNA contamination, the RNA extracts were used in Q-RT-PCR (quantitative real-time PCR combined with reverse transcription) as a negative control. The first-strand cDNA was synthesized from the total RNA using random hexamers and SuperScript® First-Strand Synthesis System for RT-PCR according to the kit’s manual (Invitrogen, Germany).
Quantitative Real-Time PCR
The primer sets and their targets were given in Table 2; 103–7 copies of the standard sequences were used to obtain the calibration curves. Roche LightCycler DNA Master SYBR Green I kit and Roche LightCycler 2.0 (Roche Diagnostics GmbH, Mannheim, Germany) were utilized for all reactions. Reaction mixes contained 25 ng template DNA, 0.5 μM of each primer and 2.5 μM MgCl2. The following thermocycling program was applied: 95°C for 10 min, 45 cycles of 10 s at 95°C, 5–10 s at primer-dependent annealing temperature, 15 s at 72°C. A melt-curve analysis was performed from 55°C to 95°C to determine if only one amplified product was generated during quantitative real-time PCR (Q-PCR). Q-PCR runs were analyzed using Roche LightCycler Software 4.05. The efficiencies were between 1.8 and 2.0, and the correlation factors (r 2) were not lower than 0.97 in all reactions. To convert gene abundances into cell numbers, averages of 3.6 and 1.6 copies of 16S rRNA gene were estimated for bacteria and archaea, respectively [27]. It was assumed that a single copy of the target functional genes exists in a prokaryotic genome [4, 12, 42, 63], and functional gene abundances were divided by total cell abundances to calculate the gene percentage.
Gas Measurements
Headspace gas (10 ml) was removed, periodically (2 weeks), throughout the course of the experiment from all microcosms and injected into evacuated gas tubes. The gas removed was replaced by 10 ml of 100% N2. Gas samples were analyzed for CH4 using a Hewlett-Packard (HP) 5972 II gas chromatograph–mass spectrometer (GC–MS). The samples were analyzed using a HP Plot Q column (30 m × 530 μm, 1 μm) with nitrogen as carrier gas. The column temperature was programmed from 60°C to a final temperature of 120°C at a rate of 8°C/min. Peak areas were calibrated using the CH4 and CO2 gas standards and the reproducibility (n = 4) of replicate standard analyses were typically less than 1% relative standard deviation.
HC Analysis
The analytical procedure for extraction of HCs derived from a modified UNEP’s protocol [58]. The samples were Soxhlet-extracted with chloroform (1:2 m/v) for a period of 1 h at 50°C and concentrated on a rotary evaporator. The total HC content of extracts was quantified by infrared spectroscopy.
The extracts were fractionated into aliphatic and aromatic HCs by adsorption liquid chromatography using a column of alumina and silica gel, and gradient solvents as eluent/n-hexane and 2:1 n-hexane/chloroform for aliphatic and aromatic fractions, respectively. The extracts for aromatic HC analyses were evaporated under gentle steam of nitrogen after addition of 50 μl dimethylformamide as keeper and diluted with acetonitrile for high-performance liquid chromatography (HPLC) analysis. The resulting solution were analyzed by a Hewlett-Packard 1046A HPLC with a programmed fluorescence detector. The column (MZ-polyaromatic HCs (PAHs) 250 × 3 mm, 5 μm, from MZ-Analysentechnik, Mainz, Germany) was maintained at 35°C and the flow of mobile phase was 0.5 ml/min. A linear gradient was applied from 52% acetonitrile in water to 100% acetonitrile and held constant for 10 min. Fourteen different PAHs and BTEX were analyzed in the samples. Certified reference materials (CRM 535) were used to assess the accuracy (> 85%) of the measurements. Another CRM (NIST-1647) was also used for recovery test and analyzed three times.
Aliphatic HC analyses were conducted on a HP 5972 II GC–MS. The samples were analyzed using a fused silica capillary column (25 m × 0.32 mm, 0.52 μm) with nitrogen as carrier gas. The column temperature was programmed from 80°C to a final temperature of 280°C at a rate of 8°C/min. The MS operating conditions were: electron ionization of 50 eV and linear scanning over the mass range 35–500 Da were used. The samples were analyzed in the splitless mode. Compound identification was based on individual mass spectra and GC retention times in comparison to the literature, library data, and authentic standards. Standards were injected and analyzed under the same conditions as the samples. Quantification was made owing to internal standards such as n-C18, n-C20, n-C22, and n-C24. Blank analyses were carried out, and all values were corrected for these blank concentrations.
