Abstract
Nitrogen fixation (nitrogenase activity, NA) of a microbial mat and a living stromatolite from Cuatro Cienegas, Mexico, was examined over spring, summer, and winter of 2004. The goal of the study was to characterize the diazotrophic community through molecular analysis of the nifH gene and using inhibitors of sulfate reduction and oxygenic and anoxygenic photosynthesis. We also evaluated the role of ultraviolet radiation on the diazotrophic activity of the microbial communities. Both microbial communities showed patterns of NA with maximum rates during the day that decreased significantly with 3-3,4-dichlorophenyl-1′,1′-dimethylurea, suggesting the potential importance of heterocystous cyanobacteria. There is also evidence of NA by sulfur-reducing bacteria in both microbial communities suggested by the negative effect exerted by the addition of sodium molybdate. Elimination of infrared and ultraviolet radiation had no effect on NA. Both microbial communities had nifH sequences that related to group I, including cyanobacteria and purple sulfur and nonsulfur bacteria, as well as group II nitrogenases, including sulfur reducing and green sulfur bacteria.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microorganisms are commonly found forming consortia [33] in which different metabolic groups act together to function as a community. Microbial mats and stromatolites are examples of microbial communities that have a great taxonomic and metabolic dynamism [13, 40, 41, 48]. Schopf [38, 50] reported the presence of diverse bacterial flora fossilized in the 3,465-million-year-old Apex Cherts of Western Australia providing the oldest morphological evidence for life on Earth. There is ongoing debate regarding the biogenicity of such ancient fossils [8, 9]. Brassier et al. [8, 9] have attributed these structures to amorphous carbon formed during hydrothermal dyke vein quartz. However, recent findings by Allwood et al. [1] propose that there is evidence of a probable biological origin to the laminated structures (stromatolites) found in the 3,430-million-year-old Strelley Pool Chert (Australia) sediment formation in which they identify seven stromatolite morphologies that cannot be explained by abiogenic hypothesis. To date, there are examples of extant stromatolites and microbial mats that usually occur in extreme environments characterized either by their high salinity, temperature, or nutrient limitation [2, 10, 14, 19, 30, 31, 35, 34, 42]. In microbial mat and living stromatolite communities, cyanobacteria will typically be involved in organic matter production and oxygen evolution; chemotrophic and phototrophic sulfur-oxidating bacteria will form sulfur and sulfate that will be used by sulfur and sulfate-reducing bacteria (SRB). Methanogenic and methanotrophic bacteria will also be involved in the transformations of organic matter [13].
Nitrogen is usually the most limiting element in marine environments. “New” N can be obtained through N2 fixation [15], the reaction that reduces dinitrogen molecules, which comprise 78% of the atmosphere, to ammonia [45]. It is catalyzed by the enzyme nitrogenase, which is formed out of two enzymatic subunits: dinitrogenase reductase (iron protein) and dinitrogenase (molybdenum–iron protein) [25]. Some bacteria have dinitrogenases in which molybdenum can be substituted by vanadium or iron [25]. During the electron transfer process that constitutes N2 fixation, two ATP molecules are needed for each electron transferred [46]. Nitrogenase is an ancient and conserved enzyme that has essentially remained conserved throughout the transition from an anoxic to oxic biosphere, suggesting that it evolved in early Earth. It has been hypothesized that either it is a loci extremely prone to horizontal gene transfer or that it was part of the cenancestor metabolism and has been lost in most lineages [36].
It is generally assumed that cyanobacteria are the most important N2-fixing microorganisms in most aquatic environments, although there is ample evidence suggesting that there are many other prokaryotic diazotrophs in both the bacterial and archaeal domains [45]. A recent study in microbial mats of Guerrero Negro suggests that bacteria of the phylum Chloroflexi formed the major percentage of the biomass throughout the mat, although cyanobacteria were the most important component in the upper millimeters [26]. Cyanobacteria have solved the conundrum between oxygen-evolving, ATP-generating metabolism and N2 fixation in a variety of ways: (1) separating temporally both metabolisms (daytime C fixation and nighttime N2 fixation), e.g., unicellular cyanobacteria such as Cyanothece, Gloeothece, and Synechococcus, as well as filamentous cyanobacteria such as Lyngbya [4, 43]; (2) separating spatially N2 fixation in specialized nonoxygenic cells called heterocysts, e.g., filamentous cyanobacteria Calothrix and Nostoc that impede entry of O2 formed in adjacent, photosynthetic cells (vegetative) [37]; (3) and by switching to diazotrophy primarily during low photosynthetic activity (e.g., filamentous cyanobacteria, Trichodesmium) [5–7]. Other bacteria will fix N2 in anoxic environments that occur during organic matter decomposition, usually during the night, when O2 evolution through photosynthesis has stopped [40].
