Abstract
Toxic blooms of the cyanobacterium Microcystis aeruginosa affect humans and animals in inland water systems worldwide, and it has been hypothesized that the development of these blooms will increase under the future scenario of global change, considering eutrophication and temperature increase as two important consequences. The importance of genetic adaptation, chance and history on evolution of growth rate, and toxin production of M. aeruginosa was studied under these new conditions. The experiment followed the idea of “replaying life’s tape” by means of the simultaneous propagation of 15 independent isolates of three M. aeruginosa strains, which were grown under doubled nutrient concentration and temperature during c. 87 generations. Adaptation by new mutations that resulted in the enhancement of growth rate arose during propagation of derived cultures under the new environmental conditions was the main component of evolution; however, chance also contributed in a lesser extension to evolution of growth rate. Mutations were selected, displacing the wild-type ancestral genotypes. In contrast, the effect of selection on mutations affecting microcystin production was neutral. Chance and history were the pacemakers in evolution of toxin production. Although this study might be considered an oversimplification of the reality, it suggest that a future scenario of global change might lead to an increase in M. aeruginosa bloom frequency, but no predictions about the frequency of toxicity can be made.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The toxin-producing cyanobacterium Microcystis aeruginosa (Kützing) Kützing frequently occurs in dense blooms in reservoirs and lakes, and its toxins (microcystins) are considered to be the most prevalent cyanobacterial toxins in freshwater ecosystems [50]. In fact, M. aeruginosa is the most important cause of toxic cyanobacterial blooms affecting humans and animals in inland water systems worldwide [9, 13, 30, 45, 50, 51]. Moreover, long-term exposure to low levels of microcystins in drinking water can be a risk factor for liver and colorectal cancer [38]. In addition, toxic cyanobacterial blooms pose a challenge for wildlife conservation because they are the cause of repeated mass mortalities of water birds and fishes [1, 10, 37].
Nowadays, humankind is considered the greatest evolution cause [42]. Civilization’s new practices have derived in a global change causing severe alterations in ecosystems worldwide [58]. In particular, inland freshwater systems are being transformed rapidly by eutrophication and temperature increase [11]. These changes will probably intensify in the next decades following both the expected increase in global temperatures and inputs of anthropogenic nitrogen. Different works have reported a warming of at least 5°C above today’s mean by 2,100 [29] and double anthropogenic nitrogen inputs by 2,050 [54]. Phytoplankton can be considered a likely target to experience this environmental forcing since nutrient availability and temperature are among the main conditions that decide competitive advantage and regulate phytoplankton species distribution [18]. Depending on which species or groups are affected and in what manner, variations have the potential to alter inland freshwater ecosystems, in particular, if toxic cyanobacteria species are favored. Recently, it has been suggested that the frequency and duration of cyanobacterial blooms are increasing due to summer heat waves [31, 40, 41]. The adaptation to new conditions linked to global change can be achieved by two different kinds of mechanisms: ecophysiological responses or genetic adaptation. Ecophysiological responses (acclimation) are the result of modification of the genes expression already present in populations while genetic adaptation counts with the selection of new genetic variants. The ecophysiological reactions of cyanobacteria under environmental change have been previously addressed [19]. In contrast, as far as we know, no experiments have been carried out to analyze evolutionary responses of cyanobacteria to environmental forcing. However, evolutionary change includes much more forces than genetic adaptation [26]. At least other two factors also contribute to evolution: chance and historical contingency. The effects of chance are usually due to genetic drift events and random mutations without value for the organisms [16, 32, 53]; the final consequence is that alleles that neither improve nor decrease adaptation are maintained in populations. Historical contingency can become important if certain genetic changes of adaptive value in the past constrain or promote evolutionary outcomes [24]. To disentangle the effects of adaptation, chance and history on evolutionary change, Gould [25] proposed a theoretical experiment, which consisted of “replaying life’s tape” to test the repeatability of evolution and thereby to evaluate their respective roles; the experiment was envisioned to demonstrate the processes involved in macroevolutionary events. Obviously, an experiment such as that envisioned by Gould cannot be performed and as Lenski and Travisano stated [34]: “the limitations reflect our lack of access to better machines for time travel”. However, this theoretical proposal can be empirically addressed in microevolution by the robust experiment of Travisano et al. [55] in which, instead of “replaying life’s tape” sequentially (where each tape record is a replication of the experiment, each of them separated in time), one can achieve the same objective by replicating independent isolates propagated simultaneously.
