Abstract
Herbivory and bacterivory by phagotrophic protists were estimated in the southern basin of the oligotrophic Lake Tanganyika at different seasons (in the rainy season in February–March 2007 and in the dry season in July–August 2006 and September 2007), using two independent methods: the selective inhibitor technique for assessing community grazing on picocyanobacteria (PCya) and fluorescently labelled bacteria (FLB) and Synechococcus (FLA) to estimate bacterivory and herbivory by phagotrophic nanoflagellates (NF) and ciliates. Protistan grazing impact on both heterotrophic bacteria and PCya was mainly due to NF, which contributed up to 96% of the microbial grazing. There was a clear selection of FLA by protists. PCya represented the main carbon source for both flagellates and ciliates in the mixolimnion, accounting for an average of 83% of the total carbon obtained from the ingestion of picoplanktonic organisms. Protists were the main consumers of particulate primary production (46–74% depending on season). Significant seasonal variation of grazing rates (0.011–0.041 h−1) was found, chiefly following variation of PCya production and biomass. Assuming a growth efficiency of 0.4, total protozoan production varied seasonally (189–313 g C m−2 day−1) and was roughly half of particulate phytoplankton production. This study provides evidence that NF and PCya were tightly coupled in Lake Tanganyika and that herbivory by protists may be one of the reasons why this great lake has high productivity. Our results bring support to the idea that microbial herbivory is a major process in oligotrophic freshwater systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Theoretical and experimental data converge to the idea that small organisms tend to dominate in warm oligotrophic aquatic environments, where most part of the living biomass is allocated in the picoplankton (fraction < 2 μm) and submitted to high grazing pressure by protists ([1] and references therein). Protistan preferences on autotrophic or heterotrophic picoplankton in such environments may have major ecological consequences on nutrient recycling, as heterotrophic bacteria (HB) or photosynthetic picoplankton (PPP) live on different sources of energy and exhibit different nitrogen-to-phosphorus ratios [2].
Photosynthetic picoplankton is a major fraction of primary producers in warm oligotrophic systems [3]. These organisms are too small to be efficiently grazed by crustacean zooplankton (e. g. [4]); therefore, the main grazers consist of phagotrophic nanoflagellates (NF) and ciliates, which prey upon both autotrophic and heterotrophic picoplankton [5–7]. A few studies [8–10] have highlighted the importance of the microbial food web in large temperate lakes in specific periods of the annual cycle, and recent works suggest that in large tropical lakes the role of the microbial food web could be of even greater importance [11–14].
The carbon fluxes in the microbial food web are highly dependent on the type of predator, grazing rates and prey preferences. Heterotrophic nanoflagellates (HNF) are often the major grazers. In the subalpine Lake Maggiore, Callieri et al. [8] estimated a grazing impact by the HNF on PPP about one order of magnitude higher than the one exerted by ciliates. Studies carried out on bacterial mortality also identified HNF as the main bacterivores in aquatic systems [6]. Conversely, ciliate grazing on HB can overcome flagellates grazing in eutrophic systems [15].
Concerning the prey preferences, it has been suggested that protistan herbivory is a major process in carbon cycling in oligotrophic oceans, probably more significant than protistan bacterivory [2, 16]. In contrast, in a coastal system, Caron et al. [17] reported a significant contribution of Synechococcus in the protist diet only when picocyanobacteria (PCya) cell abundance peaked.
PPP and HB do occupy a very different trophic position, as HB derives their energy from organic carbon and PPP from light. On the other hand, both categories of microorganisms are in the picoplankton size range, providing a major source of carbon and nutrients for protists (e.g. [18–20]). However, their fate has often been studied separately in freshwater systems. One of the rare published studies with simultaneous grazing measurements on HB and PPP by protozoan in natural freshwater systems provides evidence for a dominant role of protozoan grazing on PCya in the carbon flow and nutrient cycling in an Eastern Alps lake [21]. So far, no measurements of protozoan bacterivory and herbivory have been conducted in large tropical lakes, despite the continuously (in the complete year cycle) dominant role of the microbial food web in their productivity [11, 22, 23].
Lake Tanganyika, located in the East African Rift Valley, is one of the best studied large tropical lakes [24] with recent studies addressing mostly fish and invertebrate biodiversity [25, 26], phytoplankton and zooplankton dynamics and productivity [27–29], as well as palaeoclimatic reconstruction based on the sediment record [30–32]. Yet several aspects of its ecological functioning, among which the processes involved in controlling its productivity, are still to uncover. Indeed, studies on carbon transfer through the food web addressed the relations between hydrodynamics and production of elements of the classic food web [33], despite early ecological studies that had hypothesized a key role of bacteria in the food web [23], through their ingestion by ciliates, adding to the resources available to metazooplankton. Recent studies on microbial ecology in Lake Tanganyika have quantified bacterial abundance (2.3–5.30 × 106 cells mL−1 [34]; 1.2 × 105–4.8 × 106 cells mL−1 [35]) and biomass (1.7–2.1 g C m−2 [36]), i.e. roughly half of average phytoplankton biomass (∼3.8 g C m−2 [34]). According to Stenuite et al. [13], bacterial production (BP) in Lake Tanganyika (93–735 mg C m−2 day−1) overlaps with that of phytoplankton (150–1687 mg C m−2 day−1). Although this suggests that bacteria add up significant amounts of organic carbon for the pelagic food web, investigations on HB grazing by heterotrophic protists have provided evidence that it may not be the case. Indeed, Pirlot et al. [11] estimated an average protistan grazing rate on HB of about 190 mg C m−2 day−1. This may seem a significant flux, but because of the high losses from bacterial and protozoan respiration, the DOC taken up by bacteria could only sustain 5–8% of the estimated protozoan production [11, 36].