Cuatro Cienegas Coahuila (CCC) is a valley in central Mexico located in the middle of the Chihuahuan Desert that constitutes an oasis because it contains a series of natural wells and riverine systems [28]. A striking characteristic of this environment is the presence of different microbial communities, including microbial mats and living stromatolites that are part of the trophic web sustaining a great diversity of invertebrates [21, 44]. The focus of this study was to evaluate the potential for N2 fixation by different metabolic components of the prokaryotic community in a microbial mat and a living stromatolite from CCC. We also characterized the potential diazotrophic community through construction of DNA clone libraries for the nifH gene (dinitrogenase reductase).
Methods
Site
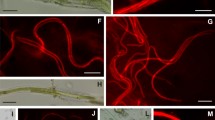
Cuatro Cienegas is a valley of approximately 30 × 40 km located at ∼740 m above sea level and surrounded by high mountains (>3,000 m) (Fig. 1). This enclosed evaporitic basin receives ∼150 mm of annual precipitation. Despite the arid climate, CCC harbors an extensive system of natural springs, streams, and pools [44]. This study focused on two contrasting systems: Poza Azul and Churince. Poza Azul is a pool formed by a spring that nurtures the larger river “Rio Mesquites.” The cretaceous karst landscape that surrounds this pool is rich in carbonates, making possible the formation of massive living stromatolites (Fig. 2A, B). Stromatolites are microbial mats that also have layers of precipitated minerals. This pool remains at a constant temperature (30–35°C) throughout the year. Meanwhile, the western part of the valley is dominated by Jurassic gypsum soil with an hydrological system called Churince that starts at a spring that forms a small river that flows toward a shallow (20–60 cm) lake (Laguna Intermedia) and continues to a desiccation pond (Laguna Grande). Microbial mats that do not form liteous stroma (Fig. 2E, F) are present in this system. Both microbial communities studied have a strong component of cyanobacteria, filamentous (with and without heterocysts) and unicellular as evidenced by microscopic observations (Fig. 2C, D, G, H). The major solutes that have been reported for CCC aquatic systems are SO4 2−, Mg+, Cl−, Na+, Ca++, and K+ in order of importance [23].
A Overview of Poza Azul living stromatolite. B Stromatolite section of ∼1 cm3 used in ARA. C, D Microphotographs showing different filamentous cyanobacteria from the Poza Azul living stromatolite (×400). E, F Overview of ARA set up in Churince microbial mat. G, H Microphotographs showing different cyanobacteria from the Churince microbial mat (×400)
N2 Fixation (Acetylene Reduction Assay)
Nitrogenase activity (NA) was measured with the acetylene reduction assay (ARA) [11] in situ in a microbial mat from the Churince system and a living stromatolite from Poza Azul, both in CCC. Serum vials (14 mL) with the bottom cut off were gently inserted in the microbial mat (Fig. 2E, F). Samples of the living stromatolite (n = 3, treatment) were collected and cut to 1 cm3 that were placed inside whole serum vials (Fig. 2B). Vials were closed with rubber stoppers and samples were covered with 0.2 μm of filtered water (4 mL) collected from the same sites as the mat and stromatolite, leaving a gas phase (3 mL) in all treatments [14, 17]. Acetylene reduction assay started with saturation of the atmosphere to 20% by replacing the gas phase with acetylene that was formed from the reaction of calcium carbide and water. NA was followed during 24-h cycles in 6-h intervals when gas samples (1 mL) were taken from the gas phase of the serum vials and collected in vacuum vials (n = 3, serum vial; n = 9, treatment/time point) (Vacutainer® 3-mL red cap, with no additives, Becton, Dickinson, Franklin Lakes, NJ, USA) for the latter analysis by gas chromatography. To maintain gas volume after sampling, we replaced the volume extracted at each time point by injecting acetylene. Time zero samples were taken immediately after saturation of the atmosphere with acetylene to verify base levels of ethylene. Experiments were ended after 24 h when incubated samples were stored in liquid N2 for further genetic characterization. Acetylene reduction assays were designed to evaluate the potential contribution to N2 fixation patterns of different bacterial metabolic groups of the microbial communities. We carried out these experiments during spring, summer, and winter of 2004 using four different metabolic treatments and filtered water as control.
Chlorophyll a (Chl a) concentration was obtained for all samples assayed for acetylene reduction after overnight extraction of ∼1 g of each microbial community using 90% aqueous methanol. The extract was filtered through a GF Whatman filter and absorbance of extract at 665 nm was read in a spectrophotometer. The procedure was repeated approximately three times for each sample until no more pigment was extracted. Temperature was measured in the overlying water of both microbial communities around noon for all seasons. Microscopic observations of bacterial communities from both the microbial mat and stromatolite was done back in the laboratory with a Zeiss Axioskop 40 microscope.
