Abstract
Previous studies of fungi in polar environments have revealed a prevalence of basidiomycetous yeasts in soil and in subglacial environments of polythermal glaciers. Ascomycetous yeasts have rarely been reported from extremely cold natural environments, even though they are known contaminants of frozen foods. Using media with low water activity, we have isolated various yeast species from the subglacial ice of four glaciers from the coastal Arctic environment of Kongsfjorden, Spitzbergen, including Debaryomyces hansenii and Pichia guillermondii, with counts reaching 104 CFU L−1. Together with the basidiomycetes Cryptococcus liquefaciens and Rhodotorula mucilaginosa, these yeasts represent the stable core of the subglacial yeast communities. Other glacial ascomycetous species isolated included Candida parapsilosis and a putative new species that resembles Candida pseudorugosa. The archiascomycete Protomyces inouyei has seldom been detected anywhere in the world but was here recovered from ice in a glacier cave. The glacier meltwater contained only D. hansenii, whereas the seawater contained D. hansenii, Debaryomyces maramus, Pichia guilliermondii, what appears to represent a novel species resembling Candida galli and Metschnikowia bicuspidata. Only P. guilliermondii was isolated from sea ice, while snow/ice in the fjord tidal zone included C. parapsilosis, D. hansenii, P. guilliermondii and Metschnikowia zobellii. All of these isolated strains were characterized as psychrotolerant and xero/halotolerant, with the exception of P. inouyei.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Large surfaces of the Earth are characterized by persistent, cold temperatures and are covered by ice. Ice is considered as a life-preserving medium that can entrap randomly deposited microbes that may remain viable for a long time. Recent studies have shown that different types of ice, i.e. snow, sea ice and accretion and glacial ice, can provide environments for active microbial life [14, 58]. While Bacteria and Archaea have been studied in a range of polar and other cold environments [12, 24], studies into the occurrence and diversity of fungi have been mainly limited to frozen Antarctic soils and Siberian permafrost [1, 3, 13, 14, 20, 36, 49–51, 54–57, 60, 73].
Contrary to soils in temperate zones, where filamentous fungi prevail, polar soil is dominated by basidiomycetous yeasts [73]. Low numbers of viable basidiomycetous yeasts have also been isolated from the upper, younger ice-sheet horizons and surface layers of ice and snow at both of the poles [1, 13, 14, 49–51, 56, 57]. Recently, large populations of basidiomycetous yeasts (4 × 106 CFU L−1) were discovered by us in Arctic subglacial environments that were previously considered to be abiotic [10]. However, reports of the presence of fungi in sea ice remain scarce to date [8].
To our knowledge, ascomycetous yeasts have only been exceptionally isolated from cold polar regions [2, 25, 69]. This is surprising, as ascomycetous yeasts represent the main spoilage agents of chilled or frozen foods [16, 63] and of food preserved with low water activity (a w) [19, 62]. Given the known adaptive behaviour of many ascomycetes to low a w, we assumed that various types of ice represent potential natural habitats also for diverse ascomycetous yeasts. To evaluate this hypothesis, media with lowered a w [9, 38, 39] and incubations at a low temperature were chosen to provide a selective advantage for the recovery of xerotolerant culturable yeasts. In this study, we present the results of the persistent occurrence and diversity of ascomycetous yeasts from the natural coastal Arctic environment, a previously unsuspected habitat of this group of fungi.
Materials and Methods
Sampling Sites and Sample Collection
Kongsfjorden is located at 79°N, 12°E and is one of the larger fjords on the western coast of Spitsbergen, in the Svalbard Archipelago. It is 26 km long and 8 km wide and stretches from ESE to WNW from the Greenland Sea. The majority of the drainage basin is covered by glaciers, which calve pieces of glacier ice into the fjord throughout the year. The annual mean temperature is around −5°C, although the water is warmer and less salty than the open sea during the summer. On average, the fjord water temperature is ≥0°C by the end of May and 3.8°C at the end of August. The mean salinity ranges from 34.00 to 35.00 PSU. Lowering of salinity can occur in summer and near the surface [43].
The glaciers studied were Conwaybreen, Kongsvegen, austre Lovénbreen and austre Brøggerbreen, and they have polythermal characteristics, and therefore, they mainly consist of ice at subfreezing temperatures [15]. Melting in the temperate cores of the glaciers and seasonal inputs of meltwater from the glacier surfaces provide liquid water at their base [66]. The unfrozen sediments beneath the glaciers are entrained into the basal ice where the meltwaters refreeze beneath the cold-based marginal regions of the glacier. The ice flow then transports them to the glacier margins, where they can be easily accessed and aseptically sampled [10, 66]. Although all of the glaciers studied are polythermal, austre Brøggerbreen is almost entirely cold-based, and thus, no prolonged interactions between the meltwaters and the glacier bed occur [41].
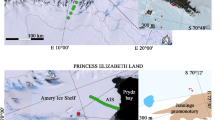
Samples from the supra- and subglacial environments were collected aseptically during the melt season in 2001 (June and August) and in 2003 (September), as previously described [10, 38]. The subglacial samples included sediment-rich and overlying clear basal ice. In 2001, 17 samples of basal ice were collected from Conwaybreen (n = 7), Kongsvegen (n = 9) and austre Lovénbreen (n = 1) coastal glaciers, while in 2003, eight samples were collected from Kongsvegen (n = 2), austre Lovénbreen (n = 4) and inland glacier austre Brøggerbreen (n = 2; Fig. 1). In the summertime, subglacial meltwater from Kongsvegen glacier was also sampled directly. Additionally, two samples of subsurface ice from cryokarst formations were collected (Fig. 1).
Map of the sampling sites at the Kongsfjorden coast (western Spitsbergen, Svalbard). 1–7 Sampling sites in 2001 of subglacial ice (1, 2, 4), glacial meltwaters (3), seawater (5a–c), sea ice (6a–c), and melted snow (7). 8–14 Sampling sites in 2003 of subglacial ice (8, 10, 12), supraglacial samples (9a–b, 11, 13, 14), and samples of subsurface ice from cryokarst formations (9c)
The supraglacial samples comprised four samples of snow/ice mixtures (austre Lovénbreen, n = 1; Kongsvegen, n = 1; austre Brøggerbreen, n = 2) and nine samples of seasonal meltwaters on the glacier surfaces (austre Lovénbreen, n = 1; Kongsvegen, n = 7; austre Brøggerbreen, n = 1; Fig. 1). In the summertime of 2001 (June and August), samples of seawater were collected from six different locations within the fjord: two samples of snow/ice mixture in the fjord tidal zone, with and without red biofilm, and samples from sea ice and ponds on its surface (Fig. 1).