Therefore, we hypothesize that NF and ciliates in Lake Tanganyika meet most their carbon requirements in the euphotic layer by feeding preferentially on the PPP, which constitutes the dominant fraction of the phytoplankton in this tropical large lake and mainly consists of PCya (Synechococcus spp. [14, 27, 29]). Sarmento et al. [12] have shown that PCya abundance and biomass in Lake Tanganyika was among the largest known worldwide. Grazing on PPP by NF and ciliates would make a direct link between primary producers and protists, increasing the efficiency of carbon transfer through the microbial food web and allowing fast nutrient recycling. Here, we assessed grazing on HB and PCya by protists in the southern basin of Lake Tanganyika, taking into account seasonal variations, addressing the question of herbivory vs. bacterivory in this oligotrophic freshwater ecosystem.
Materials and Methods
Study Site, Sampling and Environmental Conditions
Grazing experiments were performed at the southern basin of the lake (off Mpulungu, Zambia; 08°43.98′ S, 31°02.43′ E), during three sampling campaigns: in July–August 2006 and September 2007, during the dry season, and in February–March 2007, during the rainy season, totalling nine experiments. Water samples were collected offshore using a 5-L Niskin bottle, from 0-, 10-, 20- and 30-m depths, and then pooled together to get a representative sample of the epilimnetic layer, where the microbial food web compartments are known to be the more active. Pooled water samples were then gently poured through a 100-μm mesh to remove larger planktonic organisms. Environmental and limnological conditions during the study were reported in detail elsewhere [14]. Shortly, the depth of the mixed layer was ∼25 m in the rainy season (i.e. stratified conditions) and could reach 100 m in the dry season, due to the seasonal upwelling and from intense vertical mixing. Temperature varied between a maximum of 28.8°C in the rainy season and a minimum of 24.3°C in the dry season. While dissolved inorganic nitrogen was on average ∼50 μg L−1 and did not present a well-defined seasonal pattern, dissolved phosphorus concentration varied strongly, depending on the water column status, between ∼1 μg L−1 in the rainy season and 47 μg L−1 in the dry season.
Estimate of Grazing Rates by Protists
We used the selective inhibitor technique (SIT) [7, 37, 38] for estimating the whole protozoan community grazing on PCya. We also conducted ingestion experiments with fluorescently labelled algae (FLA) and bacteria (FLB) [39] to assess the specific grazing by NF and ciliates on PCya and HB in Lake Tanganyika. Ingestion estimates of fluorescent tracers are convenient and widely used methods for estimating specific grazing rates of protists on picoplankton (e. g. [40–42]).
Protistan Community Grazing Rate Assessment—SIT
The SIT technique consists in measuring prey abundance (PCya) at the beginning (T 0) and at the end (T 6) of the experiments run with and without adding cycloheximide, an inhibitor of grazers activity. The first batch, cycloheximide-free, allows assessing the prey increase rate (k), while the other one, with inhibited predators, provides estimates of the prey growth rate (μ). Previously to the all nine experiments, 100-μm filtered water samples were distributed into acid-washed 500-mL polycarbonate Nalgene bottles. Bottles were filled with water, avoiding air bubbles. Cycloheximide (100 mg L−1, final concentration) was then added in three experimental bottles, while three others were used as controls. This eukaryotic inhibitor was preferred to ampicillin because prokaryotic inhibitors were reported to inhibit mitochondrial activity of predators [43]. The efficiency of cycloheximide to stop grazer activity was previously checked by adding FMP (Fluoresbrite® YG Microspheres, Polysciences) to an experimental set. All bottles were incubated for 6 h in the lake, at a depth where light corresponded to the average light at all depths used for the pooled samples. Samples for PCya abundances (18 mL) were taken from each bottle at the beginning and the end of the experiments, fixed with cold glutaraldehyde (1%, final concentration) and filtered through a 0.2-μm pore-size polycarbonate black filter (Nuclepore, Whatman International Ltd, Maidstone, UK). Triplicate 100-mL samples for determination of predator abundances were taken from the initial 100-μm filtered water, fixed with 100 mL of cold glutaraldehyde (2%, final concentration), stained with 4,6 diamidino-2-phenylindole (DAPI; 10 μg mL−1, final concentration) and filtered through a 0.8-μm polycarbonate black filter (Nucleopore, Whatman). All these samples were stored at −20°C before enumeration of both prey and predators by epifluorescence microscopy, as explained in the FLA–FLB protocol section. Equations described by Cuevas and Morales [7] were then used to get both prey growth rate (μ) and net rate of increase (k) and the grazing rate (g):
where t is the incubation time and C t and C 0 are prey concentrations at the end and the beginning of the incubations.