At the starting point, identical isolates (replicates) from a single ancestral genotype are established and the initial mean value of a specific phenotypic trait (in our case, growth rate and microcystin production) is measured for each of them. This value is expected to be identical among isolates, within statistical limits of measurement error, at the beginning of the experiment. After a period of time, the value of each trait is measured again for each isolate. Differences between the initial and final mean values are explained as a result of the effect of adaptation, chance, or history [55]. Thus, a significant change in the mean value in relation to that of the ancestral isolate means that the trait has been a target of natural selection or that it is correlated with some other trait that has been selected. On the other hand, a significant increase in its variance represents the occurrence of divergence among the evolved isolates (the specific trait has not been a target for adaptation but reflects the effects of random mutations or drift or their interactions with other evolutionary processes). Other alternatives are the occurrence of both adaptation and chance, or on the other hand, the occurrence of neither of them (see [55]). To test the effect of history, it is necessary to carry out a similar experiment using different ancestral genotypes [55].
The experiment of Travisano et al. [55] was designed for bacterial populations, and with the appropriate modifications, has been recently addressed with digital organisms [57] and in a marine dinoflagellate species [22]. Certainly, this experimental evolutionary study can be carried out in any microorganism that can be grown asexually and easily manipulated during many generations.
The aim of this study was to investigate the evolutionary response of bloom-forming, toxic and non-toxic strains of M. aeruginosa to new environmental conditions (simulating a global change scenario, i.e., high levels of nitrate and elevated temperature). For this purpose, the relative importance of different evolutionary components (genetic adaptation, chance, and historical contingency) on the change of two relevant phenotypic traits of M. aeruginosa was analyzed. Likewise, we tried to check whether possible adaptation could be explained as acclimation or as the selection of new genetic variants. One of the selected traits was the growth rate, considered as the main component of fitness and, in consequence, an important trait in evolutionary theory [21]. The other selected trait was the toxin production, crucial for its ecological and management implications [1, 45]. We expect that growth rate is likely to exhibit directional selection (adaptation) and convergent evolution. However, knowing the weak genetic correlation of toxin production with fitness [35], as well as the unclear toxin function [28, 44, 48, 49, 59], we should expect a major influence of chance events and the retention of ancestral differences. Even though this study might be considered an oversimplification of the reality, the evolutionary ecological approach followed here constitutes a novel way to explore, throughout a rigorous experimental model, the evolutionary response of toxic cyanobacteria to anthropogenic-induced changes in environmental conditions.
Methods
Experimental Organism and Growth Conditions
Experiments were carried out using three M. aeruginosa strains (Ma3D, Ma6D, and Ma7D) from the Algal Culture Collection, Genetics Laboratory, Facultad de Veterinaria, Universidad Complutense (Madrid, Spain). Strains were collected from a pristine lagoon in Doñana National Park (SW Spain) with 0.032 M of nitrate and 22°C and were maintained in culture collection 3 years prior to the experiments under 0.035 M of nitrate and 22°C conditions (BG-11 medium. Sigma, Aldrich Chemie, Taufkirchen, Germany). Data on isolation of the strains were described by Carrillo et al. [12], and information about their ecophysiological characterization, genetic variability, and the genetic bases of resistance against several biocides of the strains are included in [3, 4, 15, 35, 36, 47]. In particular, Ma3D and Ma6D are non-toxic strains, while the strain Ma7D is toxic [12].
Cultures were grown axenically in culture flasks (Greiner, Bio-One Inc., Longwood, NJ, USA) with 20 mL of BG-11 medium (Sigma, Aldrich Chemie, Taufkirchen, Germany) at 22°C under a continuous irradiance of 60 μmol m−2 s−1 over the 400–700 nm waveband supplied by daylight fluorescent tubes. Cells were maintained axenically in mid-log exponential growth by serial transfers of an inoculum to fresh medium once every 2 weeks. Only cultures without bacteria were employed.
Experimental Design
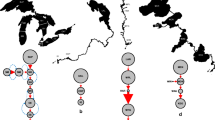
A detailed description of the experimental design is provided in reference [22]. Just before the experiment, the three strains were re-cloned from a single cell, thus assuring their genetic homogeneity at the starting point. These newly established cultures were grown to high densities in environmental conditions similar to those used to maintain the cultures started from stocks from the Algal Culture Collection (BG-11 medium, 22°C, and continuous irradiance of 60 μmol m−2 s−1 over the 400–700 nm waveband). Cultures were then used to found 15 independent isolates of each strain, and their acclimated maximal growth rate (a proxy of the Malthusian parameter of fitness) and their toxin concentration (amount of microcystin-LR equivalent per cell) were measured (see Fig. 1). These isolates were transferred to new culture conditions, which were selected in order to mimic a global change scenario with increased nitrate levels and elevated temperature. Therefore, the cultures were transferred to BG-11 medium with double concentration of nitrate (0.07 M of nitrate) and 30°C. The remaining culture conditions (i.e., photon irradiance and photoperiod) were maintained as previously described.