Physico-chemical parameters (pH, Na+, Mg2+ and K+ concentrations and total phosphorus content) were determined for five basal ice samples (originating from Kongsvegen), a sample of subglacial meltwater and three samples of seawater, as described by Gunde-Cimerman et al. [38].
Isolation and Preservation of Strains
Ice samples were transported to the laboratory, where they were processed. The surface layer of ice was aseptically melted at room temperature and discarded. The remaining ice was transferred to another sterile container and melted. The resulting water from this ice melting, directly sampled glacier meltwater and the seawater were immediately filtered (Millipore membrane filters; 0.22- and 0.45-μm pore sizes) in aliquots of up to 100 mL. The membrane filters were placed on solid media (Table 1), and a drop of the original sample water was applied onto the membrane and dispersed with a Drigalski spatula. For each sample and medium, at least four and up to ten aliquots were filtered in parallel, and the average numbers of CFU were calculated [39]. The plates were incubated for up to 14 weeks at 10°C and 24°C in the first sampling (performed in 2001) and at 4°C, 10°C and 24°C in the second sampling (performed in 2003).
For purification, the plates were examined regularly by using stereomicroscope until the colonies acquired a sufficient size to allow discriminating between the types. A representative of each colony type was recovered, and the number of colonies of this type estimated. Colonies were picked up from plates for each sample, medium and incubation temperature in order to represent the different proportions of macromorphologies present [10, 38, 46].
The isolated and identified strains are maintained in the Culture Collection of the National Institute of Chemistry (MZKI) and in the EX-F Culture Collection of the Department of Biology, Biotechnical Faculty, University of Ljubljana, Slovenia.
Cultivation Media
The general purpose isolation and enumeration media used (Table 1) were DRBC (dichloran Rose Bengal chloramphenicol agar; 5.0 g L−1 peptone, 10.0 g L−1 glucose, 1.0 g L−1 potassium dihydrogen phosphate, 0.5 g L−1 magnesium sulphate, 0.002 g L−1 dichloran, 0.025 g L−1 Rose Bengal and 15.0 g L−1 agar; pH 5.6), MEA (malt extract agar; 20 g L−1 glucose, 1 g L−1 peptone and 20 g L−1 malt extract; 20 g L−1 agar) and DG18 (dichloran 18% glycerol agar; 5.0 g L−1 peptone, 10.0 g L−1 glucose, 1.0 g L−1 potassium dihydrogen phosphate, 0.5 g L−1 magnesium sulphate, 0.002 g L−1 dichloran, 220 g L−1 glycerol and 15.0 g L−1 agar; pH 5.6), a medium for the detection of moderate xerophiles. As ice formation results in little biologically available water, additionally selective media with high concentrations of salt or sugar were used (Table 1) to decrease the a w. For prevention of bacterial growth, chloramphenicol (50 mg L−1) was added to all of the media. The a w values of the media were determined using the DECAGON CX-1 Water Activity System (Campbell Scientific Ltd.; see Table 1).
Identification of Isolates
For the preliminary identification of the isolated yeasts, a selection of physiological tests were performed, as described by Yarrow [74]: urease, Diazonium Blue B colour reaction, fermentation of glucose, assimilation of inositol and d-glucuronate as sole carbon sources, assimilation of nitrate as sole nitrogen source and production of starch-like compounds. For the micromorphological characterization, the cultures were grown on cornmeal, malt and acetate agar at 25°C and examined under phase-contrast optics.
The molecular characterisation of the isolated yeasts included analyses of the electrophoretic band patterns following minisatellite-primed polymerase chain reaction (MSP-PCR) and their identification by determining the D1/D2 domain sequences of the 26S ribosomal DNA (rDNA).
For DNA extraction, two loopfuls of MYP (7 g L−1 malt extract, 0.5 g L−1 yeast extract, 2.5 g L−1 soytone and 15 g L−1 agar) agar-grown cultures were suspended in 500 μL lysis buffer (50 mmol L−1 Tris, 250 mmol L−1 NaCl, 50 mmol L−1 EDTA and 0.3% [w/v] SDS; pH 8), and the equivalent of 1 vol of 200 μL 425–600-μm sterile glass beads (Sigma) was added. After vortexing for 3 min, the samples were incubated for 1 h at 65°C. The suspensions were then centrifuged for 30 min at 4°C. Finally, the collected supernatants were diluted 1:750, and 5 μL of each was used directly for the PCR.
The core sequence of the phage M13 (5′GAG GGT GGC GGT TCT) was used in the MSP-PCR assays [34]. The PCR reactions were performed in a total volume of 25 μL containing 1× PCR buffer (Applied Biosystems), 2 mmol L−1 of each of the four dNTPs (Promega), 0.8 μmol L−1 primer, 10–15 ng genomic DNA and 1 U Taq DNA polymerase (Applied Biosystems). DNA amplification was performed in a Uno II Thermal Cycler (Biometra), consisting of an initial denaturing step at 95°C for 5 min, followed by 40 cycles of 45 s at 93°C, 60 s at 50°C and 60 s at 72°C, with a final extension step of 6 min at 72°C. A negative control containing sterile distilled water instead of DNA was included in all of the PCR reactions. The amplified DNA fragments were separated by electrophoresis in 1.4% (w/v) agarose gels (Gibco-BRL) using 0.5× TBE (Tris-borate-EDTA) buffer at 90 V for 3 h. A molecular size marker, λ DNA cleaved with HindIII and ΦX174 DNA cleaved with HaeIII (Promega), was included in each gel as reference. The DNA banding patterns were visualised using a UV transilluminator, and the images were acquired with the Chemi Doc system (Bio-Rad). The DNA banding patterns were analysed using the Image software package.