Specific Grazing Rate Measurement—FLA, FLB
Estimates of protozoan (NF and ciliates) grazing rates on bacteria and PCya were determined from the ingestion of fluorescent tracers following the method described by Sherr and Sherr [39]. Two kinds of tracers were used: FLB (Brevundimonas diminuta) and FLA (Synechococcus sp.). FLB were prepared from a B. diminuta (syn. Pseudomonas diminuta) strain obtained from the Belgian Co-ordinated Collections of Micro-organisms (Belgium). Bacteria were stained with 5-([4,6-dichlorotriazin-2yl] amino)-fluorescein as described by Sherr et al. [44]. The size of FLB was checked by epifluorescence microscopy and image analysis, as explained below. Most cells ranged between 0.02 and 0.13 μm3 (mean 0.09 μm3), with a mean diameter of 0.52 μm, in the same size range as that of Lake Tanganyika HB [34]. FLA were produced following the same protocol as for FLB, but using a mixture of freshwater Synechococcus-like strains isolated from different Czech reservoirs (strain number 4-3 Lucina reservoir, 5-5 Jesenice reservoir, 8-3 Zlutice reservoir and 10-2 Nova Rise) by Dr J. Jezberová (Hydrobiological Institute, Czech Republic). Most cells biovolume ranged between 0.23 and 0.47 μm3 with a mean value of 0.29 μm3. In Lake Tanganyika, PCya cell volume fluctuated between 0.19 and 0.53 μm3 [14, 35]. FLA and FLB were kept frozen (−20°C) until use.
Nine grazing experiments were carried out in duplicates for both tracers, together with the incubations using SIT. Before addition to the experimental containers, each tracer working solution was thawed and gently sonicated during 15 min in an ultrasonic bath (Branson B2510) to prevent cells from clustering. Tracers were added at about 4–20% of natural HB or PCya concentrations to individual containers filled with 3 L of experimental water samples. The water with the tracers was gently mixed and distributed in equal volume into two 1.5-L transparent plastic bottles. Samples for estimation of HB, PCya (respectively 2 and 20 mL of water fixed with 10% glutaraldehyde, 1% final concentration) and tracer abundance were taken at time 0 (T 0), while 100-mL samples for ingestion of tracers by protists were taken at both T 0 and T 15 (15 min of incubation) and fixed with 100 mL of 4% cold glutaraldehyde (2%, final concentration). Samples for prey and tracers counts were then filtered through a 0.2-μm pore-size polycarbonate black filter (Nucleopore, Whatman); samples for protistan abundances and for determination of tracer ingestion rate were filtered through a 0.8-μm pore-size polycarbonate black filter. Before filtration, samples for bacterial abundances and grazing assessment were stained with DAPI (10 μg mL−1, final concentration). All filters were frozen (−20°C) before their processing using epifluorescence microscopy.
HB and PCya abundances in the experiments were determined using a Zeiss Axioplan epifluorescence microscope. Cells were enumerated at ×1,000 magnification from ten randomly chosen fields. Picoplankton cell biomass was estimated using a conversion factor of 15 fg C cell−1 for HB [13] and 71 and 93 fg C cell−1 for PCya during the rainy and the dry seasons, respectively [14]. FLA and FLB concentrations were calculated under blue light excitation, using the same filters as for HB and PCya counts, respectively. NF and ciliate abundances were assessed following the same procedure as for picoplankton. An average of 470 NF and 100 ciliates were enumerated on each filter (three replicates), totalizing 670–2,000 NF and 300–370 ciliates per experiment. The number of ingested tracers was counted on the same filter. Colourless flagellates were considered to be heterotrophic. Proven mixotrophic cells (plastidic forms with ingested particles) were included in the counts.
Ingestion rates (tracers per cell per hour) were determined from the linear slope of tracer concentration inside protists during the first 15 min of incubation. This incubation time was chosen according to Pirlot et al. [11]. An average of 200 flagellates and 60 ciliates were examined on each filter (two replicates and two times). Thus, a total of about 800 NF and 200–310 ciliates were examined for ingestion of tracers in each experiment. Clearance rates (nanolitres per cell per hour) were calculated by dividing the ingestion rate by the corresponding tracer concentration. Specific grazing rates (HB or PCya per cell per hour) for each group were estimated by multiplying the corresponding clearance rate by the HB or PCya concentration, assuming that lake picoplankton and tracers were ingested at the same rates. Grazing impact (HB or PCya per millilitre per hour) of each flagellate and ciliate group was estimated by multiplying the specific grazing rate by the abundance (individuals per millilitre) of the corresponding protists.
Phytoplankton Biomass and Production
Total phytoplankton and PPP production was measured using 14C incubations in the field [45], run as in Stenuite et al. [29]. The contribution of PPP to total phytoplankton biomass and production was estimated by filter-fractionation. Water from the pooled samples from the 0–30-m layer was screened through 28 and 10 μm Nytex plankton nets, before filtration on a Millipore membrane of 2 μm pore size. The unfiltered sample and the <2-μm fractions were collected on Macherey-Nägel GF5 filters, of 0.7 μm nominal pore size; the <2-μm fraction was considered as comprising only picoplankton. For estimating contribution of PPP to phytoplankton production, the filtrations were carried out immediately after the 14C incubations. Biomass of total phytoplankton and of PPP was obtained by HPLC measurements of chlorophyll a (chla) extracted from the filters (details in [27]), and results were expressed in milligrams of chla per cubic metre. HPLC marker pigments allowed determination of the contribution of PCya to PPP, using CHEMTAX, as described in [27].