Statistical Analysis
Bivariate correlation analyses were performed using the softwares MINITAB 15 (Minitab Ltd., England) and SPSS 17.0 (SPSS Inc., USA). Correlations were evaluated using Pearson’s method. Statistical significance was taken as p < 0.05.
Results
Correlation Analysis
Statistically significant correlations were given in Table 3. All of the measured parameters except sulfate-reducing bacteria (SRB) abundance and activity were related to the gas production. The comparative evaluation of gas production data from the limiting and the unlimited nutrient microcosms reflected the relative changes in the other parameters. This is why only the results from unlimited nutrient conditions were given for the other parameters.
CO2 and CH4 Production
The cumulative gas production data were shown in Figs. 1 and 2. CO2 and CH4 production in sterilized and inhibited control microcosms were lower than 21 μmol; their gas production data were not shown. The much higher CO2 and CH4 production in HC(+) and HC(−) microcosms compared with the sterile controls indicated that the gas production was of microbial origin.
Addition of the dissimilatory sulfate reduction (DSR) inhibitor inhibited both the DSR and methanogenic activity; the origin of CO2 production (dissimilatory sulfate, Fe(III) and Mn(IV) reduction, fermentation, and methanogenesis) was discussed based on the transcript abundance of functional genes (Fig. 10) and the previous data on microbial composition of Halic Bay sediment [24]. The initial SO4 content (120–127 μmol) was completely removed before day 126 (the data were not shown). DSR activity (dsrB transcript production) was not detected after the day 84 (Fig. 10). Methanogenesis activity (mcrA transcript production) was very high during the all incubation period. Dissimilatory nitrate reduction activity (nosZ and nrfA transcript production) was not observed in the microcosms. Dissimilatory Fe(III) and Mn(IV) reducing bacteria were not monitored in the microcosms because frequencies of 16S rRNA genes from Fe(III)- and Mn(IV)-reducing bacteria in the previously constructed clone libraries from Halic Bay sediments were very low (∼6% and ∼2%, respectively) [24].
As seen in Figs. 1 and 2, the higher initial amount of N and P was resulted in higher amount of total gas produced. Relative total gas production in HC(+) and HC(−) L/PL1/PL2/NL1/NL2/UL microcosms were ∼1/1.3/3.7/1/3.5/7.6 and ∼1/1.5/4.5/1/4.6/9.2, respectively. Addition of external HCs to the microcosms was resulted in approximately twofold higher gas production for UL microcosms. This indicated that the added HCs were biologically more available compared to the natural C sources in the sediments for the microbial growth.
C Balance
C balance in the microcosms was shown in Fig. 3 and Eq. 1 (after 224 days of incubation); 51% and 57% of the initial TOC were removed from HC(+)-UL and HC(−)-UL microcosms, respectively. These correspond to 56% and 97% HC removal in HC(+)-UL and HC(−)-UL microcosms, respectively.
C balance in the HC(+)-UL and HC(−)-UL microcosms: TOC removal, HC removal, gas production, and cell production. In order to convert the removed mg HC into μmol C, molecular formula of the HC mix was calculated based on the composition of aliphatic and aromatic HCs (Figs. 6 and 7). Cell numbers were converted into μmol C, based on the assumptions that molecular formula and average weight of a bacterial cell are C5H7NO2 and 1 pg [32, 39]
N and P Removal
Changes in TOC, N, and P content of HC(+)-UL and HC(−)-UL microcosms were shown in Fig. 4. Initial TOC/N/P ratios of both microcosms were adjusted to be 1,000/40/6. Interestingly, addition of external HCs resulted in higher N and P consumption rate for HC(+)-UL microcosms. TOC/N/P removal ratio (1,000/78/12) was lower than the initially adjusted ratio (1,000/40/6) in HC(+)-UL microcosms, which resulted in incomplete C removal. Although TOC/N/P removed from HC(−)-UL microcosms at the expected ratio (1,000/47/7), C removal stopped before the all N and P depleted. This was probably due to depletion of the biodegradable fraction of TOC.
Changes in HC Composition
Changes in aromatic and aliphatic HC levels of HC(+)-UL and HC(−)-UL microcosms were shown in Fig. 5; 55% and 57% of aromatic and aliphatic HCs in HC(+)-UL microcosms were removed, respectively. High proportion (92%) of aromatic HCs and all aliphatic HCs were removed from HC(−)-UL microcosms; 2.4× and 2.1× higher amounts of aliphatic HCs were consumed compared with those of aromatic HCs in HC(+)-UL and HC(−)-UL microcosms, respectively.
Changes in aromatic and aliphatic HC fractions were shown in Figs. 6 and 7. As seen, the short-chain HCs were degraded faster than the long-chain HCs. Aromatic HCs were only degraded after significant removal of n-alkanes and alteration of acyclic isoprenoids pristane and phytane.