Metabolic Inhibitors
3-3,4-Dichlorophenyl-1′,1′-dimethylurea (DCMU) was used at 2 × 10−5 M [2, 3] because it inhibits linear electron transport flow on the acceptor side of photosystem II (PSII) [6]. Dichlorophenyl-1′,1′-dimethylurea will block quinone A (QA) oxidation [6] and thus will affect oxygenic phototrophs that have PSII (e.g., cyanobacteria). Heterocystous cells lack PSII and most ATP used in N2 fixation comes from PSI and from vegetative cells. However, nonheterocystous cyanobacteria are affected by DCMU addition. Because the O2-forming PSII is inhibited in the presence of DCMU, microbial communities submitted to this treatment will show a decrease in O2 levels. Nitrogenase is irreversibly inhibited by oxygen [5–7], thus, DCMU blockage can facilitate N2 fixation by nonheterocystous cyanobacteria and anoxygenic phototrophs or potentially inhibit NA by heterocystous cyanobacteria during the day, depending on the carbohydrate storage present in vegetative cells and the length of exposure to this inhibitor [2, 3, 5–7]. Sodium molybdate (Na2MoO4) at 20 mM, a structural analogue to sulfate, will inhibit sulfate reduction by SRB, which occurs during anoxic conditions [14, 42]. We also submitted the samples to two light treatments: absence of IR or UV wavelengths [14] using Rosco filters (Rosco Laboratories, Stamford, CT, USA) (IR thermashield filter transmits 80% of visible light and reflects infrared wavelengths, UV filter allows less than 10% of transmission below 390 nm). Anoxygenic photoautotrophs have bacteriochlorophylls that have absorption spectra in the infrared and are visible, especially bacteriochlorophylls c, d, and e, as well as light-harvesting carotenoids in the blue to green region, which are employed for photosynthesis [27, 32]. The deleterious effect of ultraviolet radiation on microorganisms has been well documented, including damages to DNA, protein, and cellular structures [12].
DNA Extraction, nifH Amplification, Sequencing, and Phylogenic Analysis
Total environmental DNA was extracted from mat and living stromatolite samples used for ARA following the protocol of Zhou et al. [53] with modifications. Microbial communities were pulverized on ceramic mortars with liquid nitrogen and ∼3 g of the microbial mat and ∼2 g of the stromatolite were placed in 9 mL of extraction buffer (100 mM Tris–HCl, 1.5 M NaCl, 100 mM EDTA, 100 mM Na3PO4, pH 8) with 1% cetyl trimethylammonium bromide. Samples were frozen at −20°C and left to thaw at 65°C three times, incubated with lysozyme (1 mg/mL, Invitrogen, Carlsbad, CA, USA) for 30 min at 37°C, and left overnight with proteinase K (0.1 mg/mL, Invitrogen) and sodium dodecyl sulfate (3%) at 60°C. Supernatant was collected with centrifugation (5000×g, 10 min) and cleaned twice with phenol/chloroform/isoamyl alcohol (25:24:1). DNA was precipitated with isopropanol and sodium acetate (3 M) at room temperature. A 359-basepair region of the nifH gene was amplified with nested polymerase chain reaction (PCR) following the protocol of Zani et al. [51] with addition of 5% bovine serum albumin (Invitrogen) of the total PCR reaction volume (50 μl). The thermal cycle for nifH amplification was as follows: 93°C, 1 min 20 s; 50°C, 1 min; and 70°C, 1 min 30 s for 30 cycles. Thermal cycles had an initial denaturation step of 94°C for 10 min and a final extension of 72°C for 30 min. Negative controls using PCR water were run with nested nifH PCR. Amplified products were separated by cloning using Escherichia coli-competent cells and pCR®2.1 TOPO® plasmid (Invitrogen). Positive clones were sequenced in both directions in an automated capillary sequencer (ABI PRISM® 3100-Avant Genetic Analyzer, Applied Biosystems, Foster City, CA, USA) using T7 and Sp6 primers [18]. Sequences were aligned and edited with Sequencher (Gene Codes Corporation, Ann Arbor, MI, USA). Each sequence was translated to amino acid and screened in all six reading frames for stop codons. Sequences with stop codons were considered nonfunctional pseudogenes and were left out of the phylogenetic analysis. Potentially functional sequences were searched against the NCBI dataset using BLASTx, and best-hit sequences were downloaded and included in the phylogenetic analysis. Phylogenetic inferences (parsimony) were done with PAUP*4.0b8 (Sinauer Associates, Inc., Sunderland, MA, USA). We performed 500 bootstrap replicates for a dataset consisting of 368 characters, of which 235 were parsimoniously informative. The branch-swapping algorithm was tree bisection reconnection. The data matrix had 73 taxa.
Phylotype Richness Estimation
To assess if libraries were large enough to represent the diversity present in the studied sites, we analyzed our data with the software tool provided by Kemp and Aller [24] that uses the SChao1 and SACE estimators.
Statistical Analysis
Statistical analyses were done to test for differences between peaks of nitrogen fixation using Statistica-advanced software. We ran t tests for independent samples after evaluating that the distribution of the variables was normal and verifying the equality of variances with the Levene test.
Nucleotide Sequence Accession Numbers
The sequences generated in this study have been deposited in the GenBank database under the following accession numbers: DQ485349–DQ485403.