Results
Protozoan and Prey Abundance
During the experiments, the PCya abundance varied between 2.42 and 6.77 × 105 cells mL−1, with no significant difference between seasons (Student’s t test, p > 0.05). Bacterial counts varied from 2.67 × 105 and 3.21 × 106 cells mL−1 and were significantly higher during the dry season (Student’s t test, p < 0.05). Actually, HB abundances observed during the rainy season 2007 were particularly low for the usual values found in the epilimnion (average = 4.74 × 105 mL−1).
NF abundances ranged between 1.22 × 103 and 2.64 × 103 cells mL−1. Mixotrophy was clearly detected among chrysophytes, cryptophytes and dinoflagellates; these mixotrophs accounted for 0.9 to 2.9% of total phagotrophic NF abundances.
Ciliate numbers ranged between 5.35 and 21.7 cells mL−1. The average value (13.7 cells mL−1) was much higher than the maximum value (3.3 cells mL−1) reported by Pirlot et al. [11]. Such a difference can be explained by ciliate blooms during the year 2007, as abundance of these microorganisms is known to occasionally peak [46]. Among those ciliates, Strombidium sp. containing symbiotic green algae (identified as Siderocelis irregularis in [47]) were particularly abundant. Despite the fact that they can presumably obtain carbon from their symbionts, they ingested PCya and were therefore taken into account in our analysis.
Phytoplankton Biomass and Production and Contribution of PPP
In all nine grazing experiments, PPP dominated phytoplankton production and biomass (Fig. 1). The contributions of PPP to the total phytoplankton biomass and to phytoplankton production were similar, with 82.3% of chlorophyll a and 87.6% of 14C uptake in the dry season, respectively. PPP contribution was lower in the rainy season, with 53.4% of the total phytoplankton biomass and 57.8% of the total primary production. The analysis of marker pigments in the <2-μm size fraction showed that picocyanobacteria contribution to the total phytoplankton biomass ranged from to 45.1% to 97.9% throughout this study (Fig. 1).
Protozoan Community Grazing on Phototrophic Picoplankton
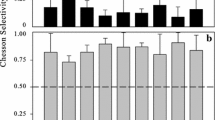
The net increase rate of PCya estimated in the SIT experiments varied from −0.005 to 0.042 h−1 (average = 0.019 h−1), while their intrinsic growth rate ranged from 0.027 to 0.072 h−1 (average = 0.046 h−1). Grazing rates ranged between 0.011 and 0.041 h−1 (Fig 2).
A comparison of the two techniques used in this study to estimate grazing rates on PCya by the total protistan assemblage is presented in Fig. 2. To get the total grazing rates (per hour) from the FLA technique, NF and ciliate grazing impacts were summed and divided by the natural PCya concentration (Table 1). Results obtained from ingestion of the fluorescent tracers were not significantly different (Student’s t test, p > 0.05) from those estimated with the selective inhibitor technique.
Protist Herbivory and Bacterivory
FLA (Synechococcus sp.) and FLB were used in parallel to evaluate specific grazing rates of NF and ciliates on PCya and HB, respectively. Because of the low abundance of mixotrophic cells, we did not discriminate their grazing impact from that associated to the heterotrophic forms. Average ingestion rates estimated from these short-term grazing experiments performed between the dry seasons 2006 and 2007 are presented in Table 1.
In terms of clearance and specific grazing rates, individual ciliates ingested around 30 times more PCya and 20 times more HB than flagellates. However, considering a mean ciliate-to-flagellate biomass ratio of 37, NF ingested more picoplanktonic cells (PCya and HB) per unit of body mass than ciliates.
Both ciliates and flagellates seemed to discriminate between types of prey, showing higher clearance rates on FLA (see Table 1). Thus, PCya represented the main carbon source for both flagellates and ciliates in the mixolimnion, accounting for an average of 83% of the total carbon obtained from the ingestion of picoplanktonic organisms.
Regarding the carbon flux, protists grazed from 8.16 to 44.56 mg C m−3 day−1 of PCya carbon vs. 1.36 to 8.15 mg C m−3 day−1 of HB carbon. There was no significant difference in protozoan community grazing on PCya between seasons (Student t test, p > 0.05).
Despite their lower specific clearance rate and owing to their larger abundance, NF had a higher grazing impact on both autotrophic and heterotrophic picoplankton than ciliates, regardless of the season (Fig. 3). In terms of carbon biomass, flagellates were responsible for 69–92% of the total PCya ingestion and for 67–96% of the total HB grazed by protists. Whereas we could not detect any significant difference in global protistan grazing on PCya between seasons, NF showed a significantly lower impact on PCya during the rainy season. The same tendency was observed for NF grazing pressure on HB (Student’s t test, p < 0.05). Such differences were not detected for ciliates.