The NL1 (5′GCA TAT CAA TAA GCG GAG GAA AAG) forward and NL4 (5′TCC TCC GTC TAT TGA TAT GC) reverse primers were used for the synthesis of amplicons and for sequencing of the 26S rDNA D1/D2 domain. Symmetrical amplifications were performed for 36 PCR cycles with denaturation for 1 min at 94°C, annealing for 1 min at 52°C and extension for 2 min at 72°C, with the final extension for 10 min at 72°C. Both of the strands of the DNAs compared were sequenced with the Big Dye® Terminator v.1.1 Cycle sequencing kit (Applied Biosystems) using an ABI 310 automated sequencer, following the manufacturer's instructions. For identification, the sequences obtained were compared with those of all of the known yeast species that were available in the GenBank database. The GenBank accession numbers for D1/D2 are listed in Table 2.
Halo/Osmotolerance and Temperature Characteristics of the Dominant Yeast Species
The morphological characteristics of the most frequently isolated yeast species were determined. Yeasts were grown at 2°C, 10°C, 25°C, 30°C and 37°C on MEA, MEA with 5% NaCl and MYG medium (200 g L−1 glucose, 20 g L−1 malt extract, 5 g L−1 yeast extract and 20 g L−1 agar) with 20% glucose (MY20G). At 40°C, the strains were grown on GPY medium (20 g L−1 glucose, 2.5 g L−1 peptone, 2.5 g L−1 yeast extract and 20 g L−1 agar) [74]. Selected strains were also grown at 10°C and 25°C on MEA with 17% NaCl, and on MYG with 50% glucose.
Multivariate Statistical Analyses
Data were analyzed by means of principal component analysis. A low-dimension (plane) projection of the data was displayed to reveal correlation between samples (six different subglacial ice samples) and variables (the 22 respective yeast species with their average counts per litre).
Results
Characteristics of the Samples
The pH of the water from which the isolates were obtained varied between 7.1 and 7.4 across all of the samples. The mean (±SE) cation concentrations for the seawater were Na+, 7,291 (±1,011) mg kg−1; K+, 107 (±170) mg kg−1; and Mg2+, 1,031 (±172) mg kg−1. The mean cation concentrations for basal ice were considerably lower: 61 (±91), 38 (±23) and 34 (±20) mg kg−1, respectively. An increased phosphorus content (2–3 mg kg−1) was detected in basal-ice-containing sediment, although otherwise, it was under the detection limit (<1.0 mg kg−1).
Identification of the Isolates
A total of 620 yeast isolates were obtained. Most of these were of basidiomycetous origin, while ascomycetous yeasts represented ~15% of all of the isolates. The isolates were grouped on the basis of the selected physiological tests and MSP-PCR fingerprinting, and representative strains were selected for sequence analyses of the D1/D2 domain of their 26S rDNA. The strains showing identical D1/D2 sequences or no more than two mismatches were considered as belonging to the same species [30, 47]. When the sequence determined differed by three or more bases, the strain was considered to be related to the closest taxon, and so qualified as a “like”, providing a possible representative of an as yet undescribed species.
The majority of ascomycetous yeasts identified to the species or genus levels were assigned to the Hemiascomycetes lineage, Order Saccharomycetales (Table 3) [47]. Within the Debaryomyces/Lodderomyces clade [47], Candida parapsilosis, Pichia guilliermondii, Debaryomyces hansenii and Debaryomyces maramus were identified. In the Metschnikowia clade [47], Metschnikowia bicuspidata and Metschnikowia zobellii were identified.
Sequence analysis of the D1/D2 domains of the 26S rDNA revealed that the sequences of two strains (MZKI K-259 and K-269) differed (three and four nucleotides, respectively) from the type strain of Candida pseudorugosa and that the sequence of one strain (MZKI K-237) differed for 11 nucleotides from the type strain of Candida galli. Only one representative, Protomyces inouyei, was found to belong to the “Archiascomycetes” lineage (Protomycetales).
The ratio of basidiomycetous towards ascomycetous yeasts changed with the a w and the solute used to increase the osmotic pressure. On media with an a w of ~1.0 (MEA and DRBC) and on media with a lowered a w due to the added glycerol (DG18) or glucose (MY20G, MY35G and MY50G), basidiomycetous yeasts prevailed (>70%). In contrast, on media with added NaCl (MY10-12, MEA10NaCl and MEA15NaCl), ascomycetous yeasts dominated (>50%; Fig. 2). On media with 17% to 30% NaCl added, no yeasts were recorded after a 14-week incubation (data not shown).
Total number of yeasts and the relative ascomycetous/basidiomycetous yeasts ratio in seawater and subglacial ice at 10°C and 24°C, on enumeration media, on media with 0–15% NaCl or 0–50% glucose added. The abundance data were log-transformed by taking log (CFU L−1 + 1), and mean ± SD were calculated
Halo/Osmotolerance and Temperature Characteristics of the Dominant Yeast Species
All of the isolated yeasts with the exception of P. inouyei showed halotolerance, as they were able to grow on the medium with 10% NaCl added. On medium with 17% NaCl, only D. hansenii, Pichia guillermondii and C. parapsilosis were able to grow, although they were not detected on selective media containing the same salt concentration. Osmotolerance was demonstrated by growth on a medium with 50% glucose, and this was observed for all of the strains tested, with the exception of P. inouyei.
The ascomycetous yeasts isolated showed a broad temperature range supporting growth. All of the strains tested were able to grow both at 2°C/10°C and at 25°C/30°C, with the exception of P. inouyei, which could not grow at 25°C/30°C. Therefore, these ascomycetous yeasts behaved as facultative psyhcrophilic (psychrotolerant). In agreement, we saw a considerably longer lag phase at the lower temperatures, as compared to the higher ones. Of all of the species tested, only D. hansenii was not able to grow at 37°C, while only P. guillermondii was able to grow at 40°C.
Yeast Abundance and Distribution in the Arctic Coastal Environments
The abundances and species diversity of basidiomycetous yeasts isolated from the supra- and subglacial environments of the four glaciers studied are well documented in Butinar et al. [9]. They were always recovered in low numbers (up to 25 × 103 CFU L−1) from supraglacial samples, whereas their counts increased approximately 15-fold (up to 4 × 105 CFU L−1) in samples from diverse cryokarst formations [10]. In comparison, the ascomycetous yeasts were absent in supraglacial samples, although they were detected in subsurface samples (up to 103 CFU L−1).
