Abstract
Pseudomonas fluorescens strains are used in agriculture as plant growth-promoting rhizobacteria (PGPR). Nontarget effects of released organisms should be analyzed prior to their large-scale use, and methods should be available to sensitively detect possible changes in the environments the organism is released to. According to ecological theory, microbial communities with a greater diversity should be less susceptible to disturbance by invading organisms. Based on this principle, we laid out a pot experiment with field-derived soils different in their microbial biomass and activity due to long-term management on similar parent geological material (loess). We investigated the survival of P. fluorescens CHA0 that carried a resistance toward rifampicine and the duration of potential changes of the soil microflora caused by the inoculation with the bacterium at the sowing date of spring wheat. Soil microbial biomass (Cmic, Nmic) basal soil respiration (BR), qCO2, dehydrogenase activity (DHA), bacterial plate counts, mycorrhiza root colonization, and community level substrate utilization were analyzed after 18 and 60 days. At the initial stage, soils were clearly different with respect to most of the parameters measured, and a time-dependent effect between the first and the second set point were attributable to wheat growth and the influence of roots. The effect of the inoculum was small and merely transient, though significant long-term changes were found in soils with a relatively low level of microbial biomass. Community level substrate utilization as an indicator of changes in microbial community structure was mainly changed by the growth of wheat, while other experimental factors were negligible. The sensitivity of the applied methods to distinguish the experimental soils was in decreasing order Nmic, DHA, Cmic, and qCO2. Besides the selective enumeration of P. fluorescens CHA0 rif+, which was only found in amended soils, methods to distinguish the inoculum effect were DHA, Cmic, and the ratio of Cmic to Nmic. The sampling time was most sensitively indicated by Nmic, DHA, Cmic, and qCO2. Our data support the hypothesis—based on ecosystem theory—that a rich microflora is buffering changes due to invading species. In other words, a soil-derived bacterium was more effective in a relatively poor soil than in soils that are rich in microorganisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microorganisms are often used in agriculture and horticulture in order to improve soil fertility, plant growth, and plant health and to facilitate bioremediation of polluted sites [1, 11, 32]. They are usually applied in high numbers in order to effectively reach the goal they are applied for. Bacterial inoculants came into agricultural use in the early 20th century, many of which were related to the N-cycle [46]. The release of Rhizobium ssp. has enhanced the N-fixation potential of leguminous plants, however, Paul and Clark [32] claimed that newly developed superior strains cannot compete with the ones introduced earlier and now being indigenous to the soil. The early use of these strains, therefore, may cause failure of later amendments. Many other organisms (e.g., Mycorrhiza, Pseudomonas ssp., Trichoderma ssp., Bacillus ssp.) have been released to agricultural crops and soils in order to improve nutrient uptake and plant health. The survival of these organisms in soil and their effects on the crop plant, however, are highly variable, and up to now, none of the released microorganisms has imposed damage to the targeted environment, where it has been released.
Microorganisms are associated with above- and belowground parts of living plants, and they are considered harmful or beneficial, depending on whether they depress or favor plant growth and health [23]. All plant parts provide niches for microorganisms, but their functional role in plant nutrition and health has attracted scientific attention only when natural processes of plant disease control were observed and investigated. The phylloplane is colonized by 106 to 108 bacteria cm−2 [19] and the rhizosphere by 109 to 1010 bacteria cm−3 [32]. The rhizosphere is inhabited by a large diversity of microbial species that may proliferate because of the presence of root exudates and debris. Strains of the genus Pseudomonas are often found in soils and especially in the rhizosphere of living plants [17], but depending on the plant species, other microorganisms may prevail like rhizobia in the rhizosphere of leguminous plants [22].
A general concept has been developed, where bacterial strains were identified that were able to colonize plant roots, stimulate plant growth, and/or reduce the incidence of plant disease. These organisms were called plant growth-promoting rhizobacteria (PGPR) that may favor plant growth by fixation or solubilization of plant nutrients and the production of growth stimulants. The aspect of biocontrol is not mandatory in this concept though not excluded. Haas and Defago [11] define the level of root colonization effectively protecting the plant from a pathogen like Gaeumannomyces graminis var. tritici responsible for take all in wheat to be 105 to 106 colony forming units (CFU) g−1 of root fresh matter. This amount may correspond to 0.1% to 1% of the total cultivable rhizobacterial population under natural conditions. The relatively low percentage of these specific organisms present on the root suggest antibiosis, induced systemic resistance, and pathogen–antagonist interactions to be the main modes of action, rather than the competitive trapping of carbon sources in the root environment [16].
Fluorescent pseudomonads have often been isolated from soils that were suppressive toward a plant pathogen, meaning that the plant remains healthy even though the pathogen is present. Among other commercially available biocontrol organisms, strains of Pseudomonas fluorescens are often used in agriculture to control soil-borne plant pathogens in order to enhance productivity and plant health [11]. Extensive work has focused on their suppressiveness and the underlying principles [47]. Early ideas put forward the mechanism of antibiosis to be responsible for a successful competition between the antagonist and a putative pathogen, but it remained hypothetic due to the lack of antibiotic production in soil and its experimental accessibility by modern techniques. Among other organisms, fluorescent pseudomonads are commercially available, and they are used for a variety of agronomic purposes [11, 38]. The organisms are generally present in soil but may be more or less abundant depending on soil characteristics and plant cover. Their application in the environment is a mass release of organisms that may proliferate. This fact alone may change the ecological balance and may impose a risk to the environment as well as to human or animal health.