Carbon Fluxes Through the Microbial Food Web
An estimation of the phytoplanktonic and bacterial carbon fluxes transiting to protozoans in the epilimnion of Lake Tanganyika is presented in Fig. 4. PCya grazing rate by NF and ciliates corresponded to 629 and 428 mg C m−2 day−1, during the dry and rainy seasons, respectively. These fluxes represented an average consumption of 49% and 39% of the PCya standing stock. Protistan grazing rate on HB during the dry and rainy seasons was 152 and 45 mg C m−2 day−1, respectively, corresponding to an average of 15% and 20% of the HB stock consumed. Considering the upper 30 m of the water column, NF and ciliates consumed on average 46% and 74% of the daily particulate primary production and 37% and 14% of the whole bacterial production, during rainy and dry seasons, respectively. To complete this carbon budget of the epilimnion of Lake Tanganyika, we estimated protozoan production from grazing rates, based on a growth efficiency coefficient of 40% [11, 48]. Based on this calculation, protist production rate was 313 mg C m−2 day−1 in the dry season and 189 mg C m−2 day−1 in the rainy season.
Average picoplankters and protist biomass (milligrams of C per square metre) integrated over 30-m depth, and carbon fluxes (milligrams of C per square metre per day) transiting from primary and bacterial producers to protists in the epilimnion of Lake Tanganyika, during the dry season (left) and the rainy season (right). Primary production values presented are averages from 2004 to 2007, integrated over the euphotic zone. PCya productions (PCyaP) were estimated considering a mean seasonal contribution of PCya to total primary production (from Stenuite et al. [29] and this study). BP values are averages from 2004 to 2007, integrated over the upper 100 m, which comprise most of the oxygenated layer (from Stenuite et al. [13]).
Discussion
Protists have an essential role in the carbon cycle of marine and temperate freshwater environments, through their grazing impact on autotrophic and/or heterotrophic picoplankton [49, 50]. Here we explored the herbivory and bacterivory patterns of protists in Lake Tanganyika, one of the world’s most productive inland fisheries, which has also one of the highest amounts of picoplankton reported [12]. We measured PCya growth and mortality from grazing by phagotrophic protists in nine experiments covering different seasons (dry and rainy seasons), using two independent methods: the selective inhibitor technique [7, 37, 38] and the uptake of fluorescently labelled prey [39] in order to discriminate herbivory from bacterivory by phagotrophic flagellates and ciliates. The estimates of protistan grazing rate on PCya obtained by the SIT and FLA incubations were not significantly different. Beyond the validation of our data, as results from two different techniques were similar, this adds further support to the view that microbial herbivores in Lake Tanganyika mainly consisted of nanoflagellates and ciliates. Indeed, community grazing rates estimated from SIT experiments included the effects of both protists and of larger potential grazers on PCya, while results from FLA experiments were obviously based on observations of protists only. Yet, small copepod nauplii which had ingested PCya were regularly detected in our samples, supporting the idea of non-accidental feeding [51]. Although nauplii feeding on picoplankton cannot be quantified from our data, the close correspondence between SIT and FLA results provides indirect evidence that the impact exerted by nauplii on picoplankton should be limited.
Measuring grazing is not straightforward, and every method has some limitations. In the case of ingestion of fluorescently labelled prey, the main limitation may have been the positive or negative selection for FLB and FLA, since they are not perfect analogues of living picoplankton [52]. As we used fluorescent prey having a biovolume very close that of natural bacteria and PCya (on average 0.31 to 0.41 μm3 for PCya and 0.07 μm3 for HB [14, 36]), we believe that the incidence of particle selection was reduced. The fact that very similar values of protozoan grazing impact on PCya were obtained with two independent methods (FLA and SIT) indicates that our estimates were not biased by particle selection.
The PCya growth rates we measured in Lake Tanganyika (0.027–0.072 h−1) were in the range reported for other oligotrophic environments (e.g. [5, 38]). During our experiments, PCya were the main primary producers, with an average of 75% of the particulate primary production and about 70% of phytoplankton biomass (Fig. 1). This estimate is close to that reported by Stenuite et al. [29]. When bacterial production [13] is added to PCya production, it becomes clear that Lake Tanganyika is a picoplankton-dominated lake.
Despite lower grazing rates per individual, high number of NF resulted in a significantly higher grazing impact than that of ciliates. NF accounted on average for 87% of the protistan grazing on HB biomass and for 82% of PCya biomass grazed by protists. The grazing rates estimated in the present study are within the range of those previously reported for natural communities [5, 7, 41]. However, one should keep in mind that our study was done at a single site of the south basin of Lake Tanganyika and that microbial community composition and production can be substantially different in the north basin [14, 27]. In particular, with a lower PCya contribution to phytoplankton biomass, protozoan consumption of particulate primary production could differ substantially from estimates obtained for the south basin. Assuming similar specific grazing rates (PCya per cell per hour) and using protozoan abundances previously found by [36], we estimated NF and ciliates grazing in the epilimnion of the north basin as respectively 138.1 and 8.9 mg C PCya m−2 day−1 during the rainy season and 269.1 and 12.2 mg C PCya m−2 day−1 during the dry season. Based on estimates of total primary production in the north basin [29], protozoan grazing would still consume about half of phytoplankton production in this basin, with little seasonal variation.