The counts of basidomycetous yeasts from the subglacial samples were two orders of magnitude greater when compared with those recovered from supraglacial samples. A similar trend was observed for ascomycetous yeasts. The ratio shifted in favour of ascomycetous yeasts in particular when the a w of the media was lowered with NaCl (Fig. 2). In subglacial ice, the highest mean values recorded for ascomycetous yeasts (with a maximum around 104 CFU L−1; Fig. 2) were obtained on media with 5% NaCl and 20% glucose (≥300 CFU L−1). On the remaining media, with few exceptions, the mean counts remained below 102 CFU L−1 (Fig. 2).
In subglacial ice, D. hansenii and P. guillermondii were the dominant species (Table 3). They primarily occurred in Kongsvegen samples (Fig. 3), while the two dominant basidiomycetous species Cryptococcus liquefaciens and Rhodotorula mucilaginosa [10] prevailed in samples originating from austre Lovénbreen and Brøggerbreen glaciers (Fig. 3). In Brøggerbreen glacier, no ascomycetous yeasts were obtained, probably due to its almost entirely cold base. The highest CFU values for D. hansenii were obtained on media with 20% glucose and 5% NaCl (up to 104 CFU L−1), whereas the P. guilliermondii numbers were approximately half (up to 7 × 103 CFU L−1) on the medium with 10% NaCl added, at 24°C (Table 3). The other ascomycetous yeast species appeared less consistently and with lower counts. C. parapsilosis occurred only in the Conwaybreen glacier, while the C. pseudorugosa-like species was detected only in the Kongsvegen glacier (Table 3 and Fig. 3). Although P. guilliermondii was recovered in high counts from the subglacial ice, surprisingly, glacier meltwater contained only D. hansenii (up to 8 × 103 CFU L−1).
Principal component analysis of yeast species (1 Candida pseudorugosa-like, 2 Cryptococcus oeirensis, Cryptococcus saitoi, Leucosporidiella fragaria and Trichosporon mucoides, 3 Rhodotorula minuta, 4 Cryptococcus magnus, 5 Rhodotorula laryngis, 6 Cryptococcus albidus, 7 Cryptococcus victoriae and Rhodosporidium diobovatum, 8 Rhodotorula mucilaginosa, 9 Cryptococcus liquefaciens, 10 Cystofilobasidium sp., 11 Pichia guilliermondii, 12 Filobasidium uniguttulatum, 13 Debaryomyces hansenii, 14 Cryptococcus albidosimilis, 15 Cryptococcus adeliensis, 16 Candida parapsilosis and Cryptococcus laurentii, 17 Cryptococcus carnescens) isolated from different samples originating from four glaciers. Letter s, following the name of glacier, indicates subglacial ice with sediment. The first two axes explained 67.3% of the variation in the species data
Sea ice bordering the fjord was sampled in the melt season of June 2001, when its surface was melting, and it was covered in brine ponds. The CFU of the ascomycetous yeasts isolated from sea ice did not exceed 84 CFU L−1 (Table 3) on media with an a w of ~1.0. Of note, the numbers for basidiomycetous yeasts (mainly due to Cryptococcus albidus and R. mucilaginosa) were one order of magnitude greater (up to 2 × 103 CFU L−1) when compared with their counts obtained in seawater (up to 400 CFU L−1). Ascomycetous yeasts were represented exclusively by P. guilliermondii, whilst the diversity of basidiomycetous yeasts was considerably higher (data not shown).
In samples of fjord seawater, no ascomycetous yeasts were detected on the enumeration media (a w > 0.946), although on selective media with lowered a w, their counts were in the range of 5–30 CFU L−1, with occasional increases up to 500 CFU L−1 on the medium with 5% NaCl at 10°C (Fig. 2 and Table 3). As in sea ice and in seawater, P. guilliermondii was present in highest numbers (500 CFU L−1), primarily on the medium with 5% to 10% NaCl added. However, D. hansenii, D. maramus, C. galli-like and M. bicuspidata were also isolated from seawater at higher incubation temperatures (24°C), although sporadically and with the last two showing counts below 60 CFU L−1 (Table 3). The diversity and counts of the basidiomycetous yeasts isolated from seawater were lower than in sea ice, and generally did not exceed 60 CFU L−1, with the exception of R. mucilaginosa (400 CFU L−1).
When the mixture of snow/ice in the tidal zone bordering the fjord was sampled, the counts of ascomycetous yeasts occasionally increased to 6.7 × 103 CFU L−1 (Table 3). In these ecological niches, C. parapsilosis, D. hansenii, P. guilliermondii and M. zobellii were found (Table 3).
Discussion
Most studies on yeasts have focused on industrially, agriculturally and medically important species. The complexity of most natural ecosystems in temperate regions poses difficulties in studying the relationship between biotic and abiotic parameters. Studies of less complex microbial communities in polar regions that are very sensitive to human influence and climatic change may thus fill important gaps in our knowledge. To date, it has been recognized that yeasts dominate in the polar desert soils [69, 71], yeast-like fungi in Antarctic cryptoendolithic communities [33] and together with mainly β Proteobacteria, in the subglacial environments of Arctic polythermal glaciers [10, 31]. For decades, these studies have revealed the almost exclusive dominance of basidiomycetous yeasts in the polar environment [1, 10, 28, 44, 54, 70, 72].