Risk assessments are aiming at minimizing the potential hazards to the environment and human beings. In contrast to abiotic threats to the environment, the introduction of living organisms comprises the risk that the organisms may proliferate, exchange their genetic information with other organisms, and thus, the genetic trait may change unpredictably in the evolutionary process. Especially in the discussion on the release of genetically modified organisms, this topic is of unequivocal importance, but it also implies to the mass release of any organisms to new environments.
In this study, we investigated the effects of a rifampicine-resistant strain of Pseudomonas fluorescens CHA0 on biological indicators of soil quality and applied a series of soil biological methods in order to evaluate their sensitivity to indicate changes. We tested the establishment of the inoculated bacterium in soils that were different in their long-term agricultural management and therefore exhibited a range of microbial biomass and activity. These soils were chosen because of their identical pedological origin. Our hypothesis in this concern was that a microbial inoculum has a greater potential to change microbial biomass and activity in soils that are poor compared to soils that are rich in microorganisms—briefly, active or fertile soils being better buffered toward disturbance than poor soils. Soils from a long-term field trial comparing organic and conventional farming systems [20] on the same loess soil were compared to a soil from a farmer’s field on a similar soil type that was obviously showing signs of degradation due to long-term monocropping of maize.
Material and Methods
Soil Sampling
Two treatments of the DOK long-term field experiment (Therwil, CH; 7°33′ E, 47°30′ N) [6, 20] and one field site that has continuously been cropped with maize (Burnhaupt-le-Haut, F; 7°07′ E, 47°43′ N) were chosen, the latter already used in a study on diversity of mycorrhiza [31]. All soils developed on deep alluvial loess and were cropped with winter wheat in that year. Soils were sampled on March 27th 2006 as a composite sample of the four replicate field plots of the DOK-experiment and as a composite sample of four 10 × 10 m subplots of the field in Burnhaupt-le-Haut (France). Soil samples were taken from the 0- to 20-cm soil layer (plough layer) with a 3 cm ∅ auger and combined to one bulk sample per treatment. Soils were sieved (8 mm mesh), and moisture was adjusted to 40–50% maximum water holding capacity. Soil samples were kept at 4°C until they were used in the pot experiment.
The soils of the DOK experiment derived from a biodynamic (D), and a conventional farming system (K); both systems are livestock based. These farming systems were run according to good agricultural practice and in line with current regulations for biodynamic (D) or Swiss-integrated production (K) in a ley rotation. D field plots received composted farmyard manure and slurry as the sole fertilizers, while K was fertilized with stacked farmyard manure, slurry, and mineral fertilizer. The soil from the field site in Burnhaupt-le-Haut (B) was cropped with maize for the preceding 4 years and has been fertilized with mineral fertilizers exclusively. The year before sampling, the farmer started to apply compost to his field because of obvious problems with soil structure, and winter wheat has been sown. Soil stability and water infiltration was poor at this site, and it also showed retarded decomposition as undecomposed organic particles from last year’s crop were still visible. Soils derived from the DOK experiment had higher soil carbon and pH than soil B from the maize monocropping site but a similar clay content (Table 1).
Inoculum P. fluorescens CHA0 rif+
The soil-derived bacterium P. fluorescens CHA0 was isolated in 1985 from a tobacco field suppressive toward Thielaviopsis basicola in the region of Morens (Fribourg, CH) [27]. This strain has shown to be suppressive also toward the root pathogen G. graminis var. tritici on wheat [14, 34]. The P. fluorescens CHA0 strain used in this study was resistant toward the antibiotic rifampicine, which is a spontaneous mutant of the one isolated from the field. Due to this resistance, a monitoring of inoculum survival was possible by using rifampicine in culture media.
Cultivation and Inoculum Preparation
The inoculum was produced by cultivating P. fluorescens CHA0 rif+ in liquid nutrient broth M-1 (0.98 g K2HPO4 × 3H2O, 0.40 g MgSO4 × 7H2O, 0.40 g CaCO3, 10 g saccharose, 3 g yeast extract in 1,000 ml H2O demin.) at 27 C for 24 h [7]. The precultivated organisms were then transferred to fresh M-1 medium and cultivated for another 24 hours at 27 C. The actively growing bacteria were then centrifuged at 4,000 rpm for 10 min and washed twice by resuspending the bacteria in sterile saline (8 g NaCl L−1) and centrifugation as described before. The cell density was adjusted to 7.2 × 108 cells ml−1 by counting appropriate dilutions of this suspension by microscopy.
Experimental Design
The experiment was laid out as a randomized pot trial under greenhouse conditions. The three field soils were filled into 3 × 20 plastic pots (Greiner pots, ∅ 14 cm, height 10.5 cm, 1,040 ml). Each pot was filled with the corresponding amount of 712 g dry matter of soil, which was slightly compacted before sowing seven spring wheat seeds (variety Greina) per pot. For each soil ten pots were inoculated with P. fluorescens CHA0 rif+, and another ten were treated with the saline only. Each pot was placed on a separate plate to avoid contamination from one pot to the other. After sowing, 20 ml of the washed bacterial suspension was poured over the soil surface, corresponding to 1.44 × 1010 cells per pot or 2 × 107 cells per gram soil (dry matter). After seed emergence, five healthy seedlings were left in each pot. Five of the set of ten pots per treatment were destructively sampled on day 18 and the other five on day 60 after sowing. The whole soil of one pot was used as a single replicate. Soils were checked for moisture on a daily base, and the water content was adjusted gravimetrically as long as the plants were relatively small; later, equal amounts of demineralized water were added. Weeds and surplus wheat seedlings were cut with a sterilized scissor at the soil surface. After 28 days, wheat plants were fertilized with 11 mg ammonium nitrate (NH4NO3) dissolved in demineralized water per pot four times in weekly intervals.