Here we provide first-hand evidence that herbivory (i.e. consumption of photosynthetic microorganisms) supply most of the carbon to the microbial food web. Indeed, protistan grazing on PCya represented 83% of the total picoplanktonic biomass grazed by NF and ciliates in the epilimnion. In terms of carbon fluxes integrated over the upper 30-m layer, grazing by NF and ciliates on PCya represented 460 and 102 mg C m−2 day−1, respectively, whereas they consumed 106 and 10 mg C m−2 day−1 of HB carbon. These estimates of grazing on HB values are lower than those from in the previous study by Pirlot et al. [11], who reported a mean grazing rate of ∼190 mg C HB m−2 day−1, i.e. ∼60–70% of bacterial production [11, 13]. However, our estimates were based on experiments performed only in the epilimnion, whereas the values reported by Pirlot et al. [11] integrated over the top 100-m water column, thus including bacterivory measured in water layers beyond the euphotic zone, where HB contribution to picoplankton increases [13].
Sherr and Sherr [2, 6] have suggested, from several studies, that microbial herbivory might be the major process in the carbon cycling within the euphotic zone of oligotrophic waters. By contrast, ‘herbivory vs. bacterivory’ studies conducted in other environments (e.g. [19, 53, 54]) presented either herbivory or bacterivory as the main carbon pathway through the microbial food web, chiefly depending on the composition of both communities of protists and their prey. Actually, as Sherr and Sherr underlined in their review [2], herbivory should dominate in oligotrophic environments because phototrophic organisms predominate in the total picoplanktonic biomass, even if this might not be true in all low-nutrient environments [21]. Here we demonstrate that herbivory is a major process providing most of the organic carbon to the microbial grazers in an oligotrophic tropical lake.
Finally, our data demonstrate that microbial grazers were an essential component of the food web in Lake Tanganyika, thereby adding support to the hypothesis drawn by Hecky et al. [23], who proposed a contribution of bacterivorous ciliates to the resources available to metazooplankton. Even though we did not consider metazooplankton grazing on protists, our study elucidates some key processes of the microbial food web in this large lake. Among phagotrophic protists, nanoflagellates were the main grazers, contributing often for more than 90% of community grazing on picoplankton, thereby confirming the conclusions by Pirlot et al. [11] on bacterial mortality in Lake Tanganyika. The main prey of phagotrophic protists in the epilimnion was photosynthetic picoplankton, which accounted for the largest part of primary production: Therefore, protozoans were the main consumers of primary production. This may be one of the explanations as to why Lake Tanganyika has such a high productivity: Its microbial food web is much more efficient by consuming directly phytoplankton, i.e. primary producers, than by depending on DOC taken up by heterotrophic bacteria, which have high respiratory losses. Even though heterotrophic bacteria are abundant in Lake Tanganyika, are distributed over the whole oxic zone and have a production which is roughly half of phytoplankton production [13], bacterivory alone could not sustain the phagotrophic protists, which are a key link within the food web of this large lake.
References
Sarmento H, Montoya JM, Váquez-Domínguez E, Vaqué D, Gasol JM (2010) Warming effects on marine microbial food web processes: how far can we go when it comes to predictions? Philos Trans R Soc, B 365:2137–2149
Sherr EB, Sherr BF (2002) Significance of predation by protists in aquatic microbial food webs. Anton Leeuw Int J G 81:293–308
Bell T, Kalff J (2001) The contribution of picophytoplankton in marine and freshwater systems of different trophic status and depth. Limnol Oceanogr 46:1243–1248
Hansen B, Bjornsen PK, Hansen PJ (1994) The size ratio between planktonic predators and their prey. Limnol Oceanogr 39:395–403
Sakka A, Legendre L, Gosselin M, Delesalle B (2000) Structure of the oligotrophic planktonic food web under low grazing of heterotrophic bacteria: Takapoto Atoll, French Polynesia. Mar Ecol Prog Ser 197:1–17
Sherr EB, Sherr BF (1994) Bacterivory and herbivory—key roles of phagotrophic protists in pelagic food webs. Microb Ecol 28:223–235
Cuevas LA, Morales CE (2006) Nanoheterotroph grazing on bacteria and cyanobacteria in oxic and suboxic waters in coastal upwelling areas off northern Chile. J Plankton Res 28:385–397
Callieri C, Karjalainen SM, Passoni S (2002) Grazing by ciliates and heterotrophic nanoflagellates on picocyanobacteria in Lago Maggiore, Italy. J Plankton Res 24:785–796