Whether as desert soil, permafrost or different ice forms, the Arctic and Antarctic environments are mainly defined by their degree of moisture, extreme temperatures and salinities. These are also the major physico-chemical factors that influence fungal growth on preserved food, with water availability probably being the single most important environmental factor affecting growth in both cases. Only a handful of food-borne yeast species of ascomycetous affinity have so far been recognized for their ability to grow on low a w substrates. The main halo/osmotolerant food-borne yeasts [4, 19, 48, 62] belong to the genera Debaryomyces (D. hansenii), Issatchenkia (Issatchenkia orientalis), Pichia (Pichia anomala, P. guillermondii, Pichia fermentans var. fermentans, Pichia ohmeri and Pichia sorbitophila), Rhodotorula (Rhodotorula glutinis) and Zygosaccharomyces (Zygosaccharomyces bailii, Zygosaccharomyces bisporus and Zygosaccharomyces rouxii). Among these listed genera, only Rhodotorula is not an ascomycete. Its widespread occurrence in natural environments, such as the soil, the phylloplane [42], hypersaline waters [9] and Arctic glaciers [10], is well recorded. In contrast, to the best of our knowledge, ascomycetous yeasts have hardly ever been isolated from extremely cold polar regions. Candida sp., D. hansenii and Torulaspora delbrueckii were reported from Greenland ice core sections [49, 51, 69, 71], and D. hansenii and P. guilliermondii from cryopegs [35] and D. hansenii from Antarctic soil [6]. From other cold habitats, Candida santamariae [6] was recorded from the Mediterranean glacier Calderone, and Candida famata together with the new species Wickerhamomyces patagonicus were found in Patagonian glacial meltwater [22, 23]. Endolythic black yeasts have been isolated primarily from Antarctic cold environments [64], while different varieties of the black yeast Aureobasidium pullulans have been isolated as well from subglacial ice and glacial melt-waters in Svalbard [75].
Other sporadic isolates originate primarily from the soil and seawater and belong to the genera Candida, Clavispora, Debaryomyces, Dipodascus, Issatchenkia, Nadsonia, Saccharomyces, Schizoblastosporion, Sporopachydermia and Sympodiomyces [7, 69, 71]. It is possible that this deficit has been a result of inappropriate selective conditions used to recover the yeasts present in those extreme ecosystems.
Coastal Arctic environments with their particularly diverse types of ice represent potential ecological habitats for xero/halotolerant ascomycetous yeasts. As freezing leads to cellular dehydration due to reduced water absorption, we used selective media with an a w below 0.95 for their isolation. On media with added NaCl, the ratio between ascomycetous and basidiomycetous yeasts increased above 50% (Fig. 2). However, on media with an a w above 0.95, basidiomycetous yeasts prevailed (Fig. 2), as reported in other studies; they constituted ~85% of all identified strains from subglacial and sea ice samples, and up to 60% in seawater samples. Although basidiomycetous yeasts are generally recognized as being more nutritionally versatile and tolerant to low temperatures than ascomycetous yeasts [61], these results also reflect the biased approach of most isolation procedures.
The biodiversity of the basidiomycetous yeasts at the genus level was similar to what has been previously reported from cold regions [35, 49, 51, 67, 69]; however, differences were seen at the species level: C. liquefaciens and R. mucilaginosa together represented more than 90% of all of the subglacial basidiomycetous yeasts [10]. Together with the dominant ascomycetous yeasts D. hansenii and P. guillermondii, C. liquefaciens and R. mucilaginosa can probably be considered autochthonous subglacial species. Sampling of ice from a glacier cave resulted in the isolation of a rare species, Protomyces inoueyi. The genus Protomyces may include up to 60 species [59], and as it is poorly studied and so far only six strains are available in culture collections, our finding is of considerable interest for future ecological investigations.
D. hansenii, with its anamorphic state C. famata, is known as a salt- and cold-tolerant species, and it is usually isolated from materials of plant and animal (including clinical) origin, and from soil and air, in temperate climates. The species is ubiquitous in the oceans of the world [29]; however, it can also be occasionally isolated from hypersaline water in solar salterns [9]. It is a common spoilage yeast of frozen food, brine-preserved food and other low a w products [4, 19]. Despite this wide distribution, reports of its occurrence in polar regions are scarce. The presence of D. hansenii has been recorded in Antarctic soils, moss and littoral mats and mats under ice [2, 55], overcooled brine cryopegs in permafrost [35], polar waters [7, 49, 51, 69] and ice from Antarctic glaciers [25]. In all cases, the isolates were present at very low densities.
P. guilliermondii was the second most frequent ascomycetous yeasts species in the subglacial ice. It was always found in association with D. hansenii, both forming a distinct phylogenetic group [47]. P. guilliermondii is also widely distributed in nature, as strains of this species are routinely isolated from exudates of various trees, and from insects [18, 65], soil, plants [11], the atmosphere and sea water [26], and it was reported as the most frequently occurring species in the Adriatic salterns [9]. Moreover, P. guilliermondii is among the yeasts that are most commonly related to human disease [46]. Although it is also known as a food-spoilage yeast of processed and refrigerated food [27], in the polar regions, P. guilliermondii has only been reported from permafrost cryopegs [35]. C. parapsilosis, which was isolated from the subglacial ice of a single glacier, is also a food-borne halotolerant yeast [16, 63]. Again, it has been frequently isolated from clinical specimens [46], and it has, somewhat surprisingly, been isolated from solar salterns and from the Dead Sea [9]. This species has been isolated from the ice tunnel samples, collected at the Amundsen-Scott IGY South Pole Station [44].
When we compare the distribution of xerotolerance and opportunistic pathogenicity, both uncommon amongst fungi, we can surprisingly observe a total overlap at the ordinal level in the fungal tree of life. Growth at decreased water activity or opportunistic pathogenicity is in most cases limited to a few species or a single genus of an order. Focusing on individual species, we notice, however, that species exhibiting xerotolerance have never or extremely rarely been encountered in medical mycology [21].
Thus, at the species level, xerotolerance and pathogenicity seem to be mutually exclusive. It is thus noteworthy, that R. mucilaginosa, Trichosporon mucoides [10], C. parapsilosis and P. guilliermondii, all amongst dominant yeast species isolated from the subglacial environment, populate as well hypersaline environments, but can also cause human infections. It seems that only in these yeasts, cellular mechanisms put in action by xerotolerance are also promotive for pathogenicity. Several genera of fungi, as they establish themselves in the mammal host, change from a longitudinal to a more invasive yeast form. Change into a more isodiametric shape occurs also as an immediate response to an increase in osmolarity. Additionally, extracellular glyocoproteins, characteristic for the listed yeasts, are known to be associated with the ability of binding water, but they have also been proven to be a major virulence factor [21]. Another group of fungi showing a similar dual behaviour are black yeasts. These melanized polymorphic fungi can be isolated from habitats on the edge of life, such as bare rock, mosses, the deep sea, salterns and nuclear power plants, but are also from brains of healthy humans, particularly genera belonging to Chaetothyriales [5].