Results
N2 Fixation
Churince Microbial Mat
All treatments, except when DCMU was added, showed the same trend of acetylene reduction activity with ethylene production peaks during both daytime measurements (sunrise–noon and noon–sunset) during all seasons sampled (Fig. 3A–C). The control treatment had maximum NA from noon (12 h) to sunset (18 h) and sunrise (6 h) to noon with acetylene reduction rates of 571 ± 101 and 811 ± 84 nmol C2H4 m−2 h−1 in the spring and 569 ± 11 and 904 ± 95 nmol C2H4 m−2 h−1 in the summer, respectively. Winter samples were significantly lower (p < 0.0140) with maximum acetylene reduction rates in control samples of 169 ± 11 nmol C2H4 m−2 h−1. Samples exposed to DCMU had a significant (p < 0.0237) drop in ethylene production during the daytime (06:12 and 12:18) of 90% in average for spring and summer, and of only 4% in the winter. Sodium-molybdate-exposed samples showed an average decrease of 57% in spring, 67% in summer, and 94% in the winter, during dusk or night (18:24 and 24:06); daytime samples showed no effect in NA for this treatment. Samples treated for absence of infrared radiation showed no significant difference in relation to the control. Although we observed higher rates of NA when UV radiation was eliminated, these were not significantly different from the control. There was no significant difference between Chl a values for the mat in spring and summer (29.9 ± 4.8 and 27.2 ± 3.3 μg Chl a cm−2, respectively) although winter values were significantly (p < 0.0210) lower (14 ± 0.9 μg Chl a cm−2). Water temperature overlying the mat was of 23°C in spring, 27°C in summer, and 12°C in winter.
Poza Azul Stromatolite
Nitrogenase activity was not significantly different between all three seasons and showed its maximum rates during the midnight to sunrise (average for all three seasons: 153 ± 5 nmol C2H4 m−2 h−1) and sunrise to noon (average for all three seasons: 248 ± 20 nmol C2H4 m−2 h−1) time points (Fig. 4A–C). Nitrogenase activity was statistically lower (p < 0.0133) to that measured in the microbial mat for spring and summer. Samples exposed to DCMU showed a significant decrease of NA during daytime points ranging from 67% in spring and summer to 91% in the winter. The stromatolite showed a peak of NA during the night that decreased 59% when exposed to Na2MoO4. There was no difference between the control and the IR treatment and no negative effect of UV radiation was evidenced in NA. There was no significant difference between Chl a values for the stromatolite during all sampling seasons (12.6 ± 1.6, 13.1 ± 1.3, and 13.4 ± 0.6 μg Chl a cm−2, respectively, for spring, summer and winter). Water temperature overlying the stromatolite was of 30°C in spring, 31°C in summer, and 30°C in winter.
Community Composition
A total of 96 nifH sequences were recovered, 39 from the microbial mat, and 57 from the stromatolite. Out of the total sequences, 14 from the Churince microbial mat and 42 from the Poza Azul stromatolite were potentially functional because they lacked stop codons in all six reading frames. A group of 15 sequences from the Poza Azul stromatolite shared highest similarity to sequences reported in the GenBank database that related to purple nonsulfur bacteria, nine sequences from the Poza Azul stromatolite, and four from the Churince microbial mat related to cyanobacteria. A separate group of sequences (17 from the Poza Azul stromatolite and 10 from the Churince microbial mat) affiliated with SRB. A sequence from the Poza Azul stromatolite showed closest affiliation to a previously reported sequence of an unknown bacteria, recovered from a denaturing gradient gel electrophoresis study in periphyton, as to the nifH sequence used as outgroup pertaining to Methanosarcina barkeri. The sequences obtained clustered in groups I and II of nitrogenases, as proposed by Raymond et al. [36] (Fig. 5). Group I sequences contained the purple sulfur and cyanobacteria, whereas group II had the anaerobic SRB and green sulfur bacteria. Sequences here reported clustered among sites and the only nifH sequence that was common to the Churince microbial mat and Poza Azul stromatolite related to a Calothrix spp. that came from Yellowstone National Park (USA) microbial mats (Fig. 5).
The number and identity of sequences recovered in this study were not enough to represent the total community of diazotrophs (nifH) covering a mere 20 to 30% of the expected richness in the microbial mat and stromatolite, respectively, as estimated with SChao1 and SACE (data not shown).
Discussion
Cuatro Cienegas is an endangered ecosystem suffering from damaging human impact due mainly to the agriculture of alfalfa in the Mexican desert, which in turn is causing the collapse of the few aquatic environments in the region [44]. We believe that it is very interesting to have living microbial communities forming mats and stromatolites in desert springs that are quite far away from coastal regions (∼800 km). The microbial communities reported here seem to function similarly to bacterial communities from coastal regions, e.g., Guerrero Negro microbial mats, Bahamas stromatolites, and North Carolina microbial mats.
The sequences recovered in this study were clearly more related among them than to any sequence previously deposited in the public database. The above suggests that there is a great diversity of potentially important N2 fixers in the pools of Cuatro Cienegas that have been isolated from other aquatic ecosystems. The phylogeny reported here clearly shows that the nifH sequences recovered from the Churince microbial mat and the Poza Azul stromatolite form separate clusters for groups I and II nitrogenases, as well as for different bacterial lineages. Nevertheless, although there are strong lineage affinities, sequences from this study can only be related at the genus level to Calothrix spp.