Schematic representation of the experimental design. Fifteen isolates for control and 15 isolates for new selective growth conditions were used for each of the three Microcystis aeruginosa strains. Cultures were maintained through serial transfer into fresh new medium for 1 year (corresponding to c. 87 generations): control conditions in BG-11 culture medium (1N) and 22°C and selective growth conditions in BG-11 with double concentration of nitrate (2N) and 30°C. Growth rate and toxin production were estimated at the beginning, every 4 months, and at the end of the experiment. In order to discriminate adaptive change due to physiological adaptation (acclimation) or by genetic adaptation, cultures were transferred to the initial culture conditions for 1 month (c. 7 generations), and growth rate and toxin production were measured again after this period
Experimental cultures were serially propagated by 32-fold dilution into fresh medium every 21 days, which allowed about five generations of binary division before the further addition of fresh medium (5 = Log232). Cultures were propagated for 1 year (corresponding to c. 87 generations). Growth rate and toxin production were checked every 4 months. Finally, averaged growth rate and toxin concentration of the evolved cells were estimated and compared with those of the ancestral ones.
The effects of adaptation, chance, and history on evolutionary change of growth rate of M. aeruginosa were estimated by using the values measured at the start of the experiment and after 12 months under the new experimental conditions (see Fig. 1). In particular, the effects of adaptation were defined by changes in the mean value, and 95% confidence limits were calculated by using the t distribution. The effects of chance and history were estimated by means of a two-level (3 strains and 15 isolates within each strain, with 3 replicates of the growth rate measurement per isolate) nested ANOVA. Thus, the contribution of the chance component corresponds to the variance measured among isolates within the same strain, whereas the history component was estimated by the variance among strains. The homogeneity of variances was checked with the F test and the normality of the data by the Kolmogorov–Smirnov test. The square root of the variance component for chance and history was reported in order to use units that were comparable to the mean change due to adaptation. Approximate 95% asymmetrical confidence limits were calculated for the variance components. In the case of toxin production, homogeneity of variances was not achieved even by transforming data. Furthermore, toxin production was just expressed in one of the strains (7D). Therefore, the non-parametrical Mann–Whitney U test was used to compare ancestral and derived values from the toxic strain 7D. All the statistical procedures were performed in accordance with reference [52].
Acclimation vs. Genetic Adaptation
In order to test if adaptation is supported by acclimation or by selection of the new genetic variants arising by mutations, the cells cultured in the selective growth conditions (c. 87 generations at double nitrate concentration, 30°C) were then newly transferred to the initial culture conditions (original nitrate concentration, 22°C) for 1 month (c. 7 generations). As control, parallel cultures were maintained in the initial growth conditions (original nitrate concentration, 22°C) for the duration of the experiment (i.e., 12 + 1 months). The growth rate and toxin production of each experimental replicate was measured and compared with that found in the controls. Changes in mean values were calculated using ANOVA. Seven generations under the initial conditions are considered enough to check if adaptation process is assured by means of acclimation or genetic mechanisms (reviewed by Cooper [14]).
It is hypothesized that similar growth rate and toxin production values mean that adaptation to selective growth conditions was achieved by acclimation because the return to the initial growth conditions yielded similar results. On the contrary, different growth rate values and toxin production mean that the genetic adaptation occurred during the time when cultures were submitted to selective growth conditions.
Measurement of Growth Rate and Toxicity
Acclimated maximal growth rate (an estimator of Malthusian parameter of fitness) was estimated under r selection conditions in exponentially growing cultures according to [16] as:
where N t and N 0 are the cell number at time t = 4 and 0 days, respectively. For this purpose, the values of N 0 and N t were determined at 3 and 7 days after the transference of cells to fresh medium. Cell numbers in experiments and controls were counted (in triplicate) using a Beckman (Brea, CA, USA) Z2 particle counter. In addition, two independent observers also made cell counts by haemocytometer (Double Neubauer ruling, Fortuna W.G. Co., Germany) to check the accuracy of the particle counter.
The toxin production of the different replicates of each strain was measured using a microcystin-specific enzyme-linked immunoabsorbent assay (ELISA) test (EnviroGard Microcystin Quantitube Test Kit, Strategic Diagnostic, Inc.), according to the manufacturer’s recommendations. This kit is a quantitative test for the detection of microcystins residues in water. Prior to the measurements, cell concentration in each replicate was assessed at the late logarithmic growth phase, when toxicity is highest [8], and an aliquot was kept. Then, the cells of each aliquot were broken by freezing and sonication. The insoluble cell debris was removed by centrifugation (15,000 g − force × 10 min). The toxin concentration was expressed as nanograms of MCYST-LR equivalent per cell.