Soil Analyses
All soil biological analyses were done with moist soil samples at a water content corresponding to 40% maximum water holding capacity.
Soil Microbial Biomass
Soil microbial biomass C (C mic) and N (N mic) was estimated by chloroform–fumigation–extraction (CFE) according to Vance et al. [45]. CFE was done on 20 g (dry matter) subsamples that were extracted with 80 ml of a 0.5-m K2SO4 solution. Total organic C (TOC) in soil extracts was determined by infrared spectrometry after combustion at 850 C (DIMA-TOC 100, Dimatec, Essen, D). Total N was subsequently measured in the same sample by chemoluminescence (TNb, Dimatec, Essen, D). Soil microbial biomass was then calculated according to the formula: C mic = E C/k EC where E C = (TOC in fumigated samples − TOC in control samples) and k EC = 0.45 [12]. N mic = E N/k EN where E N = (N t in fumigated samples − N t in control samples) and k EN = 0.54 [13].
Soil Basal Respiration
Soil basal respiration was measured in preincubated (7 days at 22 C) samples as CO2 evolved over a period of 72 h. Soil samples (20 g dry matter) were weighed into perforated centrifuge tubes and placed into screw cap glass bottles (Schott, 250 ml) in the presence of 0.025 N NaOH as CO2 trap for a 24-h preincubation period in the bottle. The actual measurement started by adding 20 ml of 0.025 N NaOH. After 72 h, the soil was taken out of the bottle, and the alkali was titrated with 0.025 N HCl. The measurement was done according to the reference methods of the Swiss agricultural research stations [5]. The metabolic quotient for CO2 (qCO2) was calculated from basal respiration rates divided by the amount of microbial biomass carbon (C mic) in the respective sample [3].
Soil Dehydrogenase Activity
Dehydrogenase activity was measured according to Tabatabai [41] in 5 g soil samples that were incubated at 30 C for 24 h in the presence of an alternative electron acceptor (triphenyltetrazoliumchloride). The red-colored product (triphenylformazan) was extracted with acetone and measured in a spectrophotometer at 546 nm.
Culturable Cell Counts
Plate counts of soil bacteria were determined at each sampling time and in each pot by using serial soil dilutions. Dilutions were adjusted to yield appropriate numbers of colonies on a glucose minimal media [1 g yeast extract, 0.25 g glucose, 50 ml solution A, 25 ml solution B, pH 7.0, 12 g agar, in 1,000 ml aqua bidest.; Solution A contained 5 g K2HPO4 L−1 H2O demin.; Solution B contained 2.5 g MgSO4, 25 g (NH4)2SO4 and 25 ml of a trace element solution (1 g FeSO4·7H2O, 0.22 g MnSO4·H2O, 0.1 g H3BO3, 0.1 g Na2Mo4·2H2O, 0.18 g ZnSO4·7H2O, 0.16 g CuSO4·5H2O, Aqua bidest ad 1,000 ml) in 1 L of demineralized water.].
Fluorescent pseudomonads were enumerated on King’s B agar (1.5 g K2HPO4, 1.5 g MgSO4·7H2O, 15 ml glycerol, 20 g proteose peptone, pH 7.2, 12 g Agar in 1,000 ml aqua bidest.), and King’s B agar with rifampicine was used for the monitoring of the inoculant according to Mascher et al. [25]. Rifampicine diluted in ethanol (10 mg ml−1 ethanol) was added at a concentration of 100 mg/L to King’s B medium.
Community Level Substrate Utilization
Community level substrate utilization (CLSU) patterns were measured according to Garland and Mills [9]. Microtitre plates (Biolog® eco-plates), prefilled with 31 different C sources and a tetrazolium dye (Biolog, Hayward, CA, USA), were filled with soil suspensions. In detail, 10 g (dry matter) of soil was suspended in 90 ml of a saline solution (0.9% NaCl) on a rotary shaker at 300 rpm for 30 min. Extracts were allowed to settle for 10 min to clear the supernatant. A tenfold dilution of the first suspension (10−2) (125 μl per well) was directly inoculated to Biolog® ecoplates, which were then incubated at 20 C. The plate absorbance was measured repeatedly at 600 nm (MRX Microplate reader, Dynex Technologies, Chantilly, VA, USA). Community level substrate utilization patterns were standardized by water blank subtraction and transformation by the average well color development (awcd) [8, 48].
After soil suspensions from experimental samples were filled into Biolog ecoplate wells, bacterial growth started with a time lag of about 20 h. Growth on single substrates—as indicated by the color formation from a tetrazolium dye—showed typical sigmoidal curve shapes. Set points after filling suspensions to the wells were chosen, when the increase in color formation of a plate became maximal. These set points occurred between 47 and 66 h of incubation at 20 C depending on the soil. Substrate utilization patterns at these set points of maximum substrate utilization were used for comparison of experimental soils.
Plant Analyses
Shoots and roots of each pot were sampled on days 18 and 60 after sowing. Aboveground biomass was measured gravimetrically after drying at 60 C for 24 h. Roots were carefully separated from the adhering soil and washed to remove soil particles.
Mycorrhiza Root Colonization
Fresh root cutlings of approximately 15 mm length were stored in ethanol (50%). For the assessment of root colonization, a modified method of Phillips and Hayman [33] was used. Briefly, roots were cleared by incubating for 15 min at 90 C in 2.5% KOH. After removal of the alkali and washing the roots with tap water, 1% HCl was added to acidify the roots. Mycorrhizal structures in the cleared roots were then stained by adding a solution of 0.5 g trypan blue, 500 ml Glycerol, 50 ml 1% HCl in 1,000 ml water and incubation for 15 min at 90 C. In order to enhance the contrast, the staining solution was decanted and replaced by the same solution without trypan blue. Here, the roots may be conserved for up to 1 year.