The CCC microbial mat and stromatolite showed highest N2 fixation rates (as measured by NA) during the day, which follows the pattern of heterocystous diazotrophic cyanobacteria [13, 33, 40, 41, 45, 47, 48]. Heterocysts, the specialized cells for N2 fixation, lack PSII activity and thus the oxygen-evolving capacity that characterizes vegetative cells. Because nitrogenase is inactivated by O2, heterocyst-forming colonies have the capacity to fix N2-coupled to photosynthetic activity by spatially separating both processes [5–7, 40, 41]. Molecular clock analyses have suggested that heterocysts evolved approximately after 2,450–2,320 Ma, when partial pressure of O2, formed as a by-product of photosynthesis, reached levels to inhibit NA [47]. Because DCMU will inhibit PSII, there can be a stimulation of N2 fixation because the community goes anoxic. Nevertheless, when heterocystous cyanobacteria are responsible for N2 fixation, DCMU-exposed samples will show a decrease in NA because the electron transfer pathway from PSII to PSI is blocked, and thus ATP formed in PSI, which is necessary to reduce N2, will stop being generated in the vegetative cells [5–7]. Our results suggest that heterocystous cyanobacteria could be important in both the microbial mat because of the 90% decrease of daytime NA when exposed to DCMU, and living stromatolite that had a decrease in daytime NA of 67% in spring and summer, and 90% in winter. The microbial mat NA data suggests that heterocystous cyanobacteria could be major components of the diazotrophic community in the Churince mat in spring and summer, whereas in the winter, Na2MoO4-exposed samples showed a decrease of 94%, suggesting that SRB could be the main diazotrophs for this season. Sulfur-reducing bacteria will utilize free sulfur as SO4 or elemental S, and thus when the atmosphere is saturated in Na2MoO4, an analogue to SO4, SRB metabolism will be affected [14]. The Poza Azul stromatolite had NA patterns that suggest that heterocystous cyanobacteria could be more important during the winter although they are still major components of NA in spring and summer. These experiments also point at the potential importance of SRB as diazotrophs in the stromatolite during all three seasons because Na2MoO4-exposed samples decreased by an average of 58% during the nighttime points. The Poza Azul stromatolite had a second NA peak from midnight to sun dawn, which suggests activity of nonheterocystous cyanobacteria, as well as of other anaerobic bacteria, for example, purple sulfur and nonsulfur, green sulfur, and SRB. Although we also found evidence of nonheterocystous cyanobacteria as potential diazotrophs in both microbial communities, our metabolic-oriented NA experiments point to the potential activity of heterocystous cyanobacteria as major diazotrophs. Nevertheless, our results showed no significant effect of lack of UV radiation in the NA of both microbial communities. Previous research has shown that Calothrix has a UV-protective pigment, scytonemin [10, 20], that shows in vivo absorption maximum at 370 nm [20]. Because both our molecular and metabolic data suggest that heterocystous cyanobacteria, possibly Calothrix, could be responsible for at least some of the N2 fixation in the microbial mat and living stromatolite, we infer that this cyanobacterium may be protected against the deleterious effects of UV radiation via scytonemin. Furthermore, Sheridan [39] hypothesizes that UVR will maintain normal three-dimensional community composition in microbial mats because most UVR-resistant bacteria, such as scytonemin-bearing cyanobacteria, will occupy the surface levels, protecting non-UVR-resistant cyanobacteria. Although our cloning effort was of only 20–30% for the total estimated richness (SChao1 and SACE) of diazotrophs in the microbial mat and living stromatolite, we were able to identify groups of nifH affiliated to green sulfur, purple sulfur, purple nonsulfur, SRB, cyanobacteria, and archaea. This coincides with the proposed metabolisms that could be held responsible for NA in both microbial communities suggested from the metabolic inhibitor NA experiments. Nevertheless, our NA experiments were not able to detect a negative effect when blocking IR radiation. This could be because of the fact that although most bacteriochlorophylls have absorption spectra in the IR, some (bacteriochlorophylls c, d, and e) also have absorption peaks in the visible, thus anoxygenic phototrophs that also have carotenoids could have obtained the energy necessary for ATP production and NA in the visible spectrum [27, 32].
