Abstract
The polymerase chain reaction coupled with denaturing gradient gel electrophoresis (PCR-DGGE) has been used widely to determine species richness and structure of microbial communities in a variety of environments. Researchers commonly archive soil samples after routine chemical or microbial analyses, and applying PCR-DGGE technology to these historical samples offers evaluation of long-term patterns in bacterial species richness and community structure that was not available with previous technology. However, use of PCR-DGGE to analyze microbial communities of archived soils has been largely unexplored. To evaluate the stability of DGGE patterns in archived soils in comparison with fresh soils, fresh and archived soils from five sites along an elevational gradient in the Chihuahuan Desert were compared using PCR-DGGE of 16S rDNA. DNA from all archived samples was extracted reliably, but DNA in archived soils collected from a closed-canopy oak forest site could not be amplified. DNA extraction yields were lower for most archived soils, but minimal changes in bacterial species richness and structure due to archiving were noted in bacterial community profiles from four sites. Use of archived soils to determine long-term changes in bacterial community structure via PCR-DGGE appears to be a viable option for addressing microbial community dynamics for particular ecosystems or landscapes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Archiving of soil samples is an important component of long-term ecological research and can provide a unique opportunity to address questions with new techniques, refine measurements of soil processes, or obtain data from a landscape or continental scale. In fact, the current understanding of the rate of soil organic matter turnover was developed using archived soil samples [14]. Archiving recommendations [2] indicate that soils be air-dried and stored at room temperature, after which samples can be held for an indeterminate length of time, depending upon the intended use.
Many soil samples were archived before the advent of polymerase chain reaction (PCR) and denaturing gel gradient electrophoresis (DGGE), but long-term comparisons of bacterial communities housed in some soil archives have been performed [4]. DNA is known to be stabilized in some soils [3, 6, 13], and a recent survey of the eukaryotic diversity of air-dried soil samples collected from soil in The Netherlands from 1942 to 1975 [7] confirmed that it was possible to use this approach for a variety of organisms. Stability of DNA in various soils must be evaluated if archived soils are to be used for historical studies of microbial species richness and community structure. However, no studies have attempted to evaluate changes in community structure incurred during the archiving process by using DGGE methodologies.
To evaluate the impacts of drying and storage on bacterial DNA in air-dried soil samples, PCR-DGGE profiles of 16S rDNA that was extracted immediately after sampling were compared with extractions of the same soils after being archived for 30 months.
Materials and Methods
Study Site
Soils were sampled from five sites along the Pine Canyon Watershed in the Big Bend National Park region of the Chihuahuan Desert [19]. Each site represents unique elevation and vegetation components [11]. The lowest elevational site is a lowland desert scrub (GS) which lies at 793 m. The second site is a creosote–bush bajada (CR) at an elevation of 1,010 m. The midelevational site is a sotol grassland (SG) at an elevation of 1,526 m. The fourth site is a closed-canopy oak forest (OF) at an elevation of 1,824 m. The highest elevational site is an oak–pine forest (LM) at an elevation of 2,098 m.
Soils along the Pine Canyon Watershed have an extremely rocky A horizon with little profile development and no litter layer, except in the forested sites or under shrubs. Soils at each location are described as a Lajitas rock outcrop complex by United States Department of Agriculture soil surveys [15]. Soils in the forested locations generally have either a mollic–argillic–rock or a mollic–rock horizon sequence, whereas vegetation zones at lower and drier elevations have an ochric–rock horizon sequence.
Sample Collection and Archiving
Composite soil samples (∼300 g) were obtained from a depth of 15 cm along two belt transects (100 m) during January 2004. Each sample was passed through 2.0-mm sieves to remove large debris, and samples were stored in freezer bags at 4°C until they could be processed (3 days). Soils were air-dried for 2 weeks by opening freezer bags and exposing samples to ambient temperature (22°C) and humidity (25%) on a laboratory bench. Subsequently, sample bags were resealed and archived at room temperature (22°C) in sealed boxes as recommended [2]. The same soil samples from which DNA was extracted for PCR-DGGE analysis in January 2004 were retrieved from the archives during June 2006, and DNA was re-extracted using the same procedure as used on fresh samples (described below).
