Abstract
Cool-season grasses often harbor obligate fungal symbionts from the genus Neotyphodium, and these symbiota can function as a single ecological unit. Previous studies have shown that gene flow in Neotyphodium in Festuca arizonica is low enough such that populations could diverge and form local adaptations. A reciprocal transplant experiment was performed between two F. arizonica/Neotyphodium populations in Arizona, Clint’s Well and Flagstaff, using symbiota with the most common Neotyphodium genotypes in each population, to test for local adaptations. The genetic difference between populations is potentially large as Neotyphodium from Clint’s Well are of hybrid origin. Local environmental variation was the most important source of variation for F. arizonica/Neotyphodium symbiota growth, with individuals at Flagstaff growing larger and individuals at Clint’s Well not reproducing. Local environment and the source population of the symbiota interacted to affect vegetative growth. Symbiota from Clint’s Well, which harbor hybrid Neotyphodium, had higher volume/wet mass and volume/dry mass ratios but only in the marginal Clint’s Well habitat. The local environment also affected F. arizonica/Neotyphodium reproduction because only symbiota transplanted to Flagstaff reproduced. Symbiota from Clint’s Well produced more panicles, whereas symbiota from Flagstaff with nonhybrid Neotyphodium produced greater seed mass per panicle. Overall seed mass production was not different, suggesting that the two strategies are functionally equivalent. We find that F. arizonica/Neotyphodium symbiota vary geographically, but potential local adaptations are only apparent in marginal habitats and may be related to the evolutionary history of the Neotyphodium part of the symbiota.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The observation that interspecific interactions vary depending upon environmental and genetic factors led to the development of the geographical mosaic theory of coevolution [1]. This theory posits that interactions evolve in metapopulations, with the interaction between local selection pressures, migration, and drift creating a range of possible outcomes including stable and dynamic antagonisms and mutualisms and equilibrium points where both may exist [2–6].
Recent empirical studies of the coevolutionary process support the importance of genetic and environmental variation on interspecific interactions. For example, seed production by Amphicarpaea bracteata (Leguminosae) is strongly affected by the genotype of its symbiotic bacteria Bradyrhizobium [7, 8], and the interaction between the fungus Colletotrichum magna and their plant hosts can change from mutualistic to parasitic by a mutation at a single locus [9]. Environmental variation can switch mutualistic interactions into parasitic ones [10, 11], and changes in population demographics [12–14] can also change the outcomes of interspecific interactions.
In this study, we tested variation in a grass/endophyte symbiosis in two environments. Neotyphodium endophytes are obligate, systemic, asexual, and vertically transmitted symbionts of the grass subfamily Pooideae and are closely related to the Epichloë endophytes. Because the interaction is vertically transmitted, the reproductive success for both plant and endophyte are directly linked, and an individual host will carry the same symbiotic Neotyphodium for its entire life. As such, an individual host/Neotyphodium combination (hereafter, we use symbiotum to designate the host/endophyte combination) will function as a single ecological unit. In some host species, Neotyphodium provides its hosts with a suite of herbivore-deterring alkaloids making the interaction highly mutualistic [15]. Herbivore deterrence is particularly well studied in the agronomic grasses tall fescue (Lolium arundinaceum=Festuca arundinacea) and perennial ryegrass (Lolium perenne). Neotyphodium does not produce alkaloids at herbivore-deterring levels in all its hosts, however [16]. Some Neotyphodium have also been shown to benefit their hosts by increasing drought resistance [17–20], herbicide resistance [21], and by increasing phosphorous [22, 23] and nitrogen uptake [24], although it is not known if these benefits are as variable between hosts as alkaloid production. These benefits are also context dependent; both environmental and genetic factors alter the outcome [25]. Neotyphodium’s effects on its hosts can be altered by nutrient availability [26, 27], herbivory [28], the surrounding community [29], and by genetic interactions between the host and endophyte [21, 30–32]. These variable outcomes because of genetic interactions are often seen in native grass–endophyte associations leading to variation in herbivore deterrence [33, 34], geographic variation in the symbiosis [35], nonrandom host–endophyte genotype combinations [36], and local adaptations [37]. These variable outcomes can even produce grass–endophyte combinations with negative outcomes for the grass host [32, 38, 39].