Our molecular data also suggest that we have a potentially important purple sulfur and nonsulfur diazotrophic bacterial community in both microbial communities studied. We found nifH sequences that related to group II of nitrogenases [36] that are characterized for being of anaerobic organisms. In this group we obtained sequences from the mat and stromatolite that related to SRB, Desulfovibrio gigas, Desulfovibrio salexigens [35], and green sulfur bacteria Chlorobium tepidum [16]. Chlorobium tepidum is capable of using both sulfide and thiosulfate as sources of sulfur and electron donors for photosynthesis [16]. The rest of the sequences clustered with nitrogenases of group I [36], which are typical Mo–Fe nitrogenases. In this group, sequences from the stromatolite clustered to Rhodopseudomonas palustris, a highly energetically versatile bacterium capable of both aerobic and anaerobic metabolisms, including photoautotrophy, photoheterotrophy, chemoautotrophy, and chemoheterotrophy [29, 49]. Rhodospirillum rubrum is a purple nonsulfur bacterium capable of living under aerobic or anaerobic conditions, making it capable of obtaining energy from a great array of substrates [52]. Heliobacterium modesticaldum [22] is another bacterium to which sequences from the stromatolite related. Halorhodospira halophila, a purple sulfur bacteria that can utilize elemental sulfur or H2S as electron donor, clustered to R. palustris and R. rubrum, forming a basal cluster to a group of sequences from the stromatolite. Our NA experiments were not able to fully identify all the metabolic bacterial groups involved in N2 fixation in both microbial communities. Previous research has suggested the potential role of archaeal methanogens and methanotrophs as part of microbial, diazotrophic ensembles [14]. Further, a nifH sequence from the stromatolite clustered to an uncultured clone obtained from periphyton and together they were more closely related to the archaeal outgroup, M. barkeri, suggesting that a greater cloning effort is needed to characterize these communities. Molecular data also evidenced the differences in composition of diazotrophs between the microbial mat from Churince and the living stromatolite from Poza Azul. Sequences from both microbial communities clustered to each other, suggesting biogeographic relationships. Poza Azul is separated in approximately 5 km from Laguna Intermedia and previous hydrological studies have proposed that the springs that feed both systems are different [23].
Although NA rates were significantly lower in the living stromatolite during spring and summer compared to the microbial mat, they were constant throughout all seasons, whereas NA rates in the microbial mat had major oscillations between spring–summer and winter. Previous research in CCC [44] has shown that temperature varies significantly in the region of the Churince system where we carried out the microbial mat experiments, whereas it remains constant (30–35°C) throughout the year in Poza Azul. Nevertheless, to date, there are no published studies in CCC that follow environmental variables such as salinity, pH, and nutrients in the different aquatic systems of CCC. Integrating our data on a yearly basis, and assuming that our spring–summer data are representative of 8 months, while our winter data represent 4 months, we can calculate a yearly NA rate of 530 mmol C2H4 m−2 h−1 for the microbial mat. Our yearly NA estimate for the stromatolite is of 192 mmol C2H4 m−2 h−1. Rates of NA from both microbial communities are comparable to those from other microbial mats and living stromatolites [14, 34], although they are much lower than NA observed in microbial mats from Guerrero Negro, Baja California, Mexico [3, 30, 31], and the McMurdo Ice Shelf, Antarctica [19].
Cuatro Cienegas Coahuila has been reported to sustain highly diverse microbial communities that have large affinities to marine bacteria [44]. In their study, Souza et al. [44] hypothesize that CCC maintained Mesozoic marine bacterial communities in the interstitial pore water and that present day microbial mats and stromatolites originated back in the Jurassic. Our study cannot shed light on the potential time of formation of the microbial mat and living stromatolite analyzed, although we should mention that previous research on modern marine stromatolites from Bahamas [34, 35] and microbial mats from North Carolina, USA [42] had already proposed the role of cyanobacteria and SRB as potential N2 fixers. Further research is needed to open the “black box” of microbial dynamics that are responsible for the biogeochemical interactions that coexist in microbial mats and living stromatolites.
References
Allwood, AC, Walter, MR, Kamber BS, Marshall CP, Burch IW (2006) Stromatolite reef from the Early Archaen era of Australia. Nature 441: 714–718