Viable microorganisms entrapped in subglacial ice can be released during summer glacial melts, or after the calving of icebergs into the ocean [49]. The meltwater of the Kongsvegen glacier almost exclusively contained in high counts (up to 8 × 103 CFU L−1) D. hansenii, a species otherwise characteristic of trophic waters [17]. Seawater in the vicinity of the glacier still contained 200 CFU L−1, decreasing at the exit of Kongsfjorden into the open sea, to 40 CFU L−1. The related D. maramus was isolated from seawater with low frequency, suggesting an exogenous origin, whilst M. bicuspidata and M. zobellii, two known autochthonous aquatic species [46, 52, 68], were also present in the fjord seawater in very low numbers, however probably due to nearing the limit of their realized niche. The halotolerant species M. bicuspidata [46] has been isolated from hypersaline waters [9] and the Antarctic Ocean [29]. Therefore, its presence on seawater bordering snow/ice is not surprising.
It is generally recognized that the seawater in the polar regions is dominated by basidiomycetous yeasts [29]. During the present study, R. mucilaginosa, a known ubiquitous species, a previously undescribed species of the genus Cystofilobasidium [10], Cryptococcus victoriae and Filobasidium uniguttulatum were isolated. They probably all originate from the subglacial environment [10], or soil [53], and apart from R. mucilaginosa, they have never been reported from aquatic environments.
During the freezing of seawater, yeasts can become entrapped in the sea ice, an extreme environment that is characterized by temperatures ranging from −1°C to −50°C, and with brine channels with salinities of up to 15% NaCl [8]. The only report on the presence of fungi in sea ice was based on the detection of characteristic fungal sequences of the small subunit of the rRNA gene in DNA extracted from Antarctic and Arctic sea ice [8]. To our knowledge, the present report is the first on the isolation of cultivatable yeasts from sea ice. The majority of basidiomycetous species fell into the non-pigmented Filobasidium/C. albidus taxa of the Tremellales [32], while the urediniomycetous yeasts were represented only by R. mucilaginosa. Besides basidiomycetous yeasts, P. guilliermondii was the only ascomycetous species detected, although with lower frequency than the basidiomycetous species. Further investigations of yeast biodiversity in sea ice are needed to complement this preliminary data.
Overall, the diversity of ascomycetous yeasts in different types of ice sampled, from sea ice to glacial ice, was very limited, although the abundance was higher than previously reported for any other natural habitat, in particular for D. hansenii and P. guilliermondii (up to 104 CFU L−1).
It seems that the occurrence of yeasts in polar and other extreme natural environments has been largely underestimated, although these micro-eukaryotes can take up and transform nutrients very efficiently, and thus serve as a carbon sink and participate in the short food webs present. Surprisingly, the deserts, glaciers and salterns may result in similar environmental and evolutionary pressure on microorganisms. Results of this study favour the hypothesis that fungal adaptation to low a w can be related to low temperatures stress. Freezing, drying and hypersaline stress lead to cellular dehydration, and can therefore activate common responses [37]. Cold-, salt- and drought-tolerant fungi may therefore belong to a limited group of extremophilic species that share more features and inhabit more extreme environments than we have imagined so far.
References
Abyzov SS (1993) Microorganisms in the Antarctic ice. In: Friedmann EI (ed) Antarctic microbiology. Wiley-Liss, New York, pp 265–297
Atlas RM, Di Menna ME, Cameron RE (1978) Ecological investigations of yeasts in Antarctic soils. Antarct Res Ser 30:27–34
Babjeva I, Reshetova I (1998) Yeast resources in natural habitats at polar circle latitude. Food Technol Biotechnol 36:1–5
Boekhout T, Robert V (2003) Yeasts in food: beneficial and detrimental aspects. Woodhead, Hamburg
Boekhout T, Gueidan C, de Hoog S, Samson R, Varga J, Walther G (2009) Fungal taxonomy: new developments in medically important Fungi. Curr Fungal Infect Rep 3:170–178
Branda E, Turchetti B, Diolaiuti G, Pecci M, Smiraglia C, Buzzini P (2010) Yeast and yeast-like diversity in the southernmost glacier of Europe (Calderone Glacier, Apennines, Italy). FEMS Microbiol Ecol 72:354–369
Bridge P, Spooner B, Roberts P (2010) List of non-lichenized fungi from the Antarctic region. Available at http://www.antarctica.ac.uk/Resources/BSD/Fungi/Speciespublic2.html#Lich
Brown MV, Bowman JP (2001) A molecular phylogenetic survey of sea-ice microbial communities (SIMCO). FEMS Microbiol Ecol 35:267–275
Butinar L, Santos S, Spencer-Martins I, Oren A, Gunde-Cimerman N (2005) Yeast diversity in hypersaline habitats. FEMS Microbiol Lett 244:229–234
Butinar L, Spencer-Martins I, Gunde-Cimerman N (2007) Yeasts in high Arctic glaciers: the discovery of a new habitat for eukaryotic microorganisms. Antonie Leeuwenhoek 91:277–289
Capriotti A, Ranieri L (1964) Yeasts in the green-house environment I. From soil, flowers and animals. Arch Microbiol 48:325–331
Cavicchioli R, Siddiqui KS, Andrews D, Sowers KR (2002) Low-temperature extremophiles and their applications. Curr Opin Biotechnol 13:253–261
Christner BC (2002) Incorporation of DNA and protein precursors into macromolecules by bacteria at −15°C. Appl Environ Microbiol 68:6435–6438