Forty stained root cutlings per pot were transferred on a microscopic slide and fixed with a cover slip. The colonization of roots was evaluated microscopically by estimating the blue-stained fungal structures along the root cutlings at 200-fold magnification.
Colonization of Roots with P. fluorescens
The remainder of the roots were put between moistened filter paper and frozen at −18 C for not more than 5 days. Approximately 500 mg of roots from each sample was put into a 50-ml Erlenmeyer flask containing 10 ml of a 0.9% sterile saline solution. The samples were shaken for 20 min at 450 rpm. From the root wash, 1 ml samples were taken to estimate Pseudomonas spp. adhering to the roots and put on ice. Twenty microliters of sample suspension were transferred to 96-well microtitre plates containing 180 μl per well of King’s B medium [15] with amendments of actidione (100 μg ml−1), chloramphenicol (13 μg ml−1), and ampicillin (40 μg ml−1). Serial tenfold dilutions were made to 10−6 in the microtitre plate, four replicates were prepared per root system, and then the plates were incubated for 4 days at 24 C temperature with continuous shaking (150 rpm). Bacterial growth was estimated visually, and the terminal dilution showing positive growth was used to calculate total population size of a sample by the most probable number technique—MPN [2, 10]. Sterile glycerol was added to all wells to achieve a final concentration of 50% for further storage at −80 C.
Statistics
The operational unit of the statistical evaluation was one replicate pot, and all analyses were performed based on five replicate pots. Data were subjected to a three-way analysis of variance (ANOVA) with soil, sampling time, and inoculum, and their interaction as factors (JMP 5.0, SAS Institute, Cary, NC, USA). In case of significant model effects, comparisons of differences between soils amended with P. fluorescens CHA0 rif+ and the respective control were performed using Tukey HSD post hoc tests. Any difference mentioned is significant at p = 0.05. Multivariate analysis of community level substrate utilization profiles were done with CANOCO 4.5 (Microcomputer Power, Ithaca, NY, USA).
Results
Crop Yield
Spring wheat emergence was almost 100% after 10 days. Five evenly developed seedlings were allowed to grow, while all other germinating seeds and weeds were removed carefully. Eighteen days after sowing, the aboveground biomass averaged at 41 mg dry matter per plant or 203 mg per pot. Sixty days after sowing, the aboveground biomass averaged at 252 mg per plant or 1,260 mg per pot (Table 2). On day 18, the average aboveground oven dry biomass from soils B and K was 34% higher than the one from soil D, while on day 60, soil D produced 22% more aboveground dry matter than the average of soils B and K.
Soil Microbial Biomass
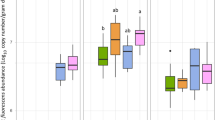
According to the objectives and the design of this study, the soils used had all different soil microbial biomass carbon (C mic) in the order D > K >B, and they retained this ranking over the time of the study. At the starting point of the experiment, soil D had 51% and soil K 34% more C mic than soil B. As an average over all soils, C mic increased from the start until day 18 by 4% and until day 60 by 21% (Fig. 1). The uninoculated soils B and K showed a slight decrease in this time span. The P. fluorescens inoculum enhanced the average C mic by 5%, but in soil B and K, C mic increased by 11% and 8%, respectively, in the inoculated soils. On day 60, only soil B showed a lasting effect of the P. fluorescens inoculum with 13% higher C mic than in the control. On the other hand, in soil D, which had the highest C mic at the start, no change due to the inoculum was observed on days 18 and 60.
Microbial biomass nitrogen (N mic) showed the same ranking of experimental soils in the order D > K >B at the start, soil D showing 56% and soil K 21% higher N mic values than soil B. The N mic average over all treatments decreased by 20% from the start to day 18 but recovered again to values 6% under the initial ones (Fig. 2). In soil K on day 18, the P. fluorescens inoculum led to 6% more microbial nitrogen, while in soil B, N mic was significantly higher on both sampling dates compared to the controls with 9% on day 18 and 7% on day 60.
The C mic-to-N mic ratio (data not shown), across all soils, increased by 30% from the start to day 18 and retained this ratio until day 60. With a C mic-to-N mic ratio of 5.86 at the start, soil K showed 14% higher values than soil D, and values of soil B were 3% higher than the ones of soil D. At an average over all treatments, the inoculum showed 2% higher values compared to the controls.
Soil Basal Respiration and qCO2
At the start of the experiment, soil basal respiration in soil B was 24% higher than in soil D and 14% higher than in soil K, and at an average over all soils, it decreased by 11% from the start to day 18 and by 28% from the start until the end of the experiment on day 60 (Fig. 3). No inoculum effect was found except for soil B that showed 14% and 12% higher values than the controls on days 18 and 60, respectively.
When basal soil respiration rates were divided by the amount of microbial biomass to calculate the metabolic quotient for CO2 (qCO2), no inoculum effect was observed, and only the effects of soils (B > K > D) and sampling time remained significant (Fig. 4). qCO2 in soil D was 47% and in soil K 34% lower than in soil B at the start. qCO2 decreased by 40% between the start and day 60.