Genetic variation within the genus Neotyphodium is likely to be large because about two thirds of all known species are the result of hybridization between either two Epichloë spp. (a closely related, sexually reproducing grass choke pathogen) or a Neotyphodium sp. and Epichloë sp., presumably via a parasexual process [40, 41]. Hybridization has occurred frequently, which is unusual for fungi [42], and some individuals within host species can harbor either hybrid or nonhybrid endophytes [43–46], sometimes within the same host population [47]. Hybridization is hypothesized to create new asexual vertically transmitted Neotyphodium spp. because of the new hybrid’s inability to form ascospores [48], and these new endophyte species could then increase in frequency by providing a selective advantage either by masking deleterious mutations (avoiding Müller’s ratchet) and/or the acquisition of new genes or gene combinations that increase benefits to their hosts [42, 49, 50]. However, to our knowledge, the hypothesis that hybrid Neotyphodium provides a selective advantage has not yet been tested other than the observation that such hybrids are common.
Festuca arizonica is the predominant range grass in the semiarid grasslands of the southwestern USA [51]. Neotyphodium commonly inhabits F. arizonica with many populations having more than 90% of the individuals infected, although the infection rate is variable (60–100% [52]). Alkaloids are generally produced at low and variable levels [31], such that neither vertebrate [53] nor invertebrate [53–55] herbivores are deterred. Three distinct genotypes of Neotyphodium have been found, and one of them is of hybrid origin [47, 56, 65]. The geographic distribution of these genotypes is highly structured [47].
To test for geographic variation in the F. arizonica/Neotyphodium symbiotum, we reciprocally transplanted symbiota from two populations; one of which has relatively high soil nitrogen and precipitation (designated Flagstaff), whereas the other is relatively nitrogen and moisture poor (Clint’s Well). These sites also differ in the evolutionary history of the Neotyphodium part of the symbiota. The majority of Neotyphodium at Clint’s Well is of hybrid origin, whereas all of the Neotyphodium at Flagstaff are nonhybrid [47]. Multiple species of Neotyphodium have been described in F. arizonica, and the Neotyphodium used in this study likely correspond to the hybrid N. tembladerae [42, 57] and the nonhybrid N. huerfanum [42, 58]. We tested how the most common symbiota types in each population, F. arizonica with nonhybrid Neotyphodium from Flagstaff and F. arizonica with hybrid Neotyphodium from Clint’s Well, vary geographically by reciprocally transplanting these symbiotum types from each population to each respective geographic locale. We then compared relative growth, reproduction, and long-term survival of each type in each geographic location to ascertain whether symbiota are locally adapted.
Materials and Methods
Field Populations
The two populations used were Clint’s Well, located in Arizona’s Coconino National Forest, and a natural area within the Arboretum at Flagstaff, AZ. These sites are approximately 90 km apart and vary in several abiotic characteristics. The Flagstaff soil is comprised primarily of shallow to moderately deep gravely clay loam over volcanic cinders and basalt flows [59] and is relatively nutrient rich containing 0.20 mg/100 g soil nitrogen [52], whereas the Clint’s Well soil has a loamy surface and clay subsoil moderately deep to deep over limestone and sandstone [59] and contains lower nitrogen concentrations (0.09/100 g soil) [52]. The Clint’s Well site is heavily forested with Ponderosa pine (Pinus ponderosa), whereas the Flagstaff site is open grassland with few trees. Precipitation as measured at nearby stations is lower at Clint’s Well (49.23 cm/year, 37 year average) compared to Flagstaff (53.87 cm/year, 54 year average; Western Regional Climate Center, http://www.wrcc.dri.edu/). When examined by month, the station near the Flagstaff site has a higher mean precipitation in 9 of the 12 months and is significantly higher overall (p = 0.03, Wilcoxon signed-rank test for paired samples). Both sites receive most of their moisture twice a year, snow melt in the spring from winter precipitation, and summer rains from late July–August.