Results
Growth Rate
Mean growth rate of the three strains increased during the first 8 months (c. 58 generations). Afterwards, growth rate apparently remained constant (Fig. 2a). Figure 2b shows derived values for growth rate after c. 87 generations in double nitrate concentration and 30°C vs. ancestral values in single nitrate concentration and 22°C in 15 isolates of the Ma3D, Ma6D, and Ma7D strains.
Evolution of growth rate during c. 87 generations in selective conditions (double nitrate concentration and 30°C) (a) and derived versus ancestral values for mean growth rate (b) in 15 isolates of three strains of Microcystis aeruginosa—Ma3D (close circles), Ma6D (open circles), and Ma7D (close triangles). Symbols in b correspond to the overall mean (CV < 1.5%) of three replicates of the growth rate measurement per isolate and per strain. Isocline (dotted line in b) represents the score location if no changes take place
Adaptation was absent (by design) at the start of the experimental selective period. The initial effect of chance on growth rate of M. aeruginosa (estimated by the variance among isolates within strains) was absent (F = 1.48; df = 42 and 90; P > 0.5). On the contrary, the initial historical contingency contribution on the growth rate (estimated by the variance among strains) was significant (F = 18.94; df = 2 and 42; P < 0.001) (Fig. 3). After 12 months under the experimental selective period, approx. two thirds of the evolutionary change of the growth rate of M. aeruginosa was explained by adaptation (t = 13.24; df = 133; P < 0.001) while approx. one third of evolution was due to chance (F = 4.32; df = 42 and 90; P < 0.001) (Fig. 3). The footprint of the historical contingency on the growth rate of the different strains of M. aeruginosa was non-significant (F = 0.33; df = 2 and 42; P > 0.5) at the end of the experiment (Fig. 3).
Mean growth rate value was found to be invariant in control cultures during c. 87 generations (i.e., single nitrate dose and 22°C during the entire length of the experiment) and similar to the growth rate value measured at the outset of the experiments (F = 2.76; df = 1 and 28; P > 0.1). However, the cells derived after c. 87 generations in double nitrate doses and 30°C, newly transferred and cultured c. 7 generations to the original conditions (single nitrate dose and 22°C) for 1 month showed significant higher growth rate values (F = 5.30; df = 1 and 28; P < 0.05) than control cultures after c. 87 + 7 generations.
Toxin Production
The toxin production in all the isolates of Ma3D and Ma6D remained below the detection limit during c. 87 generations in selective conditions (double nitrate concentration, 30°C), but the isolates from Ma7D showed abundant microcystin production (Fig. 4a). A significant difference between the initial and final 7D values was observed (c. 87 generations; U = 52; n 1, n 2 = 15, 15; P < 0.05). Figure 4b shows derived values for mean toxin production after c. 87 generations in double nitrate concentration and 30°C versus ancestral values in single nitrate concentration and 22°C in 15 isolates of Ma7D.
Evolution of toxin production during c.87 generations in selective conditions (double nitrate concentration and 30°C) in 15 isolates of three strains of Microcystis aeruginosa—Ma3D (close circles), Ma6D (open circles), and Ma7D (close triangles) (a) and derived versus ancestral values for mean toxin production in 15 isolates of the Ma7D strain (b). Isocline (dotted line in b) represents the score location if no changes take place
A significant difference between the initial variance (at the start of the experiment) and the final variance (after c. 87 generations in the new environmental conditions of doubling nitrate and 8°C temperature increase) was observed in Ma7D (F = 9.31; df = 1 and 28; P < 0.001), suggesting that chance made a significant contribution to evolution of toxin production. Although the effect of history in evolution of toxin production cannot be checked statistically by ANOVA, it could be evidenced because: (1) the non-toxic strains Ma3D and Ma6D remained non-toxic during c. 87 generations in the new environmental conditions of doubling nitrate and 8°C temperature increase; and (2) the toxin-producing Ma7D strain maintained a high level of toxicity during c. 87 generations at the new environmental conditions.
The Ma7D cells derived after c. 87 generations in double nitrate doses and 30°C, newly transferred and cultured during c. 7 generations to the original conditions (single nitrate dose and 22°C), showed significantly lower toxin production values than control cultures of Ma7D after c. 87 + 7 generations under the original conditions (U = 20; n 1, n 2 = 15, 15; P < 0.001). However, the mean toxin production value also varied in control cultures during c. 87 + 7 generations under single nitrate dose and 22°C during the entire length of the experiment (U = 34; n 1, n 2 = 30, 30; P < 0.001). This is further evidence to demonstrate that chance played an important role in the evolution of this trait.