Soil Dehydrogenase Activity
Soil dehydrogenases are microbially bound enzymes that are part of the electron transport chain. At the start of the experiment, dehydrogenase activity (DHA) in soil D was 96% and in soil K 74% higher than in soil B and this order was retained over the whole experiment. While no significant changes in DHA were found between the start and day 18, it increased by 42% between days 18 and 60 as an average over all soils (Fig. 5). The P. fluorescens inoculum produced a small but significant overall increase in DHA by 6% on day 18 compared to the controls; in soil B, however, this increase accounted for 18%. While short-term inoculum effects on day 18 were significant, they disappeared after prolonged incubation until day 60 in all soils.
Community Level Substrate Utilization
Community level substrate utilization (CLSU) with Biolog Ecoplates was evaluated 47 to 66 h after inoculating soil suspensions to the microplate wells, when maximum substrate utilization per plate was monitored. The strongest influence on the substrate utilization patterns as a result of redundancy analysis (RDA) was the sampling time with longest vectors shown in Fig. 6. CLSU patterns at the start, on days 18 and 60 were clearly different (Fig. 6), as the vectors point to different directions. The explained variance due to the different soils was substantially lower (Table 3). CLSU of soil K was different from soils B and D. The inoculation of soils with P. fluorescens CHA0 rif+ had only a minor influence on explaining the variation in CLSU patterns as indicated by the relatively short length of the respective vectors and the low percentage of explained variation of the second canonical axis in Fig. 6.
Constrained ordination of the first two canonical axes as determined by redundancy analysis (RDA) of community level substrate utilization (CLSU) patterns at the start, days 18 and 60 after inoculation with P. fluorescens CHA0 rif+ (P.f.) in soils B, D, and K (n = 5). Explained variation is displayed in parentheses along the first two ordination axes together with the overall correlation (r) of the analyzed factors (soil, inoculum, sampling time) and the dependent variable—the CLSU pattern. Vector directions indicate maximum variation due to the corresponding factor, and vector length is indicating the strength of the correlation
Root Colonization with Arbuscular Mycorrhiza and P. fluorescens
Arbuscular mycorrhizae are symbioses of specific fungi with plant roots. This symbiosis has been found to enhance plant productivity, especially in poor soils; it may also enhance plant health through induced resistance and may stabilize soils by building up soil structure. In our experiment, the colonization of wheat roots by mycorrhiza was highly variable with coefficients of variation of 44% and 58% for the average of all samples on days 18 and 60, respectively. Nonetheless, the full factorial model indicated a significant effect of the soil with 68% higher colonization of roots in soil B than in soil K (Table 4), and as an average over all soils, it increased from 9.8% on day 18 to 24% on day 60.
Numbers of P. fluorescens ssp. on root samples of spring wheat plants were not affected by the inoculum, but over all samplings and treatments, they were 4.3 times higher values in soil B than in soils D and K (Table 4). The cultivable numbers were 4.7 times higher in roots of young plants (day 18) than in older ones (day 60), the strongest decrease by a factor 17 being found on roots grown in soil B. In addition, only in soil B on day 60, numbers of P. fluorescens on the root were three times higher than in the control.
Soil Bacterial Plate Counts
Bacteria from experimental soils were isolated and cultivated on agar media. A small number of soil microorganisms are known to be able to grow on agar plates. Cultivated organisms on agar media typically account for only 0.1% to 5% of the total—directly observable—bacteria. At the start, bacterial numbers on the low concentrated glucose medium were lower in soil D by 57% compared to soils K and B. Cultivable numbers decreased by 30% from the start to the later samplings (Table 5). Over all samplings, soil B had 1.7 times higher CFU than soil K and 2.6 times higher CFU than soil D, and no inoculum effect was found.
Plate counts of fluorescent Pseudomonas ssp. on King’s B media were 1.34 times higher in soil K than the average of soils B and D (Table 5). In soil B, the inoculum produced 2.7 and 1.4 times higher cultivable numbers compared to the control soils on days 18 and 60, respectively.
P. fluorescens CHA0 rif+ on King’s B agar, containing rifampicin in order to select for resistant species, were only found in soils that have previously been inoculated with the rifampicin-resistant strain of P. fluorescens CHA0 rif+ (Table 5). The average cultivable numbers decreased by 48% as an average over all soils from days 18 to 60, and at an average over all treatments, they were 50% lower than the total number of fluorescent pseudomonads.
Discussion
The aim of the experiment presented in this paper was to analyze to what extent an introduced microbial strain may change soil microbial properties. We tested the effect of a rifampicine-resistant strain of P. fluorescens CHA0 in soils with different levels of microbial biomass and activity that developed on soils of similar parent material (loess). The differences of soil microbiological parameters at the start of the experiment were primarily due to the long-term management history of these soils. We were interested to see if (1) a mass inoculation of the soils would change microbial biomass and activity in the short and longer term, including an evaluation of method sensitivity, and if (2) this effect was depending on the initial level of microbial biomass and activity of field-derived soils.
Establishment and Survival of P. fluorescens Strain CHA0 rif+ in Soils with Low and High Microbial Biomass
The inoculation of our experimental soils resulted in a slight increase in many biological parameters of the soil bulk soil, predominantly in the short term, while most of the effects of the inoculum disappeared after 60 days. P. fluorescens CHA0 rif+ was only reisolated from soils that were previously inoculated. This result shows that the strain has not been present in our original experimental soils in considerable numbers, and that the inoculation was successful and bacteria remained present over the time and under the conditions of the experiment.