Experimental Design
Fifty symbiota from each site were randomly selected without a priori knowledge of their infection status, or if infected, the genotype of the Neotyphodium symbiont, and removed in late May to early June 1997 and taken to the greenhouse at Arizona State University. F. arizonica is a perennial bunch grass that can easily be divided into equally sized ramets, so plants were split into approximately equal sections. Two ramets from each plant were weighed and planted in soil either from their native site or from the foreign site. The plants were returned to Clint’s Well and Flagstaff in July of 1997, replanted approximately 0.5 m apart, and initial height and diameter measurements were taken. With the exception of two water treatments given in the first 14 days after transplantation to aid establishment, plants received only ambient precipitation. Height and diameter were measured in the fall of 1997 and twice a year thereafter, when the plants broke their winter dormancy (late May/early June) and when the plants reproduced (late August/early September), for 3 years. Plant size was estimated from the formula for a cylinder (volume = πr 2 h) as the growth form of bunch grasses approximates a cylinder [60]. For a given time point, both populations were measured within 3 days of each other. Panicles (culms with inflorescences) were counted, and seeds were collected at the end of every growing season starting in 1998 as the plants did not produce seeds in their first post-transplant year. Survivorship of each plant was also assessed at this time. A later assessment of long-term survivorship took place in May 2004. In the fall of 2000, the aboveground tissue was removed, the plants were dried at 70°C for 2 days, and dry mass was measured.
During the summer of 1998, each individual was screened for the presence of Neotyphodium by culturing leaf samples as described in Schulthess and Faeth [52]. Microsatellites were first used to determine Neotyphodium haplotypes (as described in [47]). A hybrid origin was suspected for some of the haplotypes because of the presence of multiple genes for some microsatellite loci, and subsequent analysis of β-tubulin intron sequence (as described in [41]) confirmed these haplotypes as hybrids ([65] Geographic and genetic variation in the Neotyphodium/Festuca arizonica interaction). We grouped these haplotypes into a single hybrid group, as all the haplotypes differed by only a single microsatellite mutation from the most common haplotype [47] and because sequences from the internal transcribed spacer (ITS) region of the ribosomal deoxyribonucleic acid indicate that the hybrid haplotypes are a single clade ([65] Geographic and genetic variation in the Neotyphodium/Festuca arizonica interaction). A single, nonhybrid Neotyphodium genotype was found in Flagstaff symbiota (hereafter referred to as FL/NH) whereas the Clint’s Well population was 81% hybrid and 19% nonhybrid [47]. From the Clint’s Well population, only symbiota with hybrid Neotyphodium (hereafter referred to as CW/H) were used in the analysis. There are three nonhybrid haplotypes found at Clint’s Well [47], and according to both ITS and b-tubulin sequences, the hybrid haplotypes are not closely related ([65] Geographic and genetic variation in the Neotyphodium/Festuca arizonica interaction). Only nine individuals with nonhybrid Neotyphodium from Clint’s Well identical to the nonhybrid Neotyphodium at Flagstaff survived the entire course of the experiment, and this sample size was deemed too small to be informative.
We analyzed alkaloid levels and types for samples from five CW/H and four FL/NH randomly selected symbiotum at the end of the growing season in September 2002. The tissue was freeze dried, and extraction and analysis were done as described in Faeth et al. [31]. Samples were analyzed for levels of peramine, the only alkaloid type heretofore found in F. arizonica/Neotyphodium [31], and for the presence of loline and ergovaline, two other major alkaloid types known from Epichloë- or Neotyphodium-infected grasses [61].
Statistical Analysis
The source population and the Neotyphodium genotype (hereafter referred to as symbiotum type) of the symbiota was treated as a single treatment, acknowledging that variation in this factor could be due to plant genotype, Neotyphodium genotype, their interaction, and/or maternal effects. We used repeated-measures analysis of covariance (ANCOVA) [62] to test for effects of local environment, symbiotum type on plant volume, panicle number, seed mass, and the seed mass/panicle ratio. Final wet mass, dry mass, volume/wet mass, and volume/dry mass were first analyzed using a multivariate analysis of variance (MANOVA) with local environment, symbiotum type, and their interaction as factors and then using analyses of variance (ANOVAs) on each variable individually. The reproductive variables, panicle number, seed mass, and the seed mass/panicle ratio were analyzed using repeated-measures ANCOVA with symbiotum type as the factor. Not all individuals were used in the analysis because some were determined to be Neotyphodium-free, died during the course of the experiment, or could not be unambiguously genotyped. Because Neotyphodium genotyping was done after the transplanting, the final sample sizes were unequal with 22 CW/H and 33 FL/NH at the Flagstaff site and 21 CW/H and 20 FL/NH at the Clint’s Well site. The response variables were non-normally distributed, so the data were Box-Cox transformed. Plant volume was analyzed with repeated-measures ANCOVA using the factors symbiotum type, local environment, and time. Plant volume at the initial transplanting was used as a covariate. Wet mass, dry mass, the volume/wet mass ratio, and the volume/dry mass ratio were analyzed using the same factors in an ANCOVA but not as a repeated measures design, as these variables were only measured once, with the symbiotum mass at the start of the experiment as a covariate. For the tests on reproductive growth and output, only the plants at the Flagstaff site were used because plants at Clint’s Well did not reproduce during the course of the experiment. We used ANCOVA with the same factors as above with the exception of local environment. Initial volume was used as a covariate, except in the analysis of the seed mass per panicle ratio.