Soil Nutrient and Edaphic Measurements
Microbial biomass carbon (MBC) was determined by the chloroform-fumigation (48 h) technique [18] with the amount of extracted carbon evaluated spectrophotometrically at 280 nm [9]. Percent soil moisture (%M) was determined by oven-drying soils at 60°C for 24–48 h. The percentage of soil organic matter (SOM) was determined by combusting 10 g of soil (dry weight) at 450°C to determine mass loss on ignition [12, 17]. Soil pH was measured with an Orion pH probe from a 2:1 paste of 5 g soil added to 10 mL of glass-distilled water (ATI Orion, Boston, MA, USA) at room temperature [10]. Extractable ammonium–nitrogen (NH4 +-N) was eluted from soils with 2.0 M KCl [17] and converted to a concentration (in milligrams per kilogram) using spectrophotometric measurements of samples and a standard curve. Extractable nitrate–nitrogen (NO3 −–N) levels were determined with ion-specific probes by A&L Plains Laboratories (Lubbock, TX, USA). Bulk densities at each location were calculated using the sand-replacement technique [1]. Soil temperatures were collected at 15 cm depths using Onset temperature probes (Bourne, MA, USA) at 36-min intervals. Soil nutrient and edaphic measurements for all sites are presented in Table 1.
Molecular Analyses
From each sample, DNA was extracted from a 0.5-g portion of sieved soils using MoBio UltraClean™ soil DNA kits. Quality of extracted DNA was checked using 0.7% (w/v) agarose gels. Extractions were quantified using absorption measurements (260 nm) on a NanoDrop ND-1000™ spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). Extractions were diluted with sterile, glass-distilled water for amplifications. Freshly extracted soil DNA was stored at −20°C permanently.
Amplification via PCR was done with the 341fgc and 534r oligonucleotides targeting 16S rDNA [8]. Each amplification contained 25 ng of DNA, 20 pmol of each oligonucleotide, 400 μM each deoxynucleotide triphosphates (dNTP), 1× polymerase buffer, and 1.5 U Taq polymerase in a final volume of 25 μL. TaKaRa Ex Taq™ DNA polymerase, polymerase buffer, and dNTP mixtures were used for all amplifications (TaKaRa Mirius Bio, Madison, WI, USA). Amplification profiles consisted of one cycle of 98°C for 1 min. This long denaturation was followed by 35 amplification cycles of 98°C for 15 s, 51°C for 45 s, and 72°C for 1 min. Amplification cycles were followed by a single extension of 72°C for 10 min. Amplifications were performed in a 96-well gradient block RoboCycler™ with a hot top (Stratagene, La Jolla, CA, USA). Products were checked on 0.7% (w/v) agarose gels prior to DGGE. Each DNA extraction was amplified twice for DGGE analyses to provide a reference for inherent variability among amplifications from a single DNA extraction.
DGGE was performed using a DCode system and 16 × 16 cm plates (Bio-Rad, Hercules, CA, USA). Gels were composed of a 30–65% urea and formamide denaturing gradient and an 8–10% acrylamide size exclusion gradient. Nondenaturing, stacking gels (8% acrylamide) were added above the gradient. All electrophoresis was done at 60°C for 1,000 V·h. After electrophoresis, gels were stained in a solution of 1× TAE and 0.5 mg/L of ethidium bromide for 20 min. Gels were visualized using a Kodak Gel Logic 440 and its bundled software (Kodak Molecular Imaging Systems, New Haven, CT, USA). Urea, acrylamide, tetramethylethylenediamine, ammonium persulfate, and 50× TAE buffer were purchased from Bio-Rad. Formamide was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Statistical Analyses
Images were cropped and enhanced using the color-leveling feature of Adobe Illustrator CS2 (Adobe Systems, San Jose, CA, USA). Replicate amplifications of each DNA extraction for both the freshly extracted and archival samples were run in contiguous lanes to avoid visualization/staining artifacts.
Bands were manually counted on screen by two individuals for each DGGE lane. Sitewise counts were compared using paired t-tests (Systat 9, Chicago, IL, USA). Amplifications from both soil samples were used as replicates for both freshly extracted and archived soils (n = 4 for each soil type). Statistical analyses linking microbial community composition to abiotic factors do not appear in this study. Such analyses are not possible with small sample sizes and were beyond the scope of the concepts presented in this article.
Results and Discussion
DNA extractions were successful for all archived soils, but yields were lower for archival than for freshly sampled soils (Table 2). However, amplifications of extracted DNA were successful for only four of the five sites sampled. Triplicate attempts at amplification failed from each of four separate extractions from archival, closed-canopy, oak forest soils (12 total amplifications).
DGGE lanes (Fig. 1) showed similar species richness profiles in amplifications of fresh and archived soil extractions from the other four sites along the watershed, but closer inspection revealed subtle differences in community profiles. Replicate amplifications of the same DNA extraction were consistent, but replicate soils did show some variation. The majority of prominent and low-intensity bands for freshly extracted soils were represented in amplifications of archived soils. However, intensity of bands commonly varied between fresh and archived soil samples for all samples of all sites. In general, both fresh and archived extractions of the low-elevation desert scrub site (GS) gave similar quality DNA banding profiles. However, soils of CR, SG, and LM showed a slight decrease in both intensity and clarity of DNA banding profiles in archived soils.