Aromatic HC Fractions
Changes in aromatic HC fractions were shown in Fig. 6. Complete removal of 1–3 ring aromatic HCs was achieved in both HC(+)-UL and HC(−)-UL microcosms. Only antracene was partially (40%) removed in HC(+)-UL microcosms. Four- to five-ring aromatic HCs were not degraded in HC(+)-UL microcosms whereas those in HC(−)-UL microcosms were completely consumed except benzo(g,h,i)perylene.
Aliphatic HC Fractions
Changes in aliphatic HC fractions were shown in Fig. 7. n-C9–31 alkanes and acyclic isoprenoids were depleted completely in HC(−)-UL microcosms. n-C9–20 alkanes, pristane, and phytane were degraded, and n-C21–31 alkanes remained unchanged in HC(+)-UL microcosms. n-C9–18 and n-C20 alkanes were completely removed from HC(+)-UL microcosms.
Changes in Microbial Abundance and Activity
Initial cell and transcript abundances in HC(+)-UL and HC(−)-UL microcosms were shown in Fig. 8. The results were in accordance with the monitoring data from Halic sediments obtained between the years 2006 and 2008 [30]. Bacteria (19 ± 2 × 109 cells/ml) dominated over archaea (7.1 ± 0.6 × 109 cells/ml); archaeal community almost completely composed of methanogens. Methanogenic archaea (MA) (6.5 ± 0.6 × 109 cells/ml) was highly dominant over SRB (2.7 ± 0.1 × 109 cells/ml), denitrifying bacteria (DB; 1.1 ± 0.1 × 109 cells/ml) and dissimilatory nitrate reduction to ammonia bacteria (DNRB; 2.2 ± 0.2 × 109 cells/ml). Anaerobic aliphatic HC-degrading bacteria (AAHDB; 5.9 ± 0.6 × 109 cells/ml), anaerobic aromatic HC-degrading bacteria (AArHDB; 6.4 ± 0.5 × 109 cells/ml), and anaerobic aromatic-degrading bacteria (AArDB; 7.7 ± 0.8 × 109 cells/ml) were as abundant as MA. All of the assessed microbial groups were active. The most active processes at the time 0 were methanogenesis (7.2 ± 0.6 × 109 mRNA/ml) and HC degradation (3.5–6.9 × 109 mRNA/ml).
Bacteria, archaea, methanogenic archaea (MA), sulfate-reducing bacteria (SRB), denitrifying bacteria (DB), dissimilatory nitrate reduction to ammonia bacteria (DNRAB), anaerobic aliphatic HC-degrading bacteria (AAHDB), anaerobic aromatic HC-degrading bacteria (AArHDB), and anaerobic aromatic-degrading bacteria (AArDB) abundances and transcription levels of the related genes in HC(+)-UL and HC(−)-UL microcosms on day 0
Bacteria, Archaea, MA and SRB Abundance, and Activity Changes
Differential changes in cell and RNA abundances compared to the levels on day 0 in HC(+)-UL and HC(−)-UL microcosms were shown in Figs. 9 and 10. The most considerable increase occurred in the abundance of SRB between the days 0 and 84 during which period sulfate concentration decreased from 1,310 to 660 and 170 μM in HC(+)-UL and HC(−)-UL microcosms, respectively. Sulfate was completely depleted in the both microcosms before the day 126 after which SRB abundance decreased and no SRB activity was observed. Initial nitrate concentration in the microcosms was very low (∼30 μM); DNRB and DB activities and nitrate were not detected throughout the incubation period.
Activity and abundance of archaea, bacteria, and MA were very high and related to the C removal (Table 3) all through the incubation period. Increase in archaea abundance was ∼1.5× higher than those of bacteria; 74% and 67% of the total C removal occurred between days 126 and 168 during which period archaeal, bacterial, and methanogenic activity levels increased to ×6–8. Overall microbiological results implied that C removal in this period could be attributable to the activities of syntrophic consortium of fermentative bacteria and MA.
Changes in Activity and Abundance of HC Degraders
Differential changes in gene and transcript abundance of enzymes related to anaerobic HC degradation were shown in Fig. 11. As given in Table 3, the changes were highly correlated to C and HC removal. Anaerobic HC-degrading bacteria (AHDB) were as abundant and active as MA in the whole incubation period. AHDB activity increased to ×9–12 and ×4–5 in HC(+)-UL and HC(−)-UL microcosms, respectively, between days 126 and 168 during which period ∼70% of the HC removal took place.