Results
Local environment had a strong effect on F. arizonica growth. All symbiota, regardless of source location, grew better at Flagstaff, the site with higher soil nitrogen and precipitation, than plants at Clint’s Well in terms of both volume (p < 0.0001; Table 1) and dry biomass (p < 0.0001; Table 2). The symbiotum type also significantly affected volume (p = 0.008, Table 1) and interacted with the local environment over time (p = 0.001, Table 1, Fig. 1).
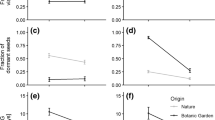
a, b Difference in mean plant volume (±1 standard error) between symbiotum types Clint’s Well/hybrid (CW/H) and Flagstaff/non-hybrid (FL/NH) over time (Fall or Summer with year) at Clint’s Well (a) and Flagstaff (b) shown with a simple linear regression line. The interaction between time, symbiota type, and local environment was significant by repeated-measures ANCOVA (p = 0.001). 167 × 234 mm (600 × 600 DPI)
As expected from the large environmental effects on volume, the local environment was a significant source of variation for variables related to mass for both the overall MANOVA and for the individual ANOVAs for each variable (Table 2). However, despite its effects on plant volume, there was no symbiotum type effect in the overall MANOVA (p = 0.08, Table 2). Symbiotum type did, however, interact with the local environment in the overall MANOVA (p = 0.04, Table 2) and was a significant source of variation for both the volume/wet mass (p = 0.01; Table 2) and volume/dry mass (p = 0.009, Fig. 2) ratios. Plants transplanted to Flagstaff were equivalent in both volume/wet mass and volume/dry mass ratios. However, at Clint’s Well, CW/H plants had a larger ratio than FL/NH. Whereas CW/H individuals were larger for both height (t test: t ratio = 3.13, p = 0.002) and diameter (t test: t ratio = 7.20, p < 0.0001) than FL/NH individuals at Clint’s Well, the difference in volume is more strongly influenced by the changes in diameter (measurements in fall, 2000: CW/H = 3.42 cm, 95% confidence interval [CI] = 2.63–4.21; FL/NH = 2.31 cm, 95% CI = 1.83–2.80), than height (measurements in fall, 2000: CW/H = 29.15 cm, 95% CI = 25.95–32.36; FL/NH = 26.67 cm, 95% CI = 23.15–30.19). Number of tillers is strongly and positively correlated with biomass [32], suggesting that the CW/H symbiotum have larger volumes or sizes but lower density of tillers per unit volume than the FL/NH symbiotum.
Interaction between symbiotum type and the local environment (Clint’s Well [CW] and Flagstaff [FL]) on mean symbiotum volume/dry mass ratio (±1 standard error). The interaction was significant by ANCOVA (p = 0.01). Differences in pairwise comparisons by Tukey’s HSD are indicated by letters above bars
Neither CW/H nor FL/NH symbiota reproduced at Clint’s Well. A logistic regression using local environment, symbiotum type, and the associated interaction on F. arizonica reproductive success found local environment as the only significant factor (likelihood ratio tests: local environment, p < 0.0001; symbiotum type, p = 0.99; local environment × symbiotum type, p = 0.99). There was no effect of symbiota type when only symbiota at Flagstaff were considered (likelihood ratio test, symbiotum type, p = 0.80). Lack of reproduction was likely due to the local environment rather than a consequence of the transplanting process because other naturally occurring F. arizonica plants in the area did not reproduce either (Sullivan, personal observation), whereas the symbiota transplanted to Flagstaff did. Therefore, none of the symbiota transplanted to Clint’s Well were used in the analysis of reproductive growth. Most symbiota at Flagstaff reproduced. For the ones that did not reproduce, symbiotum type was not a significant factor explaining the lack of reproduction (for panicles, χ 2 = 0.014, df = 1, p = 0.91; for seed production, χ 2 = 0.845, df = 1, p = 0.38). The subsequent analyses were performed only using data from individuals that reproduced in all 3 years (N for CW/H = 17, N for FL/NH = 23).