PCR-DGGE analyses of bacterial communities in fresh and archived soils of the Pine Canyon Watershed in the Big Bend National Park region of the Chihuahuan Desert. Samples shown were collected from a lowland desert scrub (GS), a creosote bajada (CR), a sotol grassland (SG), and an oak–pine forest (LM). Portions of gel images containing no bands were cropped. Each gel pane consists of two amplifications of each DNA extraction to provide a reference for variability among amplifications of the same soil extraction. Each gel pane shows replicate amplifications of a DNA extraction from the same soil sample when the soil was fresh and after the soil was archived. Two independent soil samples were used from each site. Arrows to the left of gel panes indicate bands that are present in fresh but not archived soils. Arrows to the right of gel panes indicate bands that are present in archived but not fresh soils
Some differences were observed between extractions of fresh and archived soils for several sites. Most differences in profiles were bands that were observed in freshly extracted samples but not in archived soil extractions. Such bands are indicated with arrows to the left of the gel panes in Fig. 1. In contrast, two cases were noted of bands appearing only in amplifications of archived soil extractions in CR and LM samples. Such bands are denoted with arrows to the right of the gel panes in Fig. 1. Because numbers of differences observed in soil samples were used to evaluate the utility of archived soils for molecular studies, differential bands were not excised and sequenced for identification.
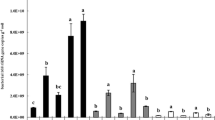
Band counts for each lane of the DGGE showed some decrease in numbers for archived soils (Fig. 2). However, differences in band counts between fresh and archived soils were significant (t = 4.284; p = 0.023) for only the lowland desert scrub site (GS). Replicate amplifications of bacterial DNA in both fresh and archived soils from GS exhibited low intrasample variation, which likely accounted for the significant difference in band counts. While not statistically significant, greater variability was observed for replicate soils sampled from the creosote bajada (CR; t = 1.954; p = 0.146), sotol grasslands (SG; t = 2.456; p = 0.091), and oak–pine forest (LM; t = 1.963; p = 0.144) sites.
Band counts from PCR-DGGE analyses of soil bacterial community structure in fresh (black) and archived (gray) soils. Samples shown were collected from a lowland desert scrub (GS), a creosote bajada (CR), a sotol grassland (SG), and an oak–pine forest (LM). Replicate amplifications of DNA extractions of fresh and archived soils were compared in an independent, paired t-test for each site. Significant differences (p < 0.05) in band counts between fresh and archived soils were found for only GS samples (indicated with a and b over the bars). Significant differences were not detected for soils of other sites (NS). Site codes correspond to those in Fig. 1
Studies of microbial detection in archived soils have been performed on soil samples much older than those of this study. Soil samples collected from The Netherlands and archived for 31–64 years were used for a PCR-DGGE study of eukaryotes [7]. A PCR-DGGE study of a Bacillus group was also performed on diverse fresh soil samples and soil samples that had been archived for 11–62 years [16]. These studies demonstrated that bacterial DNA was stable in soils that had been archived for many years, but neither study could compare molecular community profiles of freshly collected soil samples to the same samples after they were archived. Fresh and archived community profiles from four locations along the watershed were relatively stable over the 30-month timeframe for this study, but data presented in this article cannot conclusively demonstrate that longer archival periods would not cause changes to accrue.
Visualization of bands could have been impaired due to subtle differences in staining or image capture. It is also possible that bands were lost because particular DNA templates were subject to degradation over the 30-month period of archiving. Although decreases in band counts and amplification quality were observed with archived soils, most archived samples yielded bacterial community profiles that were similar to those observed from amplifications of DNA extracted immediately after sample collection. Contamination associated with the archiving process was minimal, and bands present in archived soils but not in fresh soils could represent visualization artifacts or bacteria that were introduced during the air-drying and routine handling throughout the archiving process.
DNA of bacterial communities in soils with low levels of soil organic matter (lowland desert scrub and creosote bajada sites) appeared to be more stable when archived. However, archived bacterial communities associated with soils with high levels of soil organic matter (sotol grassland and oak–pine forest sites) gave profiles comparable to those of freshly extracted soils. DNA extractions of archived soils from the closed-canopy oak forest could not be amplified. Reasons for failures could not be discerned, as all extractions were of good yield and quality. Freshly extracted OF soils could be amplified within two attempts (not shown). Amplification of DNA extractions from OF soils are always more difficult than those of other sites sampled along this watershed. Amplification difficulties in these soils could be attributed to the higher litter and humic acid content of OF soils, relative to soils from other sites along the watershed, and future studies of these soils will likely require alternative methods for removal of humics [5].
Although minimal loss of bacterial diversity was observed for the 30-month period, extended storage periods could have resulted in more extensive damage. Soils from more mesic locations or which contain high levels of SOM may result in either difficulty with DNA amplification or greater loss of bacterial DNA. Soils that are to be archived should be air-dried soon after initial molecular evaluation to possibly reduce subsequent problems with DNA degradation.