Discussion
Interactions Between the Functional Microbial Groups
Shallow marine sediments are characterized by intense and diverse microbial activities, which generate steep chemical gradients [50]. As the products of O2, NO −3 , Mn(IV), Fe(III), and SO 2−4 reduction enter consecutively deeper zones of the sediments, vertical cascades of electron-accepting processes are sustained. Methanogenesis occurs after electron acceptors that yield higher standard-free energies have been depleted.
Methanogens in Halic Bay sediments were highly abundant and active along with nitrate and sulfate-reducing bacteria in 15 cmbsf (penetration depth of the grab sampler.) This was an expected result since NO −3 and SO 24 levels in the sediments were very low compared to the exceptionally high electron donor (TOC and TPH) contents (please check “sampling and characterization of the sediment” part in “Methods”). Most of the Fe(III) and Mn(IV) in sediments form insoluble oxides, colloids, and organic complexes [31]; the low abundance of dissimilatory Fe(III) and Mn(IV) reducers was an indicator of bio-available Fe(III) and Mn(IV) limitation in Halic Bay sediments [24]. It can be speculated that limited amount of electron acceptors were quickly depleted in a very short distance below the sediment surfaces which resulted in dominancy of methanogens [50]. This was also a case for the microcosms in which no external e−-acceptor was added. The low NO −3 and SO 24 contents of the sediments were completely depleted during the initial stages of microcosm incubation, afterwards methanogenic activity and abundance increased substantially. Dissimilatory sulfate reduction was dominant over methanogenesis till the sulfate was depleted, which was apparently due to higher standard-free energy yield of sulfate reduction [50]. The overall results showed that gas production after the day 126 was originated from activities of syntrophic consortium of fermentative bacteria and methanogenic archaea.
The dominancy of DNRA over denitrification (Fig. 8) was not surprising in organic carbon rich Halic Bay sediments. Although the conditions promoting DNRA and denitrification are similar, DNRA is thought to be favored in nitrate-limited environments rich in organic carbon, while denitrification would be favored under carbon-limited conditions [6]. Tiedje [56] argued that high-labile carbon availability would favor organisms that used electron acceptors most efficiently; DNRA transfers eight electrons per mole of nitrate reduced, whereas denitrification only transfers five.
Biodegradability of the Added HC Types
The degraded HCs by Halic Bay sediments were shown in Figs. 6 and 7. Only benzo(g,h,i)perylene was recalcitrant to biodegradation and remained intact during the incubation period. The HC removal in microcosms was linked to biotic processes as attested by: (1) the much higher gas production in HC(+) and HC(−) microcosms compared with the sterile controls, (2) the increasing abundance and continuous transcription of the genes related to anaerobic HC degradation, and (3) the high correlations between C removal and microbial activity.
C9–31 n-alkanes and acyclic isoprenoid alkanes (pristane and phytane) have previously been reported to be biodegradable under nitrate- and sulfate-reducing and methanogenic conditions ([18, 62] and references therein). Studies using marine sediments as inoculum produced much of the biodegradability reports on C20–31 n-alkanes [25, 35, 36]. We confirmed that marine benthic microbiota is able to degrade long chain n-alkanes.
BTEX components are the best-studied substrates of anaerobic biodegradation since they are the most water soluble of the aromatic HCs. As expected, Halic Bay sediments degraded BTEX faster than the PAHs. Literature on PAH degradation by anoxic marine sediments was mainly obtained under sulfate-reducing conditions because of overwhelming abundance of sulfate in seawater ([14, 62] and references therein). Our study is the first that reports three- to five-ring PAHs were degraded by marine sediments under methanogenic conditions. Besides, this is the first indication of acenaphtylene biodegradation.
Our findings on biodegradation hierarchy of different HC types were coincided with the previous reports ([14, 18] and references therein): short-chain HCs were degraded faster than long chain HCs; aromatic HCs were only degraded after significant removal of n-alkanes and alteration of acyclic isoprenoids pristane and phytane.
The most biodegradable HCs under anaerobic conditions are straight-chain n-alkanes, followed by more resistant branched acyclic and monocyclic hydrocarbons, the most resistant polycyclic steroidal and triterpenoidal hydrocarbons, and aromatic hydrocarbons ([14, 18] and references therein). In this study, the sequence of removal of different HC types coincided with the previous findings.