Symbiotum type explained some of the variation in reproduction but not in a consistent manner (Table 3). CW/H individuals produced more panicles than FL/NH individuals (p = 0.04, Table 3, Fig. 3). However, FL/NH individuals produced a greater mass of seeds per panicle (p = 0.01, Table 3, Fig. 3). Reproduction varied over time (1998: mean number of panicles (±1 standard error) = 20.15 ± 2.12; mean seed mass = 1.53 ± 0.19 g; 1999: mean number of panicles = 55.0 ± 5.34; mean seed mass = 3.24 ± 0.32 g; 2000: mean number of panicles = 44.86 ± 4.47; mean seed mass = 1.47 ± 0.18 g) probably because of accumulating growth and environmental variation between years.
Peramine alkaloid levels for F. arizonica infected with either hybrid (1.96 ppm ± 1.05 [1 SD]) and nonhybrid (1.48 ppm ± 2.45) Neotyphodium strains were consistent with levels previously found for this symbiosis [31], which are likely too low to have any herbivore-deterring effect [61].
Discussion
We found geographic differences between populations of F. arizonica/Neotyphodium symbiota, but these differences are most pronounced in the more marginal habitat of Clint’s Well and suggest that the native symbiota are locally adapted. The architectural difference in F. arizonica vegetative growth appears to be due to larger sizes but reduced tiller density in the CW/H symbiotum given that volumes or sizes were greater but biomass was not different than the FL/NH symbiotum. We do not know if altered plant architecture translates into increased persistence or reproduction for CW/H symbiota in marginal habitats such as Clint’s Well.
This experiment could not test the effects of Neotyphodium hybridization independently of the original populations of the symbiota, as the Flagstaff population does not contain any symbiota with hybrid Neotyphodium. The prevalence of hybridization in Neotyphodium is hypothesized to be due to the fitness advantage of gaining novel genes for defense (e.g. [42, 50]), although this hypothesis has heretofore not been tested. This hypothesis probably does not explain the presence of hybrid Neotyphodium in F. arizonica as it does not produce either increased levels or different types of alkaloids, but then again, herbivory is not an important source of selection for F. arizonica [16]. Alternative theories on hybridization predict that it may be most advantageous in marginal habitats where each respective hybridizing population is at the edge of its range [50, 63]. This hypothesis would better explain the distribution of hybrid Neotyphodium in F. arizonica, although tests relating hybrid Neotyphodium to specific fitness advantages (e.g., drought tolerance, nutrient uptake, herbivory) still need to be done.
There are several possibilities for selective advantages for CW/H at Clint’s Well. The increased volume might aid in future reproduction. For the symbiotum transplanted to Flagstaff, volume is positively correlated with reproduction (Kendall’s tau between plant volume and panicle number = 0.449, n = 224, p < 0.0001; Kendall’s tau between plant volume and seed mass = 0.261, n = 224, p < 0.0001). In the marginal conditions at Clint’s Well, CW/H may reach the minimum size required for reproduction more quickly. Alterations in architecture (i.e., volume to mass ratio) may also influence photosynthesis via differences in light capture or may be associated with different nitrogen levels [64]. It is interesting to note that the Clint’s Well site is forested, and F. arizonica grows underneath tall and often dense Ponderosa pine trees. Alternatively, the Flagstaff site is generally unforested, open grassland. Increased volume at the expense of tiller density may be advantageous at Clint’s Well to maximize photosynthesis under lower light conditions. In addition, nitrogen acquisition can also be affected by the presence of Neotyphodium [24], although the role that genetic variation via hybridization may play in this process is not known. It is interesting to note that F. arizonica plants associated with hybrid Neotyphodium are also common in another site in Arizona, Buck Springs [47] that is also nitrogen poor [52], suggesting that for F. arizonica/Neotyphodium, the occasional genetic input from a pathogen may help the symbiota acquire genetic diversity providing them an advantage in marginal habitats because of the greater possibility for local adaptations.