Nagata T, Takai K, Kawanobe K, Kim DS, Nakazato R, Guselnikova N, Bondarenko N, Mologawaya O, Kostrnova T, Drucker V, Satoh Y, Watanabe Y (1994) Autotrophic picoplankton in Southern Lake Baikal—abundance, growth and grazing mortality during summer. J Plankton Res 16:945–959
Weisse T, Müller H (1998) Planktonic protozoa and the microbial food web in Lake Constance. Arch Hydrobiol Spec Issues Advanc Limnol 53:223–254
Pirlot S, Unrein F, Descy JP, Servais P (2007) Fate of heterotrophic bacteria in Lake Tanganyika (East Africa). FEMS Microbiol Ecol 62:354–364
Sarmento H, Unrein F, Isumbisho M, Stenuite S, Gasol JM, Descy JP (2008) Abundance and distribution of picoplankton in tropical, oligotrophic Lake Kivu, Eastern Africa. Freshw Biol 53:756–771
Stenuite S, Pirlot S, Tarbe AL, Sarmento H, Lecomte M, Thill S, Leporcq B, Sinyinza D, Descy JP, Servais P (2009) Abundance and production of bacteria, and relationship to phytoplankton production, in a large tropical lake (Lake Tanganyika). Freshw Biol 54:1300–1311
Stenuite S, Tarbe A-L, Sarmento H, Unrein F, Pirlot S, Sinyinza D, Thill S, Lecomte M, Leporcq B, Gasol JM, Descy J-P (2009) Photosynthetic picoplankton in Lake Tanganyika: biomass distribution patterns with depth, season and basin. J Plankton Res 31:1531–1544
Kisand V, Zingel P (2000) Dominance of ciliate grazing on bacteria during spring in a shallow eutrophic lake. Aquat Microb Ecol 22:135–142
Calbet A (2001) Mesozooplankton grazing effect on primary production: a global comparative analysis in marine ecosystems. Limnol Oceanogr 46:1824–1830
Caron DA, Lim EL, Miceli G, Waterbury JB, Valois FW (1991) Grazing and utilization of chroococcoid cyanobacteria and heterotrophic bacteria by protozoa in laboratory cultures and a coastal plankton community. Mar Ecol Prog Ser 76:205–217
Nagata T, Takai K, Kawabata K, Nakanishi M, Urabe J (1996) The trophic transfer via a picoplankton–flagellate–copepod food chain during a picocyanobacterial bloom in Lake Biwa. Arch Hydrobiol 137:145–160
Simek K, Hartman P, Nedoma J, Pernthaler J, Springmann D, Vrba J, Psenner R (1997) Community structure, picoplankton grazing and zooplankton control of heterotrophic nanoflagellates in a eutrophic reservoir during the summer phytoplankton maximum. Aquat Microb Ecol 12:49–63
Simek K, Macek M, Pernthaler J, Straskrabova V, Psenner R (1996) Can freshwater planktonic ciliates survive on a diet of picoplankton? J Plankton Res 18:597–613
Pernthaler J, Simek K, Sattler B, Schwarzenbacher A, Bobkova J, Psenner R (1996) Short-term changes of protozoan control on autotrophic picoplankton in an oligo-mesotrophic lake. J Plankton Res 18:443–462
Descy JP, Sarmento H (2008) Microorganisms of the East African Great Lakes and their response to environmental changes. Freshw Rev 1:59–73
Hecky RE, Fee EJ, Kling HJ, Rudd JWM (1981) Relationship between primary production and fish production in Lake Tanganyika. Trans Am Fish Soc 110:336–345
Coulter GW (1991) Lake Tanganyika and its life. Oxford University Press, London
Marijnissen SAE, Michel E, Daniels SR, Erpenbeck D, Menken SBJ, Schram FR (2006) Molecular evidence for recent divergence of Lake Tanganyika endemic crabs (Decapoda: Platythelphusidae). Mol Phylogenet Evol 40:628–634
Salzburger W, Meyer A, Baric S, Verheyen E, Sturmbauer C (2002) Phylogeny of the Lake Tanganyika cichlid species flock and its relationship to the Central and East African haplochromine Cichlid fish faunas. Syst Biol 51:113–135
Descy J-P, Hardy M-A, Sténuite S, Pirlot S, Leporcq B, Kimirei I, Sekadende B, Mwaitega S, Sinyenza D (2005) Phytoplankton pigments and community composition in Lake Tanganyika. Freshw Biol 50:668–684
Kurki H, Vuorinen I, Bosma E, Bwebwa D (1999) Spatial and temporal changes in copepod zooplankton communities of Lake Tanganyika. Hydrobiologia 407:105–114
Stenuite S, Pirlot S, Hardy MA, Sarmento H, Tarbe AL, Leporcq B, Descy JP (2007) Phytoplankton production and growth rate in Lake Tanganyika: evidence of a decline in primary productivity in recent decades. Freshw Biol 52:2226–2239
Cohen AS, Lezzar KE, Cole J, Dettman D, Ellis GS, Gonneea ME, Plisnier PD, Langenberg V, Blaauw M, Zilifi D (2006) Late Holocene linkages between decade-century scale climate variability and productivity at Lake Tanganyika, Africa. J Paleolimnol 36:189–209
Stager JC, Cocquyt C, Bonnefille R, Weyhenmeyer C, Bowerman N (2009) A late Holocene paleoclimatic history of Lake Tanganyika, East Africa. Quat Res 72:47–56
Tierney JE, Mayes MT, Meyer N, Johnson C, Swarzenski PW, Cohen AS, Russell JM (2010) Late-twentieth-century warming in Lake Tanganyika unprecedented since AD 500. Nat Geosci 3:422–425