Christner BC, Mosley-Thompson E, Thompson LG, Zagorodnov V, Sandman K, Reeve JN (2000) Recovery and identification of viable bacteria immured in glacial ice. Icarus 144:479–485
Copland L, Sharp M (2001) Mapping thermal and hydrological conditions beneath a polythermal glacier with radio-echo sounding. J Glaciol 47:232–242
Davenport RR (1980) Cold-tolerant yeasts and yeast-like organisms. In: Skinner FA, Passmore SM, Davenport RR (eds) Biology and activities of yeasts. Academic, London, pp 215–230
de Almeida JMGCF (2005) Yeast community survey in the Tagus estuary. FEMS Microbiol Ecol 53:295–303
de Araújo FV, Soares CA, Hagler AN, Mendonça-Hagler LC (1995) Ascomycetous yeast communities of marine invertebrates in a southeast Brazilian mangrove ecosystem. Antonie Leeuwenhoek 68:91–99
Deak T, Beuchat L (1996) Handbook of food spoilage yeasts, CRC series in contemporary food science. CRC, Boca Raton
Deegenaars ML, Watson K (1998) Heat shock response in psychrophilic and psychrotrophic yeast from Antarctica. Extremophiles 2:41–49
de Hoog GS, Zalar P, van den Ende BG, Gunde-Cimerman N (2005) Relation of halotoleranve to human pathogenicity in the fungal tree of life: an overview of ecology and evolution under stress. In: Gunde-Cimerman N, Oren A, Plemenitaš A (eds) Adaptation to life at high salt concentrations in Arcahea, Bacteria and Eucarya. Springer, Dordrecht, pp 371–395
de Garcia V, Brizzio S, Libkind D, Buzzini P, van Broock M (2007) Biodiversity of cold-adapted yeasts from glacial meltwater rivers in Patagonia, Argentina. FEMS Microbiol Ecol 59:331–341
de Garcia V, Brizzio S, Libkind D, Rosa CA, van Broock M (2010) Wickerhamomyces patagonicus sp. nov., an ascomycetous yeast species from Patagonia, Argentina. Int J Syst Evol Microbiol 60:1693–1696
Deming JW (2002) Psychrophiles and polar regions. Curr Opin Microbiol 5:301–309
Di Menna ME (1966) Yeasts in Antarctic soils. Antonie Leeuwenhoek 32:29–38
Diriye FU, Giansante C, Scorzetti G, Zilli R (1993) Indagine preliminare sulla ecologia dei lieviti delle acque del litorale Abruzzese. Ann Fac Agr Perugia XLV:339–345
Diriye FU, Scorzetti G, Martini A (1993) Methods for the separation of yeast cells from the surfaces of processed, frozen foods. Int J Food Microbiol 19:27–37
Dmitriev VV, Gilichinsky DA, Faizutdinova RN, Shershunov IN, Golubev WI, Duda VI (1997) Occurrence of viable yeasts in 3-million-year-old permafrost in Siberia. Mikrobiologiya 66:655–660
Fell JW (1976) Yeasts in oceanic regions. In: Jones GEB (ed) Recent advances in aquatic mycology. Elek Science, London, pp 93–124
Fell JW, Boekhout T, Fonseca A, Scorzetti G, Statzell-Tallman A (2000) Biodiversity and systematics of basidiomycetous yeasts as determined by large-sub-unit rDNA D1/D2 domain sequence analysis. Int J Syst Evol Microbiol 50:1351–1371
Foght J, Aislabie J, Turner S, Brown CE, Ryburn J, Saul DJ, Lawson W (2004) Culturable bacteria in subglacial sediments and ice from two southern hemisphere glaciers. Microb Ecol 47:329–340
Fonseca A, Scorzetti G, Fell JW (2000) Diversity in the yeast Cryptococcus albidus and related species as revealed by ribosomal DNA sequence analysis. Can J Microbiol 46:7–27
Friedmann EI (1982) Endolithic microorganisms in the Antarctic cold desert. Science 215:1045–1053
Gadanho M, Almeida JM, Sampaio JP (2003) Assessment of yeast diversity in a marine environment in the south of Portugal by microsatellite-primed PCR. Antonie Leeuwenhoek 84:217–227
Gilichinsky D, Rivkina E, Bakermans C, Shcherbakova V, Petrovskaya L, Ozerskaya S, Ivanushkina N, Kochkina G, Laurinavichuis K, Pecheritsina S, Fattakhova R, Tiedje JM (2005) Biodiversity of cryopegs in permafrost. FEMS Microbiol Ecol 53:117–128
Golubev WI (1998) New species of basidiomycetous yeasts, Rhodotorula creatinovora and R. yakutica, isolated from permafrost soils of Eastern-Siberian Arctic. Mykologiya Phytopathologiya 32:8–13
Gunde-Cimerman N, Butinar L, Sonjak S, Turk M, Uršič V, Zalar P, Plemenitaš A (2005) Halotolerant and halophilic fungi from coastal environment in the Arctics. In: Gunde-Cimerman N, Oren A, Plemenitaš A (eds) Adaptation to life at high salt concentrations in Arcahea, Bacteria and Eucarya. Springer, Dordrecht, pp 397–423
Gunde-Cimerman N, Sonjak S, Zalar P, Frisvad JC, Diderichsen B, Plemenitaš A (2003) Extremophilic fungi in Arctic ice: a relationship between adaptation to low temperature and water activity. Phys Chem Earth 28:1273–1278
Gunde-Cimerman N, Zalar P, de Hoog GS, Plemenitaš A (2000) Hypersaline water in salterns—natural ecological niches for halophilic black yeasts. FEMS Microbiol Ecol 32:235–240
Hocking AD, Pitt JI (1980) Dichloran-glycerol medium for enumeration of xerophilic fungi from low moisture foods. Appl Environ Microbiol 39:488–492
Hodson AJ, Mumford PN, Kohler J, Wynn PM (2005) The High Arctic glacial ecosystem: new insights from nutrient budgets. Biogeochemistry 72:233–256
Inacio J, Pereira P, de Carvalho M, Fonseca A, Amaral-Collaco MT, Spencer-Martins I (2002) Estimation and diversity of phylloplane mycobiota on selected plants in a Mediterrenean-type ecosystem in Portugal. Microb Ecol 44:344–353
Ito H, Koduh S (1997) Characteristics of water in Kongsfjorden, Svalbard. Proc NIPR Symp Polar Meteorol Glaciol 11:221–232
Jacobs MPH, Taylor HC, Shafer JC (1964) Studies of fungi at Amundsen-Scott IGY South Pole Base (1957). Arch Dermatol 89:117–123
King AD, Hocking AD, Pitt JI (1979) Dichloran-rose bengal medium for enumeration of molds from foods. Appl Environ Microbiol 37:959–964
Kurtzman CP, Fell JW (1998) The yeasts a taxonomic study. Elsevier, Amsterdam