Countless laboratories and research institutes have archived soil samples that were used for routine soil chemistry measurements. It is likely that microbiological studies of archived soils have been neglected because culture methods are no longer an option for most microbes that were archived within the soils. Based upon results presented in this study and others [4, 7, 16], community structure preserved in archived soils collected from arid regions with low levels of soil organic matter can be analyzed using modern molecular techniques with confidence. Use of molecular techniques for community structure analysis in soils with higher moisture content and dense vegetation were more variable and warrant further studies.
References
Blake GR, Hartage KH (1986) Bulk density. In: Klute A (ed) Methods of soil analysis: part 1: physical and mineralogical methods monograph no. 9. American Society of Agronomy and Soil Science Society of America, Madison, WI, pp 363–375
Boone RD, Grigal DF, Sollins P, Ahrens RJ, Armstrong DE (1999) Soil sampling, preparation, archiving, and quality control. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, NY, pp 3–28
Cai P, Huang Q-Y, Zhang X-W (2006) Interactions of DNA with clay minerals and soil colloidal particles and protection against degradation by DNase. Environ Sci Technol 40:2971–2976
Dolfing J, Vos A, Bloem J, Ehlert PA, Naumova NB, Kuikman PJ (2004) Microbial diversity in archived soils. Science 306:813
Dong D, Yan A, Liu H, Zhang X, Xu Y (2006) Removal of humic substances from soil DNA using aluminium sulfate. J Microbiol Methods 66:217–222
Franchi M, Braanti E, Bonzi LM, Orioli PL, Vettori C, Gallori E (1997) Clay-nucleic acid complexes: characteristics and implications for the preservation of genetic material in primeval habitats. Orig Life Evol Biosph 29:297–315
Moon-van der Staay S, Tzeneva VA, van der Staay GWM, de Vos WM, Smidt H, Hackstein JHP (2006) Eukaryotic diversity in historical soil samples. FEMS Microbiol Ecol 57:420–428
Muyzer G, de Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700
Nunan N, Morgan MA, Herlihy M (1998) Ultraviolet absorbance (280 nm) of compounds released from soil during chloroform fumigation as an estimate of the microbial biomass. Soil Biol Biochem 30:1599–1603
Robertson GP, Sollins P, Boyd GE, Lajitha K (1999) Exchangeable ions, pH, and cation exchange capacity. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, NY, pp 106–114
Sobek EA, Zak JC (2003) The soil FungiLog procedure: method and analytical approaches toward understanding fungal functional diversity. Mycologia 95:590–602
Sollins P, Glassman C, Paul EA, Swanston C, Lajitha K, Heil JW, Elliot ET (1999) Soil carbon and nitrogen: pools and fractions. In: Robertson GP, Coleman DC, Bledsoe CS, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, NY, pp 89–105
Trevors JT (1996) DNA in soil: adsorption, genetic transformation, molecular evolution and genetic microchip. Antonie Van Leeuwenhoek 70:1–10
Trumbore SE (1993) Comparisons of carbon dynamics in tropical and temperate soils using radiocarbon measurements. Glob Biogeochem Cycles 7:275–290
Turner AJ (1977) Soil survey of Jeff Davis County, Texas. USDA, Texas, pp 3–12
Tzeneva VA, Li Y, Felske ADM, de Vos WM, Akkermans ADL, Vaughan EE, Smidt H (2004) Development and application of a selective PCR-denaturing gradient gel electrophoresis approach to detect a recently cultivated Bacillus group predominant in soil. Appl Environ Microbiol 70:5801–5809
Van Miegroet H (1995) Inorganic nitrogen determined by laboratory and field extractions of two forest soils. Soil Sci Soc Am J 59:549–553
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Zak JC, Ziehr LL, Urbanczyk K, McHam JB, Yarborough K (1997) Big Bend National Park Watershed Program: monitoring microbial activity and diversity along an elevational gradient. Presented at the 9th Conference on Research and Management in Parks and Public Lands
Acknowledgments
This work was funded by United States Geological Survey-BRD Small Watershed Program and National Park Service grants to JCZ. Access to sites was made possible by Joe Sirotnak (Big Bend National Park). Many helpful discussions were provided by Dora Ogles (Microbial Insights), Edward Sobek (Clean Air Labs), and Jennifer Humphries (Texas Tech University).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Campbell, J.H., Clark, J.S. & Zak, J.C. PCR-DGGE Comparison of Bacterial Community Structure in Fresh and Archived Soils Sampled along a Chihuahuan Desert Elevational Gradient. Microb Ecol 57, 261–266 (2009). https://doi.org/10.1007/s00248-008-9479-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-008-9479-3