Bebout, BM, Paerl, HW, Crocker, KM, Prufert, LE (1987) Diel interactions of oxygenic photosynthesis and N2 fixation (acetylene reduction) in a marine microbial mat community. Appl Environ Microbiol 53: 2353–2362
Bebout, BM, Fitzpatrick, MW, Paerl, HW (1993) Identification of the sources of energy for nitrogen fixation and physiological characterization of nitrogen-fixing members of a marine microbial mat community. Appl Environ Microbiol 59: 1495–1503
Bergman, B, Gallon, JR, Rai, AN, Stal, LJ (1997). N2 fixation by non-heterocystous cyanobacteria. FEMS Microbiol Rev 19: 139–185
Berman-Frank, I, Cullen, JT, Shaked, Y, Sherrell, RM, Falkowski, PG (2001). Iron availability, cellular iron quotas and nitrogen fixation in Trichodesmium. Limnol Oceanogr 46: 1249–1260
Berman-Frank, I, Lundgren, P, Chen, YB, Küpper, H, Kolber, Z, Bergman, B, Falkowsky, P (2001). Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science 294: 1532–1537
Berman-Frank, I, Lundgren, P, Falkowski, P (2003) Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Res Microbiol 154: 157–164
Brassier, MD, Green, OR, Jephcoat, AP, Kleppe, AK, Van Kranendonk, MJ, Lindsay, JF, Steele, A, Grassineau, NV (2002) Questioning the evidence for earth’s oldest fossils. Nature 422: 76–81
Brassier, MD, Green, O, Lindsay, J, Steele, A (2004) Earth’s oldest (∼3.5 Ga) fossils and the “Early Eden Hypothesis”: Questioning the evidence. Orig Life Evol Biosph 34: 257–269
Brenowitz, S, Castenholz, RW (1997) Long-term effects of UV and visible irradiance on natural populations of a scytonemin-containing cyanobacterium (Calothrix sp.). FEMS Microbiol Ecol 343–352
Capone, DG (1993) Determination of nitrogenase activity in aquatic samples using the acetylene reduction procedure. In: Kemp PF, Sherr, BF, Sherr, EB, Cole, JJ (Eds.) Handbook of Methods in Aquatic Microbial Ecology, Lewis Press, Boca Raton, FL, pp 621–631
Castenholz, RW, Garcia-Pichel, F (2000) Cyanobacterial responses to UV-radiation. In: Whitton, BA, Potts, M (Eds.) Ecology of Cyanobacteria: Their Diversity in Time and Space, Kluwer, Dordrecht, pp 591–611
Des Marais, DJ (2003) Biogeochemistry of hypersaline microbial mats illustrates the dynamics of modern microbial ecosystems and the early evolution of the biosphere. Biol Bull 204: 160–167
de Wit, R, Falcón, LI, Charpy-Roubaud, C (2005) Heterotrophic dinitrogen fixation (acetylene reduction) in phosphate fertilized Microcoleus chthonoplastes microbial mat from the hypersaline inland lake “la Salada de Chiprana” (NE Spain). Hydrobiology 534: 245–253
Dugdale, RC, Goering, JJ (1967) Uptake of new and regenerated forms of nitrogen in primary productivity. Limnol Oceanogr 12: 196–206
Eisen, JA et al (2002) The complete genome sequence of Chlorobium tepidum TLS, a photosynthetic, anaerobic, green-sulfur bacterium. Proc Natl Acad Sci U S A 99: 9509–9514
Falcón, LI, Escobar-Briones, E, Romero, D (2002) Nitrogen fixation patterns displayed by cyanobacterial consortia in Alchichica crater-lake, Mexico. Hydrobiology 467: 71–78
Falcón, LI, Carpenter, EJ, Cipriano, F, Bergman, B, Capone, DG (2004) N2-fixation by unicellular bacterioplankton in the Atlantic and Pacific Oceans: phylogeny and in situ rates. Appl Environ Microbiol 70: 765–770
Fernández-Valiente, E, Quesada, A, Howard-Williams, C, Hawes, I (2001) N2 fixation in cyanobacterial mats from ponds on the McMurdo ice shelf, Antarctica. Microb Ecol 42: 338–349
Garcia-Pichel, F, Castenholz, RW (1991) Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. J Phycol 27: 395–409
Garcia-Pichel, F, Al-Horani, FA, Farmer, JD, Ludwig, R, Wade, BD (2004) Balance between microbial calcification and metazoan bioerosion in modern stromatolitic oncolites. Geobiology 49–57
Gest, H (1994) Discovery of the heliobacteria. Photosynth Res 41: 17–21
Johannesson, KH, Cortés, A, Kilroy, KC (2004) Reconnaissance isotopic and hydrochemical study of Cuatro Ciénegas groundwater, Coahuila, México. J South Am Earth Sci 17: 171–180
Kemp, PF, Aller, JY (2004) Estimating prokaryotic diversity: when are 16S rDNA libraries large enough? Limnol Oceanogr: Methods 2: 114–125
Kirshtein, JD, Paerl, HW, Zehr, J (1991) Amplification, cloning and sequencing of a nifH segment from aquatic microorganisms and natural communities. Appl Environ Microbiol 57: 2645–2650
Ley, RE, Harris, JK, Wilcox, J, Spear, JR, Miller, SR, Bebout, BM, Maresca, JA, Bryant, DA, Sogin, ML, Pace, NR (2006) Unsuspected diversity and complexity of the Guerrero Negro hypersaline microbial mat. Appl Environ Microbiol 72: 3685–3695
Madigan, MT, Martinko, JM, Parker, J (2000) Brock Biology of Microorganisms. Prentice-Hall International, London, UK, pp 991
McKee, JW, Jone, NW, Long, LE (1990) Stratigraphy and provenance of strata along the San Marcos fault, central Coahuila, Mexico. Geol Soc Amer Bull 102: 593–614