Discussion
The understanding of the biological response to global change will be one of the main tasks of evolutionary biologists in the coming decade [5]. Investigating the capacity of phytoplankton to respond to the predicted changes in environmental conditions has become a key issue in order to further understand future repercussions on the functioning of aquatic ecosystems [18]. In this context, the importance of estimating the adaptive potential of toxic cyanobacteria to environmental forcing is obvious, but not much is known about the mechanisms implicated in this process [31, 40]. Assuming the complexity of possible responses that may arise to cope with the changing environmental conditions, the experimental approach followed here constitutes a novel way to explore the roles played by physiological adaptation (acclimation), genetic adaptation (selection of favored mutants), chance (genetic drift and neutral mutations), and historical contingency in the adaptation of toxic cyanobacteria under an experimental scenario of increasing temperature and nitrate. Evidently, this study might be considered an oversimplification of the reality, but reductionist approaches have propitiated the development of modern biology [60].
Ever since Fisher [21], the importance of fitness on evolution is well known. For that reason, the evolution of growth rate (the main estimator of fitness) of M. aeruginosa after a rapid environmental change was one of the considered parameters in the current study. Genetic adaptation was the predominant component of growth rate evolution. Since asexually growing clonal cultures were used, the evolutionary changes are due to new mutations, which occurred during propagation of derived cultures under the new environmental conditions. Numerous mutations should have arisen in each isolate (due to the huge number of cells growing in each culture). Some mutations increased growth rate and were selected, displacing the wild-type genotypes [22]. Mutations decreasing growth rate were eliminated by natural selection. Increased nutrient concentrations and temperature not only induced selection for increased growth rate, but this selective effect seemed to be strong enough to constrain the evolution of all the experimental isolates, whose growth rates converged at the end of the experiment, as was demonstrated by the low contribution of chance and history. Finally, chance contributed in a lesser extension than adaptation to evolution of growth rate of M. aeruginosa, suggesting that some neutral mutations which affected growth rate could arose during the experiment.
Previous studies using viruses [7, 17], bacteria [5, 33] parasitic protozoa [43], a dinoflagellate [22], and even digital organisms ([57]; in this case, award rate, a variable closely related to average fitness, was used) have also reported the occurrence of fitness convergence in sets of isolates propagated under identical, novel environmental conditions. As long as a similar derived growth rate value was achieved, not only within each set of evolving isolates, but also between the strains, it seems that environmental conditions determined the outcome for growth rate to a large extent. Consequently, adaptation was the main component of growth rate evolution and the footprint of history was eliminated, as may occur for traits that are subject to strong selection pressure [35].
It has been observed that the majority of the evolutionary change in a long-term experiment with bacteria occurred during the first generations, followed by evolutionary stasis [34]. Thus, it could be assumed that the c. 87 generations of M. aeruginosa along the experiment was enough to detect evolutionary change. In particular, the greater change in growth rate occurred during the first 58 generations after the population was placed in the experimental environment, followed by a period of quasi-stasis. Moreover, some previous studies demonstrated very rapid adaptive evolution of M. aeruginosa to lethal selective agents [23, 36].
On the other hand, toxin production by cyanobacteria is crucial from a practical point of view (i.e., management of water supply reservoirs and wildlife conservation). For this reason, evolution of toxicity after a rapid change in environmental conditions was also considered as an important parameter to study. Chance was the predominant component in the evolution of microcystin production in M. aeruginosa. Taking into account the constant experimental conditions (in contrast with field conditions) as well as the high number of cells in the experimental cultures, we should consider that the probability of drift events was low and most of the chance effects could be attributed to random mutations. The evolutionary changes are due to new mutations arising during propagation of the derived cultures under the new environmental conditions. Since each culture has a huge number of cells, numerous mutations should have arisen during cell growth in each isolate. Some mutations increased toxin production whereas other mutations decreased toxin production, suggesting that the effect of selection for microcystin production in derived population (i.e., under double nitrate and +8°C) was neutral. Thus, neither toxin-increased nor toxin-decreased mutants have selective advantage. Moreover, it could be supposed that wild-type ancestral genotypes have no selective advantage. Consequently, natural selection was not strong enough to constrain the evolution of the experimental isolates. So, the effect of history was maintained during evolution of microcystin production under new environmental conditions.