The Pathway of Anaerobic HC Degradation in Halic Bay Sediments
Overall microbiological results obtained in this study implied that assA, bssA, and bcrA genes were carried by syntrophic methanogenic consortium, and fermentative bacteria most probably carried out the initial attack on HCs during the exponential growth phase. Initial activation of aromatic HCs is crucial for anaerobic biodegradation, and four general enzymatic reactions are recognized: (1) addition of fumarate, catalyzed by a glycyl radical enzyme such as bss; (2) methylation of unsubstituted aromatics; and (3) hydroxylation of an alkyl substituent ([15] and references therein). The other proposed pathways represent a combination of these reactions. These activation reactions feed into pathways that result in production of central metabolites such as benzoyl-coenzyme A, which are eventually incorporated into biomass or completely oxidized.
Bss has been the only identified enzyme, which specifically attacks on aromatic HCs (toluene and xylene) [14]; bcr catalyzes dearomatization of the central metabolite benzoyl-coenzyme A. This is why we chose bcr and bss genes and their transcripts as indicators of anaerobic aromatic HC degradation. Abundance of these genes gradually increased during the incubation period and their transcription levels were very high even after depletion of toluene and xylene. These implied that fumarate addition was the main route of initial activation of the other aromatic HCs. Fumarate addition has previously been proposed to be included in degradation pathways of the other monoaromtic HCs (benzene, alkylbenzenes, and ethylbenzene) and PAHs (naphtalene and phenanthrene) ([14] and references therein).
The two main mechanisms of anaerobic n-alkane degradation involves (1) activation at the subterminal carbon of the alkane and addition to a molecule of fumarate and (2) the alkane carboxylation at C-3 [18 and references therein]. Recently, Callaghan et al. [7] reported a glycyl radical type enzyme (assA) which involve in alkane activation through fumarate addition in the SRB strain AK-01. This was the first description of a gene specifically involved in anaerobic n-alkane metabolism. Our results imply that fumarate addition was the main mechanism of initial activation of n-alkanes since both abundance and transcription level of the ass gene was very high throughout the incubation period.
Anaerobic Bioremediation Feasibility of the Chronically Polluted Sediments
Anaerobic biodegradation processes are a significant component of natural attenuation owing to the abundance of anoxic electron acceptors relative to dissolved oxygen. Furthermore, clean-up systems based on anaerobic biodegradation require less human intervention [21]. Studies on the anaerobic/anoxic biodegradation of the HCs in natural habitats, microcosms and enrichment cultures were initiated to determine whether or not bioremediation processes are possible in petroleum/fuel-contaminated anoxic sediments, groundwaters and aquifers [62]. Although numerous reports have been published documenting natural attenuation of HCs in these environments [3, 14, 32, 33], bioremediation strategies based on anaerobic microbial processes are very limited because they proceed at much lower rates than the aerobic ones [19, 35, 36].
Recent reports on the anaerobic HC degradation rates of marine sediments or marine enrichment cultures were summarized in Table 4. As seen, none has achieved anaerobic HC degradation rates comparable to the aerobic ones. In this study, we obtained anaerobic HC degradation rates as fast as SRB enrichment cultures’ rates via addition of the limiting nutrients to Halic Bay sediments which resulted in substantial microbial activity increases (approximately ninefold). Although the obtained rates were comparable to the aerobic ones, they are still much lower than the aerobic HC degradation rates. Moreover, aerobic microorganisms degrade a wider range of HC compounds than anaerobic microorganisms [8, 20]. Nevertheless, an aerobic bioremediation strategy is unfeasible for Halic Bay since oxygen penetration into the anoxic sediments is poor and oxygen mass transfer enhancement by mechanical means is inappropriate for the inaccessible sediments. Moreover, economical long-term solution for the chronic HC pollution by continuous aeration of the huge anoxic area in Halic Bay is out of the question. Under these conditions anaerobic HC degradation is the only alternative.
In summary, we have obtained three lines of evidence for demonstrating anaerobic bioremediation feasibility of petroleum HC pollution in Halic Bay sediments: (1) the anaerobic HC-degrading microorganisms were highly abundant in the sediments [30]; (2) anaerobic HC degradation was taking place in the sediments [30]; (3) the sediments were able to degrade wide range of HCs under anaerobic/anoxic conditions (this study); (4) high anaerobic HC degradation rates were achieved via biostimulation of the sediments through nutrient amendment (this study).