Larimer, FW et al (2004) Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nat Biotechnol 22: 55–61
Omoregie, EO, Crumbliss, LL, Bebout, BM, Zehr, JP (2004) Determination of nitrogen-fixing phylotypes in Lyngbya sp. and Microcoleus chthonoplastes cyanobacterial mats from Guerrero Negro, Baja California, Mexico. Appl Environ Microbiol 70: 2119–2128
Omoregie, EO, Crumbliss, LL, Bebout, BM, Zehr, JP (2004) Comparison of diazotrophic community structure in Lyngbya sp. and Microcoleus chthonoplastes dominated microbial mats from Guerrero Negro, Baja, Mexico. FEMS Microbiol Ecol 47: 305–318
Overmann, J, García-Pichel, F (2005) The phototrophic way of life. In: Dworkin, M (Eds.) The Prokaryotes: An Evolving Electronic Resource for the Microbiological Community, 3rd ed., Springer, Berlin Heidelberg New York, p 89
Paerl, HW, Pinckney, JL, Steppe, TF (2000) Cyanobacterial-bacterial mat consortia: examining the functional unit of microbial survival and growth in extreme environments. Environ Microbiol 21: 11–26
Pinckney, JL, Paerl, HW (1997) Anoxygenic photosynthesis and nitrogen fixation by a microbial mat community in a Bahamian hypersaline lagoon. Appl Environ Microbiol 63: 420–426
Pinckney, J, Paerl, HW, Reid, RP, Bebout, B (1995) Ecophysiology of stromatolitic microbial mats, Stocking Island, Exuma-Cay, Bahamas. Microb Ecol 29: 19–37
Raymond, J, Siefert, JL, Staples, CR, Blankenship, RE (2004) The natural history of nitrogen fixation. Mol Biol Evol 21: 541–554
Rippka, R, Deruelles, J, Waterbury, JB, Herdman, M, Stanier, RY (1979) Generic assignments, Strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111: 1–61
Schopf, JW, Packer, BM (1987) Early Archean (3.3 billion to 3.5 billion-year-old) microfossils from Warrawoona Group, Australia. Science 237: 70–73
Sheridan, RP (2001) Role of ultraviolet radiation in maintaining the three dimensional structure of a cyanobacterial mat community and facilitating nitrogen fixation. J Phycol 37: 731–737
Stal, LJ (1995) Physiological ecology of cyanobacteria in microbial mats and other communities (Tansley Review 84). New Phytol 131: 1–32
Stal, LJ, Caumette, P (1994) Microbial mats, structure, development and environmental significance. NATO ASI Series 35, Springer, Berlin Heidelberg New York, p 463
Steppe, TF, Paerl, HW (2002) Potential N2 fixation by sulfate-reducing bacteria in a marine intertidal microbial mat. Aquat Microb Ecol 28: 1–12
Steunou, AS, Bhaya, D, Bateson, MM, Melendrez, MC, Ward, DM, Brecht, E, Peters, JW, Kuhl, M, Grossman, AR (2006) In situ analysis of nitrogen fixation and metabolic switching in unicellular themophilic cyanobacteria inhabiting hot spring microbial mats. Proc Natl Acad Sci USA 103(7): 2398–2403
Souza, et al (2006) An endangered oasis of aquatic microbial biodiversity in the Chihuahuan desert. Proc Natl Acad Sci USA 103: 6545–6570
Sprent, JI, Sprent, P (1990) Nitrogen-fixing organisms: Pure and applied aspects. Chapman & Hall, London, pp 256
Stryer, L (1995) Biochemistry. Freeman and Co., NY, USA, p 1064
Tomitani, A, Knoll, AH, Cavanaugh, CM, Ohno, T (2006) The evolutionary diversification of cyanobacteria: Molecular-phylogenetic and paleontological perspectives. Proc Natl Acad Sci USA 103: 5442–5447
Van Gemerden, H (1993) Microbial mats: a joint venture. Mar Geol 113: 3–25
Wall, JD (2004) Rain or shine—a phototroph that delivers. Nat Biotechnol 22: 40–41
Walter, MR, Grotzinger, JP, Schopt, JW (1992) Proterozoic stromatolites. In: Schopf, JW, Klein, C (Eds.) The Proterozoic Biosphere: A Multidisciplinary Study. Cambridge University Press, Cambridge, pp 253–260
Zani, S, Mellon, MT, Collier, JL, Zehr, JP (2000) Expression of nifH genes in natural microbial assemblages of Lake George, NY detected with RT-PCR. Appl Environ Microbiol 66: 3119–3124
Zhang, Y, Pohlmann, EL, Ludden, PW, Roberts, GP (2000) Mutagenesis and functional characterization of glnB, glnA and nifA genes from the photosynthetic bacterium Rhodospirillum rubrum. J Bacteriol 182: 983–992
Zhou, J, Bruns, MA, Tiedje, JM (1996) DNA recovery from soils of diverse composition. Appl Environ Microbiol 62: 316–322
Acknowledgements
This work was supported by Consejo Nacional de Ciencia y Tecnología, Mexico. L.F. had a postdoctoral fellowship from Universidad Nacional Autonoma de Mexico. We thank Comisión Nacional para el Conocimiento y Uso de la Biodiversidad for elements used in Fig. 1 and are also grateful to L. Espinosa-Asuar for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Falcón, L.I., Cerritos, R., Eguiarte, L.E. et al. Nitrogen Fixation in Microbial Mat and Stromatolite Communities from Cuatro Cienegas, Mexico. Microb Ecol 54, 363–373 (2007). https://doi.org/10.1007/s00248-007-9240-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-007-9240-3