We inoculated 2 × 107 actively growing cells of P. fluorescens CHA0 rif+ per gram of soil and reisolated numbers of colony-forming units that were smaller by two orders of magnitude. Consequently, around 99% of the inoculated cells were lost. This may have several reasons: (1) The strain is not competitive in soils and is suppressed by the existing microflora. This is a natural process that occurs after mass release of organisms and has been found in several studies in the field and under controlled conditions [4, 27, 36]. Moreover, the used strain was isolated from tobacco rhizosphere and may not be adapted to the conditions on the wheat root [40]. Nevertheless, Pseudomonas ssp. are competitive colonizers of wheat roots as we were able to show also for our wheat root samples. Aguirre et al. [1] found that a P. fluorescens strain, genetically modified for bioremediation purpose, changed the community of rhizosphere organisms but not the one in the bulk soil. (2) The reisolation procedure was not quantitative including change of viability and cultivability. The ongoing “plate debate” on cultivability of soil organisms concludes on only a small percentage of soil organisms being cultivable and questions its being representative for microbial ecology [30, 35]. Additionally the used strain of P. fluorescens CHA0 has been found to loose its ability to grow on artificial media and agar plates once inoculated to soils, but it still remained viable in soil [24, 25]. (3) The strain has lost its antibiotic resistance, which is often found when the resistance is located on plasmids. The P. fluorescens strain we used, however, carried a constitutive resistance toward rifampicine and appeared to be relatively stable with respect to this resistance [28, 29]. In conclusion, the main explanation for decreasing cell counts is the lack of competition and cultivability after soil inoculation.
Sensitivity of Soil Biological Measures to Detect Effects of the Bacterial Inoculum
Most of the parameters tested showed a temporal change, which was most likely due to the growth of spring wheat in the experimental soils. Plant growth usually stimulates the activity of microorganisms in soils due to root exudates and plant residues that are used by the existing organisms as substrates for growth and maintenance. So the effect of sampling time on soil biological parameters may be interpreted at least partly as being driven by plant growth. No significant differences in plant growth were obtained between the inoculated and the control pots.
The design of our experiment allowed for the separation of soil, sampling time, and inoculum effects as well as their interactions. Significant inoculum effects were detectable with the following methods: (1) reisolation of rifampicine resistant P. fluorescens CHA0 from soil, (2) dehydrogenase activity (DHA), (3) microbial biomass carbon (C mic), and (4) the of C mic-to-N mic ratio. The magnitude of the effect as indicated by the F value of the three-way ANOVA decreased from the first to the last method mentioned before (see Tables 4, 5, and 6). While inoculum effects were highly significant for methods 1 and 2, the significant interaction with sampling time showed that the effect on DHA disappeared until day 60. The effects of the inoculum on C mic (3) and the C mic-to-N mic ratio (4) were found to be significant over the whole time of the study, as no interaction with sampling time was found. None of the other methods was able to detect changes caused by the P. fluorescens inoculum.
Effects of Inoculated P. fluorescens CHA0 rif+ on Soil Biology in Soils with Different Initial Level of Microbial Biomass and Activity
The soils chosen for the experiment was derived from the well-documented DOK-experiment with a 7-year ley rotation [6, 20, 21] and from an intensely cropped and heavily degraded monoculture site that also developed on the same type of alluvial loess. The soils were similar in their parent material, but showed differences in soil organic matter and pH that were due to the previous long-term soil management.
The different microbial biomass and activity of our experimental soils did not change the numbers of reisolated P. fluorescens CHA0 rif+. Only the total CFU of P. fluorescens was enhanced by the inoculum, but remained higher over 60 days only in soil B with the lowest microbial biomass of our set of soils, which indicates an approval of our second hypothesis that a soil poor in microorganisms is reacting more to fertility inputs than a “rich” soil.
Differences between the three soils used in the study were significantly indicated by all the applied methods except for the reisolated numbers of P. fluorescens CHA0 rif+ from soils. The strongest differences between soils were detectable by the methods for N mic, DHA, C mic, qCO2, in decreasing order as indicated by the F values of the three-way ANOVA. Interactions of soil and inoculum were significant for N mic, crop biomass, and C mic. N mic and C mic only showed an inoculum effect for soil B, while the other soils remained unaffected. This is supporting our hypothesis that poor soils as soil B are reacting stronger to microbial inocula than soils rich in microorganisms as soils D and K. Without inoculum, crop biomass in soil D was higher than in the other soils, whereas it was identical in all inoculated soils. This may indicate an integrative increase in soil fertility of soils K and B as initiated by the bacterial inoculum.
Changes in microbial biomass and basal respiration rates due to the inoculum were going in the same direction. The effect points toward typical soil values that are not influenced by a bacterial inoculum even if abundantly present under natural soil conditions. The absence of an inoculum effect supports the underlying concept that the qCO2 is indicating the living conditions for soil microorganisms, which are mainly driven by the physicochemical soil conditions.
Subtle effects of soil inoculation with microorganisms have been found in several studies [18, 39] and focus mainly on survival of a labeled strain that has been introduced or on effects on microbial community structure [36, 37, 42–44]. We tested changes of microbial communities by the use of a substrate utilization assay, which primarily yielded significant effects of the different sampling dates. The experimental soils explained a small part of the variation found in the community profiles, but the inoculation with P. fluorescens CHA0 was obviously not driving the substrate utilization patterns. Matos et al. [26], investigating the survival of P. aeruginosa in wheat rhizospheres with different levels of diversity, found an inverse relationship between diversity and invasibility, which is partly supported by our data. Our data merely point in the direction that a soil-derived bacterial strain may enhance the level of microbial biomass in poor soils, while rich soils remain almost unaffected. Further studies are needed to reproduce these findings, to analyze microbial community diversity and define the specific mechanisms behind.