As given in Table 4, almost all of the studies on anaerobic hydrocarbon degradation in marine sediments were conducted ex situ. There were two studies reporting in situ anaerobic hydrocarbon degradation in marine sediments: (1) Miralles et al. [35, 36] subjected Mediterranean coastal sediments to massive crude oil inputs and observed alteration of n-alkanes by SRB; (2) Tang et al. [55] enhanced in situ anaerobic phenanthrene biodegradation rates by factors up to 2–3 in undisturbed marine sediments via controlled-release of nitrate and sulfate. The obtained in situ hydrocarbon degradation rates in these studies were very low compared to those from ex situ incubations (Table 4), which highlights the gap between microcosm results and reality of the field. Hence, we are planning to confirm our ex situ findings via a pilot-scale in situ biostimulation trial: Halic Bay sediments will be (1) sampled, (2) reworked by addition of slow release fertilizers which prolong duration of nutrient release up to 6 months [38], (3) placed in PVC cores, and (4) reinserted into sampling site. If a remarkable enhancement of natural attenuation is observed in the cores, the slow release fertilizers will be applied over a large area of Halic Bay subsurface to remove the accumulated HCs. The fertilizers will be supplied in the form of granules that were shown to adhere tightly to the oiled sediment material [45].
We are now making the preliminary preparations in conjunction with Maritime Undersecretariat of Turkish Republic, ISKI and IBB to scale-up our microcosm trials, the success of which will certainly lead to less human intervened and more economical field-scale bioremediation applications for highly polluted anoxic marine environments worldwide.
References
Ahmed A, Webster L, Pollard P, Davies I, Russell M, Walsham P, Packer G, Moffat C (2006) The distribution and composition of hydrocarbons in sediments from the Fladen ground, North Sea, an area of oil production. J Environ Monit 8:307–316
Anderson RT, Lovley DR (2000) Anaerobic bioremediation of benzene under sulfate-reducing conditions in a petroleum-contaminated aquifer. Environ Sci Technol 34:2261–2266
Andreoni V, Gianfreda L (2007) Bioremediation and monitoring of aromatic-polluted habitats. Appl Microbiol Biotechnol 76:287–308
Beller HR, Kane SR, Legler TC, Alvarez PJJ (2002) A real-time polymerase chain reaction method for monitoring anaerobic, HC degrading bacteria based on a catabolic gene. Environ Sci Technol 36:3977–3984
Boopathy R (2003) Anaerobic degradation of no. 2 diesel fuel in the wetland sediments of Barataria-Terrebonne estuary under various electron acceptor conditions. Bioresour Technol 86:171–175
Burgin AJ, Hamilton SK (2007) Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ 5:89–96
Callaghan AV, Wawrik B, Chadhain SMN, Young LY, Zylstra GJ (2008) Anaerobic alkane-degrading strain AK-01 contains two alkylsuccinate synthase genes. Biochem Biophys Res Commun 366:142–148
Cao B, Nagarajan K, Loh K (2009) Biodegradation of aromatic compounds: current status and opportunities for biomolecular approaches. Appl Microbiol Biotechnol 85:207–228
Colwell FS, Boyd S, Delwiche ME, Reed DW, Phelps TJ, Newby DT (2008) Estimates of biogenic methane production rates in deep marine sediments at hydrate ridge, cascadia margin. Appl Environ Microbiol 74:3444–3452
Cross KM, Biggar KW, Semple K, Foght J, Guigard SE, Armstrong JE (2006) Intrinsic bioremediation of diesel-contaminated cold groundwater in bedrock. J Environ Eng Sci 5:13–27
Cunningham JA, Rahme H, Hopkins GD, Leb-ron C, Reinhard M (2001) Enhanced in situ bioremediation of BTEX-contaminated groundwater by combined injection of nitrate and sulfate. Environ Sci Technol 35:1663–1670
Da Silva ML, Alvarez PJJ (2002) Effects of ethanol versus MTBE on BTEX migration and natural attenuation in aquifer columns. ASCE J Env Eng 128:862–867
Davidova IA, Gieg LM, Duncan KE, Suflita JM (2007) Anaerobic phenanthrene mineralization by a carboxylating sulfate-reducing bacterial enrichment. ISME J. doi:10.1038/ismej.2007.48
Foght J (2008) Anaerobic biodegradation of aromatic HCs: pathways and prospects. J Mol Microbiol Biotechnol 15:93–120
Fuchs G (2008) Anaerobic metabolism of aromatic compounds. Ann NY Acad Sci 1125:82–99
Gavala HN, Angelidaki I, Ahring BK (2003) Kinetics and modeling of anaerobic digestion process. Adv Biochem Eng Biotechnol 81:57–93
Geets J, Borremans B, Diels L, Springael D, Vangronsveld J, van der Lelie D, Vanbroekhoven K (2006) dsrB gene-based DGGE for community and diversity surveys of sulfate-reducing bacteria. J Microbiol Methods 66:194–205
Grossi V, Cravo-Laureau C, Guyoneaud R, Ranchou-Peyruse A, Hirschler-Rea A (2008) Metabolism of n-alkanes and n-alkenes by anaerobic bacteria: a summary. Org Geochem 39:1197–1203
Harayama S, Kasai Y, Hara A (2004) Microbial communities in oil-contaminated seawater. Curr Opin Biotechnol 15:205–214
Haritash AK, Kaushik CP (2009) Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater 169:1–15
Head IM, Swannell RP (1999) Bioremediation of petroleum HC contaminants in marine habitats. Curr Opin Biotechnol 10:234–239
Henry S, Bru D, Stres B, Hallet S, Philippot L (2006) Quantitative detection of the nosZ Gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl Environ Microbiol 72:5181–5189
Ince O, Akarsubasi AT, Sayi N, Eyice O, Ovez s, Ince BK (2006) Analysis of anaerobic microbial diversity in Haliç (marine inlet) sediment by molecular tools. Adv Mol Med 2:71–77
Ince O, Kolukirik M, Ince BK (2009) Anaerobic degradation of petroleum HCs in anoxic marine environments. Final Report of TUBITAK 105Y307 Project (Turkish Academy of Sciences).