Symbiota with hybrid Neotyphodium are not found naturally at Flagstaff, the population with higher soil nitrogen and precipitation [47], although transplanted symbiota from Clint’s Well with hybrid Neotyphodium grew as well or better at Flagstaff than native symbiota. Previous work on the Neotyphodium gene flow between Clint’s Well and Flagstaff indicates that Neotyphodium migration between Flagstaff and Clint’s Well is low [47]. As such, rare migrant seeds carrying hybrid symbionts to Flagstaff may be lost because of stochastic processes. Another interesting possibility is that CW/H plants transplanted to Flagstaff experience pollen limitation or incompatibility, given that they produced more panicles than nonhybrid infected plants but lowered seed mass per panicle (Fig. 3). The Flagstaff site is composed of only plants infected with a single nonhybrid Neotyphodium strain. Because Neotyphodium is strictly maternally transmitted, this may indicate that the Flagstaff population of F. arizonica is relatively narrow genetically (at least for maternal host plant genotype), and cross-pollination with other F. arizonica genotypes is less likely. If so, then pollen limitation or incompatibility may limit success of invading symbiota with hybrid Neotyphodium, providing another mechanism that maintains the high genetic structuring of Neotyphodium populations [47].
Coevolutionary theory predicts that interspecific interactions should vary between populations because of specific genotypes interacting with varying environments creating local adaptations. In this study, we find that there is variation in F. arizonica/Neotyphodium symbiotum based on their native population, the environment in which they grew, and the interaction between these factors with symbiota from a marginal habitat having an advantage in a marginal habitat. We suggest that these differences may be related to the hybrid origin of the Neotyphodium genotype commonly found at Clint’s Well.
References
Thompson JN (1994) The coevolutionary process. University of Chicago Press, Chicago
Gandon S (1998) Local adaptation and host–parasite interactions. Trends Ecol Evol 13:214–216
Gandon S (2002) Local adaptation and the geometry of host–parasite coevolution. Ecol Lett 5:246–256
Gandon S, Capowiez Y, Dubois Y, Michalakis Y, Olivieri I (1996) Local adaptation and gene-for-gene coevolution in a metapopulation model. Proc R Soc Lond B Biol Sci 263:1003–1009
Nuismer SL, Thompson JN, Gomulkiewicz R (1999) Gene flow and geographically structured coevolution. Proc R Soc Lond B Biol Sci 266:605–609
Nuismer SL, Thompson JN, Gomulkiewicz R (2000) Coevolutionary clines across selection mosaics. Evolution 54:1102–1115
Parker MA (1995) Plant fitness variation caused by different mutualist genotypes. Ecology 76:1525–1535
Wilkinson HH, Parker MA (1996) Symbiotic specialization and the potential for genotypic coexistence in a plant–bacterial mutualism. Oecologia 108:361–367
Freeman S, Rodriguez RJ (1993) Genetic conversion of a fungal plant pathogen to a nonpathogenic, endophytic mutualist. Science 260:75–78
Douglas AE, Smith DC (1983) The cost of symbionts to their host in green hydra. In: Schenk HEA, Schwemmler W (eds) Endocytobiology II. Walter de Gruyter, Berlin, pp 631–648
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism–parasitism continuum. New Phytol 135:575–586
Cushman JH, Whitham TG (1989) Conditional mutualism in a membracid ant association - temporal, age-specific, and density-dependent effects. Ecology 70:1040–1047
Pellmyr O, Huth CJ (1994) Evolutionary stability of mutualism between yuccas and yucca moths. Nature 372:257–260
Burdon JJ, Thrall PH (1999) Spatial and temporal patterns in coevolving plant and pathogen associations. Am Nat 153:S15–S33
Bush LP, Wilkinson HH, Schardl CL (1997) Bioprotective alkaloids of grass–fungal endophyte symbioses. Plant Physiol 114:1–7
Faeth SH (2002) Are endophytic fungi defensive plant mutualists? Oikos 98:25–36
Elbersen HW, West CP (1996) Growth and water relations of field-grown tall fescue as influenced by drought and endophyte. Grass Forage Sci 51:333–342