Kurtzman CP, Robnett CJ (1998) Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie Leeuwenhoek 73:331–371
Lages F, Lucas C (1995) Characterization of a glycerol/H+ symport in the halotolerant yeast Pichia sorbitophila. Yeast 11:111–119
Ma LJ, Catranis CM, Starmer WT, Rogers SO (1999) Revival and characterization of fungi from ancient polar ice. Mycologist 13:70–73
Ma LJ, Catranis CM, Starmer WT, Rogers SO (2005) The significance and implications of the discovery of filamentous fungi in glacial ice. In: Castello JD, Rogers SO (eds) Life in ancient ice. Princeton University Press, Princeton
Ma LJ, Rogers SO, Catranis CM, Starmer WT (2000) Detection and characterization of ancient fungi entrapped in glacial ice. Mycologia 92:286–295
Mendonca-Hagler LC, Hagler AN, Kurtzman CP (1993) Phylogeny of Metschnikowia species estimated from partial rRNA sequences. Int J Syst Bacteriol 43:368–373
Montes MJ, Belloch C, Galiana M, Garcia MD, Andres C, Ferrer S, Torres-Rodriguez JM, Guinea J (1999) Polyphasic taxonomy of a novel yeast isolated from antarctic environment; description of Cryptococcus victoriae sp. nov. Syst Appl Microbiol 22:97–105
Onofri S, Selbmann L, Zucconi L, Pagano S (2004) Antarctic microfungi as models for exobiology. Planet Space Sci 52:229–237
Onofri S, Zucconi L, Tosi S (2007) Continental Antarctic Fungi. IHW-Verlag, Eching bei Munchen
Poglazova MN, Mitskevich IN, Abyzov SS, Ivanov MV (2001) Microbiological characterization of the accreted ice of subglacial Lake Vostok, Antarctica. Mikrobiologiia 70:838–846
Price PB (2000) A habitat for psychrophiles in deep Antarctic ice. Proc Natl Acad Sci USA 97:1247–1251
Price PB, Sowers T (2004) Temperature dependence of metabolic rates for microbial growth, maintenance, and survival. Proc Natl Acad Sci USA 101:4631–4636
Reddy MS, Kramer CL (1975) A taxonomic revision of the Protomycetales. Mycotaxon 3:1–50
Rivkina EN, Friedmann EI, McKay CP, Gilichinsky DA (2000) Metabolic activity of permafrost bacteria below the freezing point. Appl Environ Microbiol 66:3230–3233
Sampaio JP (2004) Diversity, phylogeny and classification of basidiomycetous yeasts. In: Agerer R, Blanz P, Piepenbring M (eds) Frontiers in basidiomycote mycology. IHW-Verlag, Eching, pp 49–80
Samson RA, Hoekstra ES, Frisvad JC, Filtenborg O (2000) Introduction to food- and airborne fungi. Centraalbureau voor Schimmelcultures, Utrecht
Schmidt-Lorenz W (1982) Indicator organisms in frozen foods in relation to spoilage. Antonie Leeuwenhoek 48:625–633
Selbmann I, de Hoog GS, Mazzaglia A, Friedmann EI, Onofri S (2005) Fungi at the edge of life: cryptoendolithic black fungi from Antarctic desert. Stud Mycol 51:1–32
Sibirny AA (1996) Pichia guilliermondii. In: Wolf K (ed) Nonconventional yeasts in biotechnology: a handbook. Springer, Berlin, pp 255–275
Skidmore ML, Foght JM, Sharp MJ (2000) Microbial life beneath a high Arctic glacier. Appl Environ Microbiol 66:3214–3220
Turchetti B, Buzzini P, Goretti M, Branda E, Diolaiuti G, D’Agata C, Smiraglia C, Vaughan-Martini A (2008) Psychrophilic yeasts in glacial environments of Alpine glaciers. FEMS Microbiol Ecol 63:73–83
van Uden N, Fell JW (1968) Marine yeasts. Adv Microbiol Sea 1:167–201
Vishniac HS (1987) Psychrophily and the systematics of yeast-like fungi. In: de Hoog GS, Smith MT, Weijman ACM (eds) Proceedings of an international symposium on the perspectives of taxonomy, ecology and phylogeny of yeasts and yeast-like fungi, CBS, Delft. Elsevier Science Publishers, Amsterdam, pp 389–402
Vishniac HS (2002) Cryptococcus tephrensis, sp. nov., and Cryptococcus heimaeyensis, sp. nov.; new anamorphic basidiomycetous yeast species from Iceland. Can J Microbiol 48:463–467
Vishniac HS (2006) A multivariate analysis of soil yeasts isolated from a latitudinal gradient. Microb Ecol 52:90–103
Vishniac HS, Klinger J (1986) Extreme environments. Yeasts in the Antarctic deserts. In: Megušar F, Gantar M (eds) Perspectives in microbial ecology. Slovene Society for Microbiology, Ljubljana, pp 46–51
Vishniac HS, Onofri S (2003) Cryptococcus antarcticus var. circumpolaris var. nov., a basidiomycetous yeast from Antarctica. Antonie Leeuwenhoek 83:231–233
Yarrow D (1998) Methods for the isolation, maintenance and identification of yeasts. In: Kurtzman CP, Fell JW (eds) The yeasts a taxonomic study. Elsevier, Amsterdam, pp 77–100
Zalar P, Gostinčar C, de Hoog GS, Uršič V, Sudhadham M, Gunde-Cimerman N (2008) Redefinition of Aureobasidium pullulans and its varieties. Stud Mycol 61:21–38
Acknowledgements
The work in Kongsfjorden was funded by EU Large Scale Facility Fund. The work on identification of yeasts was supported by the Slovenian Ministry of Education, Science and Sport and Slovenia-Portugal bilateral collaboration. Prof. Isabel Spencer-Martins (New University of Lisbon, Biotechnology Unit, Faculty of Sciences and Technology, Centro de Recursos Microbiológicos (CREM), Caparica, Portugal) contributed a lot to our research work on yeast diversity from extreme environments and also contributed to this manuscript, but she unfortunately passed away during the final preparation of the manuscript. The authors would like to thank Nick Cox (NERC Station) for his logistic support and to Martin Grube for help with sequencing of yeasts.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Butinar, L., Strmole, T. & Gunde-Cimerman, N. Relative Incidence of Ascomycetous Yeasts in Arctic Coastal Environments. Microb Ecol 61, 832–843 (2011). https://doi.org/10.1007/s00248-010-9794-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-010-9794-3