Jones DM, Head IM, Gray ND, Adams JJ, Rowan AK, Aitken CM, Bennett B, Huang H, Brown A, Bowler BF, Oldenburg T, Erdmann M, Larter SR (2008) Crude-oil biodegradation via methanogenesis in subsurface petroleum reservoirs. Nature 451:176–180
Kim M, Bae SS, Seol M, Lee JH, Oh YS (2008) Monitoring nutrient impact on bacterial community composition during bioremediation of anoxic PAH-contaminated sediment. J Microbiol 46:615–623
Klappenbach JA, Saxman PR, Cole JR, Schmidt TM (2001) rrndb: the ribosomal RNA operon copy number database. Nucleic Acids Res 29:181–184
Kniemeyer O, Musat F, Sievert SM, Knittel K, Wilkes H, Blumenberg M (2007) Anaerobic oxidation of short-chain HCs by novel marine sulphate-reducing bacteria. Nature 449:898–901
Kolukirik M, Ince O, Ince BK (2011a) Local and seasonal changes in microbial diversity of the Marmara sea sediments. Mar Pollut Bull MPB-D-09-00623 (in press).
Kolukirik M, Ince O, Ince BK (2011b) Metabolic activity variations in Marmara sea sediments. Microb Ecol MECO-2010-0253 (in press).
Lovley DR (1991) Dissimilatory Fe(III) and Mn(IV) reduction. Microbiol Rev 55:259–287
Madigan MT, Martinko JM, Dunlap PV, Clark DP (2009) Brock biology of microorganisms, 12th edn. Pearson Benjamin-Cummings, San Francisco
Meckenstock RU, Safinowski M, Griebler C (2004) Anaerobic degradation of polycyclic aromatic HCs. FEMS Microbiol Ecol 49:27–36
Millero FJ (1996) Chemical oceanography. CRC Press, Boca Raton. pp, 469
Miralles G, Grossi V, Acquaviva M, Duran R, Bertrand JC, Cuny P (2007) Alkane biodegradation and dynamics of phylogenetic subgroups of sulfate-reducing bacteria in an anoxic coastal marine sediment artificially contaminated with oil. Chemosphere 68:1327–1334
Miralles G, Nerini D, Mante C, Acquaviva M, Doumenq P, Michotey V, Nazaret S, Bertrand JC, Cuny P (2007) Effects of spilled oil on bacterial communities of mediterranean coastal anoxic sediments chronically subjected to oil HC contamination. Microb Ecol 54:646–661
Musat F, Galushko AS, Jacob J, Widdel F, Kube M, Reinhardt R, Wilkes H, Schink B, Rabus R (2008) Anaerobic degradation of naphthalene and 2-methylnaphthalene by strains of marine sulfate-reducing bacteria. Environ Microbiol 11:209–219
Nikolopoulou M, Kalogerakis N (2010) Biostimulation strategies for fresh and chronically polluted marine environments with petroleum hydrocarbons. J Chem Technol Biotechnol 84:802–807
Orhon D, Artan A (1994) Modelling of activated sludge systems. Technomic Publishing Company, Pennsylvania
Pekey B, Karakas D, Ayberk S (2007) Atmospheric deposition of polycyclic aromatic HCs to Izmit Bay, Turkey. Chemosphere 67:537–547
Phelps CD, Young LY (1999) Anaerobic biodegradation of BTEX and gasoline in various aquatic sed- iments. Biodegradation 10:15–25
Philippot L (2005) Tracking nitrate reducers and denitrifiers in the environment. Biochem Soc Trans 33:200–204
Powell SM, Ferguson SH, Snape I (2006) Siciliano SD:fertilization stimulates anaerobic fuel degradation of Antarctic soils by denitrifying microorganisms. Environ Sci Technol 40:2011–2017
Prince RC, JM Suf lita (2007) Anaerobic biodegradation of natural gas condensate can be stimulated by the addition of gasoline. Biodegradation. doi:10.1007/s10532–006–9084–4
Pritchard PH, Mueller JG, Rogers JC, Kremer FV, Glaser JA (1992) Oil spill bioremediation: experiences, lessons and results from the Exxon Valdez oil spill Alaska. Biodegradation 3:109–132
Rothermich MM, Hayes LA, Lovley DR (2002) Anaerobic, sulfate-dependent degradation of polycyclic aromatic HCs in petroleum-contaminated harbour sediment. Environ Sci Technol 36:4811–4817