Conclusions
A new bacterial strain introduced into natural soils with different levels of biological activity has been shown to remain viable over a time frame of 60 days in the presence of growing wheat plants. Some inoculated soils showed elevated soil microbial biomass in the short term, but only in the soil with the lowest microbial biomass did this effect remained present over the longer term. Bacterial community level substrate utilization and qCO2 were clearly showing effects of time and soils, whereas the effect of the inoculum was negligible. This indicates that the bacterial community and its maintenance requirement are not changed by the inoculum. This may also be explained by the fact that the inoculated Pseudomonas strain is derived from soils and cannot be regarded as an invading species with new properties. We conclude that soils poor in microbial biomass and activity are slightly more susceptible to inoculants than soils that are inhabited by a rich and diverse microflora. The methods we used for evaluating the inoculum effects were suitable and able to indicate subtle changes in soil biological activities.
References
Aguirre de Carcer D, Martin M, Mackova M, Macek T, Karlson U, Rivilla R (2007) The introduction of genetically modified microorganisms designed for rhizoremediation induces changes on native bacteria in the rhizosphere but not in the surrounding soil. ISME J 1:215–223
Alexander M (1982) Most probable number method for microbial populations. In: Segoe S (ed) Methods of soil analysis part 2, chemical and microbiological properties. ASA-SSSA, Madison, US, pp 815–820
Anderson TH, Domsch KH (1993) The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol Biochem 25:393–395
Compeau G, Al-Achi BJ, Platsouka E, Levy SB (1988) Survival of rifampin-resistant mutants of Pseudomonas fluorescens and Pseudomonas putida in soil systems. Appl Environ Microbiol 54:2432–2438
Fal R, Faw R, Rac R (1996) Referenzmethoden der Eidg. landwirtschaftlichen Forschungsanstalten - 1. Bodenuntersuchung zur Düngeberatung, Zürich-Reckenholz
Fließbach A, Oberholzer H-R, Gunst L, Mäder P (2007) Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agric Ecosys Environ 118:273–284
Fuchs J-G, Moënne-Loccoz Y, Défago G (2000) The laboratory medium used to grow biocontrol Pseudomonas sp. Pf153 influences its subsequent ability to protect cucumber from black root rot. Soil Biol Biochem 32:421–224
Garland JL (1996) Analytical approaches to the characterization of samples of microbial communities using patterns of potential C source utilization. Soil Biol Biochem 28:213–221
Garland JL, Mills AL (1991) Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl Environ Microbiol 57:2351–2359
Garthright WE, Blodgett RJ (2003) FDA’s preferred MPN methods for standard, large or unusual tests, with a spreadsheet. Food Microbiol 20:439–445
Haas D, Defago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nature Rev Microbiol 3:307–319
Joergensen RG, Mueller T (1996a) The fumigation extraction method to estimate soil microbial biomass: calibration of the kEC-factor. Soil Biol Biochem 28:25–31
Joergensen RG, Mueller T (1996b) The fumigation extraction method to estimate soil microbial biomass: calibration of the kEN-factor. Soil Biol Biochem 28:33–37
Keel C, Schnider U, Maurhofer M, Voisard C, Laville J, Burger U, Wirthner P, Haas D, Defago G (1992) Suppression of root diseases by Pseudomonas fluorescens CHA0: importance of the bacterial secondary metabolite 2,4-diacetylphloroglucinol. Mol Plant-Microb Interact 5:4–13
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med 44:301–307
Kloepper JW, Leong J, Teintze M, Schroth MN (1980) Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286:885–886
Kloepper JW, Lifshitz R, Zablotowicz RM (1989) Free-living bacterial inocula for enhancing crop productivity. Trends Biotechnol 7:39–44
Kozdroj J (1995) Effect of genetically modified Pseudomonas fluorescens introduced into soil contaminated with copper(II) on microbial community diversity in the soil and rhizosphere. World J Microbiol Biotechnol 11:546–548
Lindow SE, Brandl MT (2003) Microbiology of the phyllosphere. Appl Environ Microbiol 69:1875–1883
Mäder P, Fließbach A, Dubois D, Gunst L, Fried P, Niggli U (2002) Soil fertility and biodiversity in organic farming. Science 296:1694–1697
Mäder P, Fließbach A, Dubois D, Gunst L, Jossi W, Widmer F, Oberson A, Frossard E, Oehl F, Wiemken A, Gattinger A, Niggli U (2006) The DOK experiment (Switzerland). In: Raupp J, Pekrun C, Oltmanns M, Köpke U (eds) Long-term field experiments in organic farming. Koester, Bonn, pp 41–58
Marilley L, Vogt G, Blanc M, Aragno M (1998) Bacterial diversity in the bulk soil and rhizosphere fractions of Lolium perenne and Trifolium repens as revealed by PCR restriction analysis. Plant and Soil 198:219–224
Marx J (2004) The roots of plant–microbe collaborations. Science 304:234–236
Mascher F, Hase C, Moenne-Loccoz Y, Defago G (2000) The viable-but-nonculturable state induced by abiotic stress in the biocontrol agent Pseudomonas fluorescens CHA0 does not promote strain persistence in soil. Appl Environ Microbiol 66:1662–1667
Mascher F, Moenne-Loccoz Y, Schnider-Keel U, Keel C, Haas D, Defago G (2002) Inactivation of the regulatory gene algU or gacA can affect the ability of biocontrol Pseudomonas fluorescens CHA0 to persist as culturable cells in nonsterile soil. Appl Environ Microbiol 68:2085–2088
Matos A, Kerkhof L, Garland JL (2005) Effects of microbial community diversity on the survival of Pseudomonas aeruginosa in the wheat rhizosphere. Microb Ecol 49:257–264