Sarvala J, Salonen K, Jarvinen M, Aro E, Huttula T, Kotilainen P, Kurki H, Langenberg V, Mannini P, Peltonen A, Plisnier PD, Vuorinen I, Molsa H, Lindqvist OV (1999) Trophic structure of Lake Tanganyika: carbon flows in the pelagic food web. Hydrobiologia 407:149–173
Pirlot S, Vanderheyden J, Descy J-P, Servais P (2005) Abundance and biomass of heterotrophic micro-organisms in Lake Tanganyika. Freshw Biol 50:1219–1232
Stenuite S (2009) Le picoplancton du lac Tanganyika: nature, biomasse et production. Ph.D., University of Namur
Pirlot S (2006) Le bactérioplancton du Lac Tanganyika: Biomasse, production et devenir trophique. Ph.D., University of Namur
Fuhrman JA, McManus GB (1984) Do bacteria-sized marine eukaryotes consume significant bacterial production? Science 224:1257–1260
Liu HB, Campbell L, Landry MR (1995) Growth and mortality rates of Prochlorococcus and Synechococcus measured with a selective inhibitor technique. Mar Ecol Prog Ser 116:277–287
Sherr E, Sherr B (1993) Protistan grazing rates via uptake of fluorescently labeled prey. In: Kemp PF, Sherr E, Sherr B, Cole JJ (eds) Handbook of methods in aquatic microbial ecology. Lewis, Boca Raton, pp 695–702
Cho BC, Na SC, Choi DH (2000) Active ingestion of fluorescently labeled bacteria by mesopelagic heterotrophic nanoflagellates in the East Sea, Korea. Mar Ecol Prog Ser 206:23–32
Callieri C, Piscia R (2002) Photosynthetic efficiency and seasonality of autotrophic picoplankton in Lago Maggiore after its recovery. Freshw Biol 47:941–956
Vaqué D, Gasol JM, Marrasé C (1994) Grazing rates on bacteria—the significance of methodology and ecological factors. Mar Ecol Prog Ser 109:263–274
Landry MR (1994) Methods and controls for measuring the grazing impact of planktonic protists. Mar Microb Food Webs 8:37–57
Sherr B, Sherr E, Fallon R (1987) Use of monodispersed, fluorescently labeled bacteria to estimate in situ protozoan bacterivory. Appl Environ Microbiol 53:958–965
Steeman-Nielsen E (1952) The use of radio-active carbon (14 C) for measuring organic production in the sea. J Cons Int Explor Mer 18:117–140
De Wever A (2006) Spatio-temporal dynamics in the microbial food web in Lake Tanganyika. Ph.D., University of Gent
Stoyneva M, Ingolič E, Kofler W, Vyverman W (2008) Siderocelis irregularis (Chlorophyta, Trebouxiophyceae) in Lake Tanganyika (Africa). Biologia 63:799–805
Straile D (1997) Gross growth efficiencies of protozoan and metazoan zooplankton and their dependence on food concentration, predator–prey weight ratio, and taxonomic group. Limnol Oceanogr 42:1375–1385
Sanders RW, Caron DA, Berninger UG (1992) Relationships between bacteria and heterotrophic nanoplankton in marine and fresh waters—an inter-ecosystem comparison. Mar Ecol Prog Ser 86:1–14
Hambright KD (2007) Microzooplankton dominate carbon flow and nutrient cycling in a warm subtropical freshwater lake. Limnol Oceanogr 52:1018–1025
Roff JC, Turner JT, Webber MK, Hopcroft RR (1995) Bacterivory by tropical copepod nauplii: extent and possible significance. Aquat Microb Ecol 9:165–175
Sherr EB, Sherr BF, Mcdaniel J (1991) Clearance rates of less-than-6 mu-M fluorescently labeled algae (FLA) by estuarine protozoa—potential grazing impact of flagellates and ciliates. Mar Ecol Prog Ser 69:81–92
Putland JN (2000) Microzooplankton herbivory and bacterivory in newfoundland coastal waters during spring, summer and winter. J Plankton Res 22:253–277
Shinada A, Ban S, Ikeda T (2003) Seasonal changes in nano/micro-zooplankton herbivory and heterotrophic nano-flagellates bacterivory off Cape Esan, southwestern Hokkaido, Japan. J Oceanogr 59:609–618
Acknowledgements
The authors are indebted to Dr. H. Phiri and all the technical staff from the Department of Fisheries (DOF) of Mpulungu, Zambia. We are grateful to Dr. J. Jezberová for providing the Synechococcus-like strains. This study was supported by the Belgian Science Policy Office project CLIMFISH and by the National Fund for Scientific Research, through the FNRS Ph.D. scholarship to A. L. Tarbe, FRIA Ph. D. scholarship to S. Stenuite and FNRS Postdoctoral scholarship to F. Unrein. H. Sarmento benefited from a grant from the Spanish MCyI (Juan de la Cierva Fellowship JCI-2008-2727). The Spanish grant PICOTANGA (CGL2005-24219-E), obtained by Dr. J. M. Gasol, partly supported F. Unrein during his mission in February 2005. A. L. Tarbe also benefited from a ‘Communauté Française de Belgique’ grant for her mission in July–August 2006.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tarbe, AL., Unrein, F., Stenuite, S. et al. Protist Herbivory: a Key Pathway in the Pelagic Food Web of Lake Tanganyika. Microb Ecol 62, 314–323 (2011). https://doi.org/10.1007/s00248-011-9817-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-011-9817-8