Elmi AA, West CP (1995) Endophyte infection effects on stomatal conductance, osmotic adjustment and drought recovery of tall fescue. New Phytol 131:61–67
Hill NS, Pachon JG, Bacon CW (1996) Acremonium coenophialum mediated short- and long-term drought acclimation in tall fescue. Crop Sci 36:665–672
Malinowski D, Leuchtmann A, Schmidt D, Nosberger J (1997) Growth and water status in meadow fescue is affected by Neotyphodium and Phialophora species endophytes. Agron J 89:673–678
Vila-Aiub MM, Martinez-Ghersa MA, Ghersa CM (2003) Evolution of herbicide resistance in weeds: vertically transmitted fungal endophytes as genetic entities. Evol Ecol 17:441–456
Zabalgogeazcoa I, Ciudad AG, de Aldana BR, Criado BG (2006) Effects of the infection by the fungal endophyte Epichloe festucae in the growth and nutrient content of Festuca rubra. Eur J Agron 24:374–384
Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40:923–940
Arachevaleta M, Bacon CW, Hoveland CS, Radcliffe DE (1989) Effect of the Tall fescue endophyte on plant response to environmental stress. Agron J 81:83–90
Saikkonen K, Lehtonen P, Helander M, Koricheva J, Faeth SH (2006) Model systems in ecology: dissecting the endophyte–grass literature. Trends Plant Sci 11:428–433
Cheplick GP, Clay K, Marks S (1989) Interactions between infection by endophytic fungi and nutrient limitation in the grasses Lolium perenne and Festuca arundinacea. New Phytol 111:89–97
Saikkonen K, Faeth SH, Helander M, Sullivan TJ (1998) Fungal endophytes: a continuum of interactions with host plants. Annu. Rev Ecol Syst 29:319–343
Bultman TL, Bell G, Martin WD (2004) A fungal endophyte mediates reversal of wound-induced resistance and constrains tolerance in a grass. Ecology 85:679–685
Lehtonen P, Helander M, Wink M, Sporer F, Saikkonen K (2005) Transfer of endophyte-origin defensive alkaloids from a grass to a hemiparasitic plant. Ecol Lett 8:1256–1263
Cheplick GP (1998) Genotypic variation in the regrowth of Lolium perenne following clipping: effects of nutrients and endophytic fungi. Funct Ecol 12:176–184
Faeth SH, Bush LP, Sullivan TJ (2002) Peramine alkaloid variation in Neotyphodium-infected Arizona fescue: effects of endophyte and host genotype and environment. J Chem Ecol 28:1511–1526
Faeth SH, Sullivan TJ (2003) Mutualistic, asexual endophytes in a native grass are usually parasitic. Am Nat 161:310–325
Brem D, Leuchtmann A (2001) Epichloe grass endophytes increase herbivore resistance in the woodland grass Brachypodium sylvaticum. Oecologia 126:522–530
Tintjer T, Rudgers JA (2006) Grass–herbivore interactions altered by strains of a native endophyte. New Phytol 170:513–521
Faeth SH, Gardner DR, Hayes CJ, Jani A, Wittlinger SK, Jones TA (2006) Temporal and spatial variation in alkaloid levels in Achnatherum robustum, a native grass infected with the endophyte Neotyphodium. J Chem Ecol 32:307–324
Brem D, Leuchtmann A (2003) Molecular evidence for host-adapted races of the fungal endophyte Epichloe bromicola after presumed host shifts. Evolution 57:37–51
Hesse U, Schoberlein W, Wittenmayer L, Forster K, Warnstorff K, Diepenbrock W, Merbach W (2003) Effects of Neotyphodium endophytes on growth, reproduction and drought-stress tolerance of three Lolium perenne L. genotypes. Grass Forage Sci 58:407–415
Brem D, Leuchtmann A (2002) Intraspecific competition of endophyte infected vs uninfected plants of two woodland grass species. Oikos 96:281–290
Saikkonen K, Ion D, Gyllenberg M (2002) The persistence of vertically transmitted fungi in grass metapopulations. Proc R Soc Lond B Biol Sci 269:1397–1403
Tsai HF, Liu JS, Staben C, Christensen MJ, Latch GCM, Siegel MR, Schardl CL (1994) Evolutionary diversification of fungal endophytes of tall fescue grass by hybridization with Epichloë species. Proc Natl Acad Sci USA 91:2542–2546
Schardl CL, Leuchtmann A, Tsai HF, Collett MA, Watt DM, Scott DB (1994) Origin of a fungal symbiont of perennial ryegrass by interspecific hybridization of a mutualist with the ryegrass choke pathogen, Epichloë typhina. Genetics 136:1307–1317
Moon CD, Craven KD, Leuchtmann A, Clement SL, Schardl CL (2004) Prevalence of interspecific hybrids amongst asexual fungal endophytes of grasses. Mol Ecol 13:1455–1467