There are diverse hypothesis about the role of microcystins in natural populations of cyanobacteria. It has been hypothesized that the microcystins are involved in basic metabolism, protective effects against predators and competitive organisms, alleophatic interactions and others [28, 44, 48, 49, 56, 59]. However, biological functions of microcystin remain controversial. For instance, cyanobacteria are among the most ancient organisms on the Earth [27], and it has been proposed that microcystin synthesis is an ancient process, developed long before the evolution of eukaryotic photoautotrophs [2]. Microcystin synthetase genes were in the past more widely present in cyanobacteria than today [46]. This apparently loss of microcystin synthetase genes during evolution indicates that selection pressure on microcystin production has decreased. It could be possible that toxin production is purely fortuitous, and “non-toxic” strains could have similar structurally related but non-toxic compounds serving the same physiological function [39]. Recently, it has been showed that under environmental conditions that favor cyanobacteria growth, the cost of microcystin production prevails over its benefits, and consequently, toxin production is lowered [6].
An evolutionary approach to this controversy could be useful. It is well established in evolutionary theory that traits that are strongly correlated with fitness evolve by adaptation (selection of favorable mutations); whereas traits that are not (or are very weakly) correlated with fitness evolve by chance [32, 55]. The effect of history is preserved only in traits that are less important to evolution [25, 26, 55]. As it has been previously hypothesized, our study demonstrates that growth rate, which is strongly correlated with fitness, evolved mainly by adaptation, although chance events also contributed to some extent. In contrast, microcystin production was not (r = −0.091, df = 14, P > 0.05 in this study) correlated with growth rate (the main component of fitness); thus, toxin production evolution was modulated by chance and history, but not by adaptation. Similarly, previous papers showed a very high phenotypic: genetic variance ratio of microcystin production in experimental populations of M. aeruginosa [35]. Quantitative genetic theory proves that only the traits that are not (or are scarcely) important in evolution have high phenotypic: genetic variance ratio [reviewed in 20]. Consequently, toxin production of cyanobacteria had no adaptive value (it seems to be purely fortuitous).
Finally, these results could lead to tentatively hypothesize that the increase of temperature and eutrophication might increase cyanobacteria blooms via growth rate increase. In contrast, since chance is the pacemaker of evolution of toxin production, predictions of the future of toxicity may be impossible. However, the mere fact of increasing cyanobacterial blooms could aggravate problems in water reservoirs due to the unpredictability of the toxin evolution.
References
Alonso-Andicoberry C, García-Villada L, López-Rodas V, Costas E (2002) Catastrophic mortality of flamingos in a Spanish National Park caused by cyanobacteria. Vet Rec 151:706–707
Babica P, Blaha L, Marsalek B (2006) Exploring the natural role of microcystins—a review of effects on photoautotrophic organisms. J Phycol 42:9–20
Bañares-España E, López-Rodas V, Salgado C, Costas E, Flores-Moya A (2006) Inter-strain variability in the photosynthetic use of inorganic carbon, exemplified by the pH compensation point, in the cyanobacterium Microcystis aeruginosa. Aquat Bot 85:159–162
Bañares-España E, López-Rodas V, Costas E, Salgado C, Flores-Moya A (2007) Genetic variability associated with photosynthetic pigment concentration, and photochemical and non-photochemical quenching, in strains of the cyanobacterium Microcystis aeruginosa. FEMS Microbiol Ecol 60:449–455
Bell G, Collins S (2008) Adaptation, extinction and global change. Evol Appl 1:3–16
Briand EC, Yepremian C, Humbert JF, Quiblier C (2008) Competition between microcystin- and non-microcystin-producing Planktothrix agardhii (Cyanobacteria) strains under different environmental conditions. Environ Microbiol 10:3337–3348
Bull JJ, Badgett MR, Wichman HA, Huelsenbeck JP, Hillis DM, Gulati A, Ho C, Molineux IJ (1997) Exceptional convergent evolution in a virus. Genetics 147:1497–1507
Carmichael WW (1997) The cyanotoxins. Adv Bot Res 27:211–256