Röling WFM, Milner MG, Jones DM, Lee K, Daniel F, Swannell RJP, Head IM (2002) Robust HC degradation and dynamics of bacterial communities during nutrient-enhanced oil spill bioremediation. Appl Environ Microbiol 68:5537–5548
Ruppel S, Rühlmann J, Merbach W (2006) Quantification and localization of Bacteria in plant tissues using quantitative real-time PCR and online emission fingerprinting. Plant Soil 286:21–35
Schreiber ME, Bahr JM (2002) Nitrate-enhanced bioremediation of BTEX-contaminated groundwater: parameter estimation from natural-gradient tracer experiments. J Contam Hydrol 55:29–56
Schulz HD, Zabel M (2000) Marine Geochemistry. Springer, Berlin, pp 85–128, and 173–207
Smith CJ, Nedwell DB, Dong LF, Osborn AM (2007) Diversity and abundance of nitrate reductase genes (narG and napA), nitrite reductase genes (nirS and nrfA), and their transcripts in estuarine sediments. Appl Environ Microbiol 73:3612–3622
Song B, Ward BB (2005) Genetic diversity of benzoyl coenzyme A reductase genes detected in denitrifying isolates and estuarine sediment communities. Appl Environ Microbiol 71:2036–2045
Syakti AD, Mazzella N, Nerini D, Guiliano M, Bertrand JC, Doumenq P (2006) Phospholipid fatty acids of a marine sedimentary microbial community in a laboratory microcosm: responses to petroleum HC contamination. Org Geochem 37:1617–1628
Takai K, Horikoshi K (2000) Rapid detection and quantification of members of the archaeal community by quantitative PCR using fluorogenic probes. Appl Environ Microbiol 66:5066–5072
Tang YJ, Carpenter S, Deming J, Krieger-Brock-ett B (2005) Controlled release of nitrate and sulfate to enhance anaerobic bioremediation of phenanthrene in marine sediments. Environ Sci Technol 39:3368–3373
Tiedje JM (1988) Ecology of denitrification and dissimilatory nitrate reduction to ammonium. In: Zehnder AJB (ed) Biology of anaerobic microorganisms. Wiley, New York
Tolun LT, Martens D, Okay O, Schramm KW (2006) Polycyclic aromatic hydrocarbon contamination in coastal sediments of İzmit Bay (Marmara Sea): case studies before and after Marmara earthquake. Environ Int 32:758–765
UNEP (United Nations Environment Programme) (1991) Determinations of petroleum HCs in sediments. Ref Meth Mar Pollut Stud 20:97
Unlu S, Alpar B (2006) Distribution and sources of HCs in surface sediments of Gemlik Bay (Marmara Sea, Turkey). Chemosphere 64:764–777
Vrede K, Heldal M, Norland S, Bratbak G (2002) Elemental composition (C, N, P) and cell volume of exponentially growing and nutrient-limited bacterioplankton. Appl Environ Microbiol 68:2965–2971
Widdel F, Bak W (1992) In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer K-H (eds) The Prokaryotes, vol. 4, 2nd edn. Springer, New York, pp 3352–3379
Widdel F, Rabus R (2001) Anaerobic biodegradation of saturated and aromatic HCs. Curr Opin Biotechnol 12:259–276
Zhang T, Fang HHP (2006) Applications of real-time polymerase chain reaction for quantification of microorganisms in environmental samples. Appl Microbiol Biotechnol 70:281–289
Acknowledgments
This work was supported by the Scientific and Technological Research Council of Turkey grant TUBITAK 105Y307. Authors would also like to acknowledge Turkish Petroleum Corporation (TPAO) for their cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kolukirik, M., Ince, O. & Ince, B.K. Increment in Anaerobic Hydrocarbon Degradation Activity of Halic Bay Sediments via Nutrient Amendment. Microb Ecol 61, 871–884 (2011). https://doi.org/10.1007/s00248-011-9825-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-011-9825-8