Christensen MJ, Leuchtmann A, Rowan DD, Tapper BA (1993) Taxonomy of Acremonium endophytes of tall fescue (Festuca arundinacea), meadow fescue (F. pratensis) and perennial ryegrass (Lolium perenne). Mycol Res 97:1083–1092
Moon CD, Scott B, Schardl CL, Christensen MJ (2000) The evolutionary origins of Epichloë endophytes from annual ryegrasses. Mycologia 92:1103–1118
Craven KD, Blankenship JD, Leuchtmann A, Hignight K, Schardl CL (2001) Hybrid fungal endophytes symbiotic with the grass Lolium pratense. Sydowia 53:44–73
Moon CD, Miles CO, Jarlfors U, Schardl CL (2002) The evolutionary origins of three new Neotyphodium endophyte species from grasses indigenous to the Southern Hemisphere. Mycologia 94:694–711
Sullivan TJ, Faeth SH (2004) Gene flow in the endophyte Neotyphodium and implications for coevolution with Festuca arizonica. Mol Ecol 13:649–656
Selosse MA, Schardl CL (2007) Fungal endophytes of grasses: hybrids rescued by vertical transmission? An evolutionary perspective. New Phytol 173:452–458
Clay K, Schardl CL (2002) Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am Nat 160:S99–S127
Schardl CL, Craven KD (2003) Interspecific hybridization in plant-associated fungi and oomycetes: a review. Mol Ecol 12:2861–2873
Kearney TH, Peebles RH (1960) Arizona Flora. University of California Press, Berkeley, CA
Schulthess FM, Faeth SH (1998) Distribution, abundances, and associations of the endophytic fungal community of Arizona fescue (Festuca arizonica). Mycologia 90:569–578
Saikkonen K, Helander M, Faeth SH, Schulthess F, Wilson D (1999) Endophyte–grass–herbivore interactions: the case of Neotyphodium endophytes in Arizona fescue populations. Oecologia 121:411–420
Lopez JE, Faeth SH, Miller M (1995) Effect of endophytic fungi on herbivory by redlegged grasshoppers (Orthoptera: Acrididae) on Arizona fescue. Environ Entomol 24:1576–1580
Tibbets TM, Faeth SH (1999) Neotyphodium endophytes in grasses: deterrents or promoters of herbivory by leaf-cutting ants? Oecologia 118:297–305
An ZQ, Liu JS, Siegel MR, Bunge G, Schardl CL (1992) Diversity and origins of endophytic fungal symbionts of the North American grass Festuca arizonica. Theor Appl Genet 85:366–371
Cabral D, Cafaro MJ, Saidman B, Lugo M, Reddy PV, White JF (1999) Evidence supporting the occurrence of a new species of endophyte in some South American grasses. Mycologia 91:315–325
White JF, Cole GT, Morganjones G (1987) Endophyte-host associations in forage grasses. VI. A new species of Acremonium isolated from Festuca arizonica. Mycologia 79:148–152
US Department of Agriculture (1967) General soil map—Coconino county, Arizona. US Department of Agriculture—Soil Conservation Service, Washington, DC
Moore KJ, Moser LE (1995) Quantifying developmental morphology of perennial grasses. Crop Sci 35:37–43
Siegel MR, Bush LP (1996) Defensive chemicals in grass-fungal endophyte associations. In: Romero JT, Saunders JA, Barbosa P (eds) Phytochemical diversity and redundancy in ecological interactions, vol. 30. Plenum, New York, pp 81–117
SAS Institute (2003) JMP IN. SAS Institute, Cary, NC
Rieseberg LH (1997) Hybrid origins of plant species. Annu Rev Ecol Syst 28:359–389
Singer JW (2002) Species and nitrogen effect on growth rate, tiller density, and botanic composition in grass hay production. Crop Sci 42:208–214
Sullivan TJ (2003) Geographic and genetic variation in the Neotyphodium/Festuca arizonica interaction. Ph.D. dissertation
Acknowledgments
The authors wish to thank Natalie Fuller and Phil Steiner for their field assistance and Dr. Thomas Dowling for his aid with the microsatellites and DNA sequencing. We also thank Andrea Jani and Tamaru Hunt-Joshi for their helpful comments on the manuscript. This research was supported by grants from the Associated Graduate Students of A.S.U. and by an anonymous donor to the ASU foundation to TJS and DEB 0128343 to SHF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sullivan, T.J., Faeth, S.H. Local Adaptation in Festuca arizonica Infected by Hybrid and Nonhybrid Neotyphodium Endophytes. Microb Ecol 55, 697–704 (2008). https://doi.org/10.1007/s00248-007-9312-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-007-9312-4