Carmichael WW, Azevedo SMFO, An JS, Molica RJR, Jochimsen EM, Lau S, Rinehart KL, Shaw GR, Eaglesham GK (2001) Human fatalities from cyanobacteria: chemical and biological evidence for cyanotoxins. Environ Health Perspect 109:663–668
Carmichael WW, RenHui L (2006) Cyanobacteria toxins in the Salton Sea. Saline Syst 2:5
Carpenter SR, Fisher SG, Grimm NB, Kitchell JF (1992) Global change and freshwater ecosystems. Annu Rev Ecol Systemat 23:119–139
Carrillo EL, Ferrero M, Alonso-Andicoberri C, Basanta A, Martín A, López-Rodas V, Costas E (2003) Interstrain variability in toxin production in populations of the cyanobacterium Microcystis aeruginosa from water-supply reservoirs of Andalusia and lagoons of Doñana National Park (southern Spain). Phycologia 42:269–274
Codd GA, Poon GK (1988) Cyanobacterial toxins. In: Rogers LJ, Gallon JG (eds) Proceedings of the phytochemical society of Europe. Oxford University Press, Oxford, pp 283–296
Cooper S (1991) Bacterial growth and division. Biochemistry and regulation of prokaryotic and eukaryotic division cycles. Academic, San Diego
Costas E, López-Rodas V (2006) Copper sulphate and DCMU-herbicide treatments increase asymmetry between sister cells in the toxic cyanobacterium Microcystis aeruginosa: implications for detecting environmental stress. Water Res 40:2447–2451
Crow JF, Kimura M (1970) An introduction to population genetics theory. Harper and Row, New York
Cuevas JM, Elena SF, Moya A (2002) Molecular basis of adaptive convergence in experimental populations of RNA viruses. Genetics 162:533–542
De Senenport Domis LN, Mooij WM, Huisman J (2007) Climate-induced shifts in a phytoplankton community: a mechanistic approach. Hydrobiologia 584:403–413
Downing JA, Watson SB, McCauley E (2001) Predicting cyanobacteria dominance in lakes. Can J Fish Aquat Sci 58:1905–1908
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics. Longman, Essex
Fisher RA (1930) The genetical theory of natural selection. Oxford University, Oxford
Flores-Moya A, Costas E, López-Rodas V (2008) Roles of adaptation, chance and history in the evolution of the dinoflagellate Prorocentrum triestinum. Naturwissenschaften 95:697–703
García-Villada L, Rico M, Altamirano M, Sánchez-Martín L, López-Rodas V, Costas E (2004) Occurrence of copper resistant mutants in the toxic cyanobacterium Microcystis aeruginosa: characterization and future implications in the use of copper sulphate as an algaecide. Water Research 38:2207–2213
Gould SJ, Lewontin RC (1979) Spandrels of San-Marco and the Panglossian Paradigm—a critique of the adaptationist program. Proc R Soc B 205:581–598
Gould SJ (1989) Wonderful life: the Burguess shale and the nature of history. Norton, New York
Gould SJ (2002) The structure of evolutionary theory. Harvard University Press, Cambridge
Graham L, Graham J, Wilcox LW (2009) Algae, 2nd edn. Benjamin Cummings, Pearson, San Francisco
Gross EM (2003) Allelopathy of aquatic autotrophs. Crit Rev Plant Sci 22:313–339
Hansen J, Sato M, Ruedy R, Lacis A, Oinas V (2000) Global warming in the twenty-first century: an alternative scenario. P Natl Acad Sci USA 97:9875–9880
Jochimsen EM, Carmichael WW, An J, Cardo DM, Cookson ST, Holmes CEM, Antunes MB, Filho DA, Lyra FM, Barreto VST, Azevedo SMFO, Jarvis WR (1998) Liver failure and death after exposure to microcystins at a hemodialysis center in Brazil. New Engl J Med 338:873–878
Jöhnk KD, Huisman J, Sharples J, Sommeijerz B, Visser PM, Strooms JM (2008) Summer heatwaves promote blooms of harmful cyanobacteria. Glob Chang Biol 14:495–512
Kimura M (1983) The neutral theory of molecular evolution. Cambridge University Press, Cambridge
Korona R (1996) Genetic divergence and fitness convergence under uniform selection in experimental populations of bacteria. Genetics 143:637–644
Lenski RE, Travisano M (1994) Dynamics of adaptation and diversification: a 10.000 generation experiment with bacterial populations. Proc Natl Acad Sci USA 91:6808–6814
López-Rodas V, Costas E, Bañares E, García-Villada L, Altamirano M, Rico M, Salgado C, Flores-Moya A (2006) Analysis of polygenic traits of Microcystis aeruginosa (Cyanobacteria) strains by restricted maximum likelihood (REML) procedures: 2. Microcystin net production, photosynthesis and respiration. Phycologia 45:243–248
López-Rodas V, Flores-Moya A, Maneiro E, Perdigones N, Marva F, García ME, Costas E (2007) Resistance to glyphosate in the cyanobacterium Microcystis aeruginosa as result of pre-selective mutations. Evol Ecol 21:535–547