Natsch A, Keel C, Hebecker N, Laasik E, Defago G (1997) Influence of biocontrol strain Pseudomonas fluorescens CHA0 and its antibiotic overproducing derivative on the diversity of resident root colonizing pseudomonads. FEMS Microbiol Ecol 23:341–352
Natsch A, Keel C, Pfirter HA, Haas D, Defago G (1994) Contribution of the global regulator gene gacA to persistence and dissemination of Pseudomonas fluorescens biocontrol strain CHA0 introduced into soil microcosms. Appl Environ Microbiol 60:2553–2560
Natsch A, Keel C, Troxler J, Zala M, Von Albertini N, Defago G (1996) Importance of preferential flow and soil management in vertical transport of a biocontrol strain of Pseudomonas fluorescens in structured field soil. Appl Environ Microbiol 62:33–40
Nichols D (2007) Cultivation gives context to the microbial ecologist. FEMS Microbiol Ecol 60:351–357
Oehl F, Sieverding E, Ineichen K, Mäder P, Boller T, Wiemken A (2003) Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agro-ecosystems of central Europe. Appl Environ Microbiol 69:2816–2824
Paul EA, Clark FE (1996) Soil microbiology and biochemistry. Academic, San Diego, p 340, Cal
Phillips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc 55:158–160
Raaijmakers JM, Weller DM, Thomashow LS (1997) Frequency of antibiotic-producing Pseudomonas ssp. in natural environments. Appl Environ Microbiol 63:881–887
Ritz K (2007) The plate debate: cultivable communities have no utility in contemporary environmental microbial ecology. FEMS Microbiol Ecol 60:358–362
Schwieger F, Tebbe CC (2000) Effect of field inoculation with Sinorhizobium meliloti L33 on the composition of bacterial communities in rhizospheres of a target plant (Medicago sativa) and a non-target plant (Chenopodium album) linking of 16S rRNA gene-based single-strand conformation polymorphism community profiles to the diversity of cultivated bacteria. Appl Environ Microbiol 66:3556–3565
Schwieger F, Willke B, Munch JC, Tebbe CC (1997) Ecological pre-release risk assessment of two genetically engineered, bioluminescent Rhizobium meliloti strains in soil column model systems. Biol Fertil Soils 25:340–348
Speiser B, Tamm L, Maurer V, Berner A, Walkenhorst M, Böhler K, Früh B, Chevillat V (2007) Hilfsstoffliste 2007. Zugelassene und empfohlene Hilfsstoffe für den biologischen Landbau. FiBL, Frick, p 80
Steddom K, Menge JA, Crowley D, Borneman J (2002) Effect of repetitive applications of the biocontrol bacterium Pseudomonas putida 06909-rif/nal on citrus soil microbial communities. Phytopathology 92:857–862
Stutz E, Defago G, Kern H (1986) Naturally occurring fluorescent pseudomonads involved in suppression of black root rot of tobacco. Phytopathology 76:181–185
Tabatabai MA (1982) Soil Enzymes. In: Page AL, Miller RH, Keeney DR (eds) Methods of Soil Analysis, Part 2 Chemical and Microbiological Properties. American Society of Agronomy & Soil Science Society of America, Madison, Wisconsin, pp 903–947
Tebbe CC (2003) Dissemination of genetically engineered microorganisms in terrestrial ecosystems: case studies for identifying risk potentials. In: Ecological impact of GMO dissemination in agro-ecosystems, Grossrussbach, Austria, September 27–28, 2002, 2003. OECD (ed.), pp 31–44
Vahjen W, Munch J-C, Tebbe CC (1997) Fate of three genetically engineered, biotechnologically important microorganism species in soil: impact of soil properties and intraspecies competition with non-engineered strains. Can J Microbiol 43:827–834
Vahjen W, Munch JC, Tebbe CC (1995) Carbon source utilization of soil extracted microorganisms as a tool to detect the effects of soil supplemented with genetically engineered and non-engineered Corynebacterium glutamicum and a recombinant peptide at the community level. FEMS Microbiol Ecol 18:317–328
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Vogel (1922) Impfung von Hülsenfrüchten und Nichtleguminosen. Z Pflanzenern Düng 1:531–535
Weller DM, Raaijmakers JM, McSpadden Gardener BB, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol 40:309–348
Widmer F, Rasche F, Hartmann M, Fließbach A (2006) Community structures and substrate utilization of bacteria in soils from organic and conventional farming systems of the DOK long-term field experiment. Appl Soil Ecol 33:294–307
Acknowledgements
We thank Prof. Geneviève Défago for fruitful discussions in the planning of this project and for providing the Pseudomonas fluorescens strain CHA0 rif+. Skilful technical assistance of Bruno Nietlispach and Anja Vieweger as well as proof reading of an earlier version of this manuscript by Peter Weisskopf is gratefully acknowledged. This project “Bewertungskonzept und Methodenempfehlungen zur Abschätzung von Risiken von GVO und anderen biologischen Belastungen auf das Ökosystem Boden” was part of the research program “Biosafety in non-human genetic engineering,” which was funded by the Swiss Federal Office of the Environment. The European community provided additional funds in the frame of the integrated project “QualityLowInputFood”: Improving quality and safety and reduction of cost in the European organic and low-input supply chain (FP6-FOOD-CT-2003-506358).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fließbach, A., Winkler, M., Lutz, M.P. et al. Soil Amendment with Pseudomonas fluorescens CHA0: Lasting Effects on Soil Biological Properties in Soils Low in Microbial Biomass and Activity. Microb Ecol 57, 611–623 (2009). https://doi.org/10.1007/s00248-009-9489-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-009-9489-9