López-Rodas V, Maneiro E, Lanzarot MP, Perdigones N, Costas E (2008) Mass wildlife mortality due to cyanobacteria in the Doñana National Park, Spain. Vet Rec 162:317–318
Martínez-Hernández J, López-Rodas V, Costas E (2009) Microcystins from tap water could be a risk factor for liver and colorectal cancer: a risk intensified by global change. Med Hypotheses 72:539–540
Orr PT, Jones GJ (1998) Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol Oceanogr 43:1604–1614
Paerl HW, Huisman J (2008) Blooms like it hot. Science 320:57–58
Paerl HW, Huisman J (2009) Climate change: a catalyst for global expansion of harmful cyanobacterial blooms. Environ Microbiol Rep 1:27–37
Palumbi SR (2001) Evolution—humans as the world’s greatest evolutionary force. Science 293:1786–1790
Pérez-Zaballos FJ, Ortega-Mora LM, Álvarez-García G, Collantes-Fernández E, Navarro-Lozano V, García-Villada L, Costas E (2005) Adaptation of Neospora caninum isolates to cell-culture changes: an argument in favor of its clonal population structure. J Parasitol 91:507–510
Pflugmacher S (2002) Possible allelopathic effects of cyanotoxins, with reference to microcystin-LR, in aquatic ecosystems. Environ Toxicol 17:407–413
Pouria S, de Andrade A, Barbosa J, Cavalcanti RL, Barreto VTS, Ward CJ, Preiser W, Poon GK, Neild GH, Codd GA (1998) Fatal microcystin intoxication in haemodialysis unit in Caruaru, Brazil. Lancet 352:21–26
Rantala A, Fewer DP, Hisbergues M, Rouhiainen L, Vaitomaa J, Börner T, Sivonen K (2004) Phylogenetic evidence for the early evolution of microcystin synthesis. P Natl Acad Sci USA 101:568–573
Rico M, Altamirano M, López-Rodas V, Costas E (2006) Analysis of polygenic traits of Microcystis aeruginosa (Cyanobacteria) strains by restricted maximum likelihood (REML) procedures: 1. Size and shape of colonies and cells. Phycologia 45:237–242
Sedmak B, Elersek T (2006) Microcystins induce morphological and physiological changes in selected representative phytoplanktons. Microb Ecol 51:508–515
Singh DP, Tyagi MB, Kumar A, Thakur J (2001) Antialgal activity of a hepatotoxin-producing cyanobacterium, Microcystis aeruginosa. World J Microbiol Biotechnol 17:15–22
Sivonen K, Jones G (1999) Cyanobacterial toxins. In: Chorus I, Bartram J (eds) Cyanobacteria in water: a guide to public health significance, monitoring and management. Routledge, London, pp 41–111
Skulberg OM, Carmichael WW, Codd GA, Skulberg R (1993) Taxonomy of toxic Cyanophyceae (Cyanobacteria). In: Falconer IR (ed) Algal toxins in seafood and drinking water. Academic, London, pp 145–164
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. Freeman, New York
Spiess EB (1989) Genes in populations. Wiley, New York
Tilman D, Lehman C (2001) Human-caused environmental change: impacts on plant diversity and evolution. P Natl Acad Sci USA 98:5433–5440
Travisano MJA, Mongold JA, Bennett AF, Lenski RE (1995) Experimental tests of the roles of adaptation, chance, and history in evolution. Science 267:87–90
Utkilen H, Gjolme N (1995) Iron-stimulated toxin production in Microcystis aeruginosa. Appl Environ Microbiol 61:797–800
Wagenaar DA, Adami C (2004) Influence of chance, history, and adaptation on digital evolution. Artif Life 10:181–190
Walther GR, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F (2002) Ecological responses to recent climate change. Nature 416:389–395
Yoshida M, Yoshida T, Takashima Y, Hosoda N, Hiroishi S (2007) Dynamics of microcystin-producing and non-microcystin-producing Microcystis populations is correlated with nitrate concentration in a Japanese lake. FEMS Microbiol Lett 266:49–53
Stanford Encyclopedia of Philosophy (2008) Reductionism in biology. Available at http://plato.stanford.edu/entries/reduction-biology/
Acknowledgments
This work was financially supported by the Spanish Ministry of Sciences and Innovation through the grants CTM2008-05680 C02-01/MAR and CGL2008-00652/BOS, and Junta de Andalucía Research Group RNM-115. We thank to Dr Héctor Rodriguez and Eva M. Salgado for their technical support. Dr. Eric C. Henry kindly revised the English style and usage. The suggestions from three anonymous reviewers helped us to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rouco, M., López-Rodas, V., Flores-Moya, A. et al. Evolutionary Changes in Growth Rate and Toxin Production in the Cyanobacterium Microcystis aeruginosa Under a Scenario of Eutrophication and Temperature Increase. Microb Ecol 62, 265–273 (2011). https://doi.org/10.1007/s00248-011-9804-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-011-9804-0